Abstract
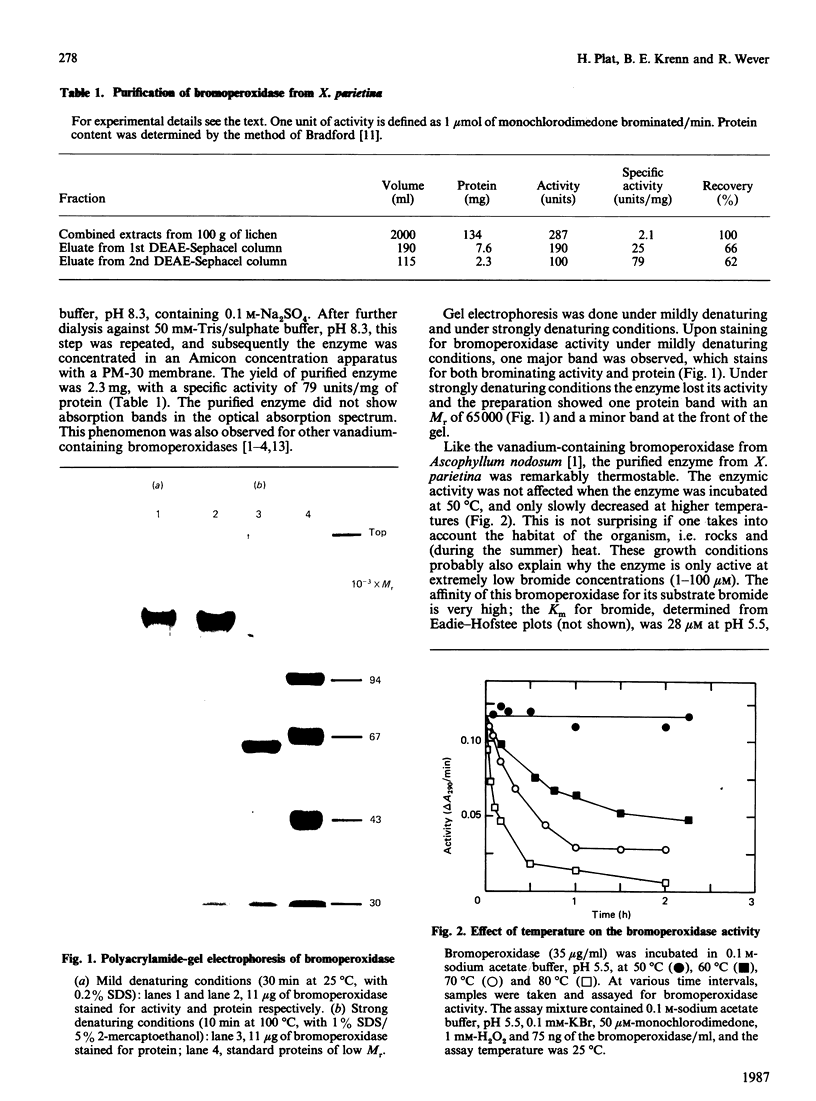
A novel bromoperoxidase was isolated from the lichen Xanthoria parietina. The enzyme contained vanadium, which is essential for enzymic activity. Under denaturating conditions the preparation showed a single protein band with an Mr of 65,000. Thermal-denaturation studies showed that this bromoperoxidase could tolerate high temperatures. The affinity of the enzyme for its substrate bromide is high; the Km for bromide was 29 microM. Excess halides (50 mM) inhibited enzymic activity considerably.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Hales B. J., Case E. E., Morningstar J. E., Dzeda M. F., Mauterer L. A. Isolation of a new vanadium-containing nitrogenase from Azotobacter vinelandii. Biochemistry. 1986 Nov 18;25(23):7251–7255. doi: 10.1021/bi00371a001. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]