Introduction
In recent years, with the rapid development of biological therapies, cancer treatment has progressed substantially. As a result, a range of side effects associated with antitumor therapy are receiving increased attention. Cancer therapy-related cardiac dysfunction (CTRCD) includes myocarditis caused by immune checkpoint inhibitors (ICIs), cardiotoxicity of targeted drugs, and vasospasm associated with chemotherapy drugs, etc. Among these, ICI-induced myocarditis has the highest mortality rate, at more than 27% (1-3). The cumulative incidence of moderate or severe CTRCD is 1.7%, while the cumulative incidence of mild asymptomatic CTRCD is 9.3% to 49.2% (4). Myocardial injury may lead to the interruption of treatment and a change of antitumor strategy, which can ultimately affect patient prognosis (5). Therefore, early diagnosis and proper management of CTRCD are crucial.
The diagnosis of CTRCD depends on the symptoms and signs, laboratory tests, imaging examination, and myocardial biopsy (6). However, myocardial biopsy has not been widely performed due to the risks of the procedure. With the development of magnetic resonance technology, cardiac magnetic resonance (CMR) has become the gold standard for volumetric assessment and noninvasive myocardial tissue characterization (7). However, due to the low incidence of CTRCD, few studies have reported the role of CMR images in diagnosing and intervening in the CTRCD process.
In this paper, we present and discuss the CMR imaging features of three patients with CTRCD with reference to the processes of diagnosis, management, and prognosis.
Case presentation
All procedures in this study were performed in accordance with the ethical standards of the ethics committee of Henan Cancer Hospital (No. 2021-KY-0159) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for publication of this article and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Case 1
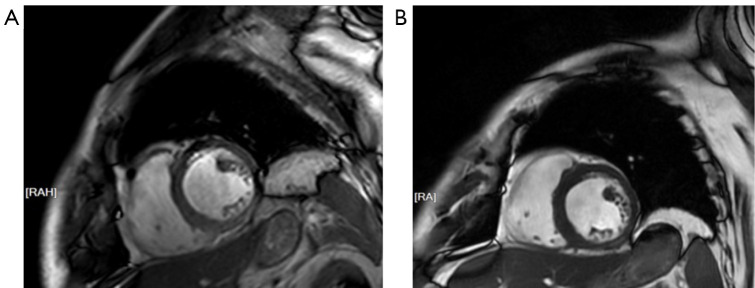
A 68-year-old male with primary liver cancer received donafenib and toripalimab combined with transarterial chemoembolization (TACE) treatment for 6 cycles (clinical study). He then experienced palpitations and interval chest tightness. The troponin (Tn) level increased to 1.624 ng/mL. Cardiac ultrasound showed a slightly larger left atrium and decreased left ventricular diastolic function. Computed tomography (CT) indicated multiple calcium plaques present in the aorta and coronary arteries. He was diagnosed with myocardial infarction at a local hospital and received aspirin, hydroclopidogrel, rosuvastatin, bisexolol, and levamlodipine. However, he complained of feeling worse. Electrocardiography (ECG) of the patient showed sinus rhythm and complete right bundle branch block. Cardiac ultrasound showed left atrium enlargement with an anterior and posterior diameter of 41 mm and a left ventricular ejection fraction (LVEF) of 69%. The Tn level was 2.21 ng/mL, and N-terminal pro b-type natriuretic peptide (NT-proBNP) level was 1,150.19 pg/mL. As a result, he was diagnosed with ICI-related myocarditis and treated immediately with methylprednisolone sodium succinate (2 mg/kg/d). However, 2 days after treatment, the Tn and NT-proBNP levels had risen drastically, and the patient’s chest tightness was significantly aggravated. He was the transferred to department of Immunotherapy. He exhibited orthopnea, and fulminant myocarditis could not be ruled out. CMR indicated the following: (I) left atrial enlargement and mild mitral valve regurgitation, (II) a slight decrease in the thickness of the midventricular inferior and inferolateral walls, and (III) bilateral pleural effusion (Figure 1A). Meanwhile, ECG indicated the following: (I) sinus rhythm, (II) complete right bundle branch block, and (III) frequent premature ventricular contractions. He was prescribed methylprednisolone sodium succinate (500 mg/d) and gamma globulin (25 g/d) for 5 days. Next, antithymocyte globulin (75 mg/d) was slowly administered. In addition, cardiac stimulants, antiarrhythmic drugs, and intermittent furosemide were used to alleviate symptoms. As a result, the patient’s Tn and Pro-BNP levels returned to the normal range, and his symptoms gradually resolved. A second ECG indicated (I) sinus rhythm, (II) complete right bundle branch block, and (III) a QT of 460 ms. Thus, the dose of glucocorticoids was gradually reduced. A second CMR scan suggested a recovery of the myocardial thickness (Figure 1B). The patient was discharged several days after the condition was stable. Oral methylprednisolone was gradually reduced to complete withdrawal after discharge.
Figure 1.
CMR images of the patient before and after treatment in our department. (A) The thickness of midventricular inferior and inferolateral wall seemed to be slightly lower than normal. (B) The thickness of corresponding ventricular appeared normal in subsequent follow-up. RA, right anterior; RAH, right anterior head; CMR, cardiac magnetic resonance.
Case 2
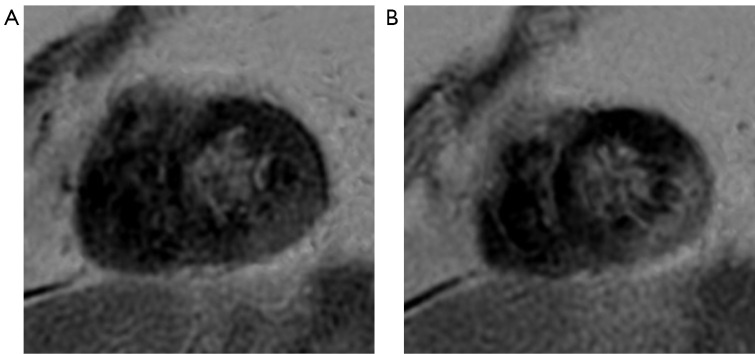
A 57-year-old male was diagnosed with transverse colon adenocarcinoma with multiple metastases. He had received multiline salvage therapy (previous treatments including FOLFOX, oxaliplatin, capecitabine, and bevacizumab), and the final treatment regimen (irinotecan, leucovorin calcium, fluorouracil, and bevacizumab) had concluded. The patient occasionally experienced shoulder and back pain, and the Tn level was abnormal before treatment. CTRCD could not be ruled out, so the originally planned treatment was postponed. ECG and cardiac ultrasound showed no abnormalities, and no abnormalities were found on an MR scan of the right shoulder joint. CMR indicated (I) a slightly reduced thickness of the inner inferolateral wall at the mid-to-apical portion of the ventricle and (II) focal patchy delayed enhancement in the inferior and partial inferolateral wall of the apical ventricle showed indicating myocardial injury (Figure 2). Treatment recommendations were as follows: myocardial polarization fluid [potassium and magnesium aspartate + 10% glucose and sodium chloride (GS) + insulin], ubiquinone Q10, and trimetazidine. The Tn level gradually decreased to normal 6 days after treatment, and 7 days after treatment, the pain in the shoulder and back had almost completely disappeared. Ultimately, the originally planned antitumor treatment was continued.
Figure 2.
Late gadolinium enhancement images of case 2 six days after diagnosis. Focal patchy delayed enhancement lesion was observed in the inferior and inferolateral wall at the apical portion of the ventricle.
Case 3
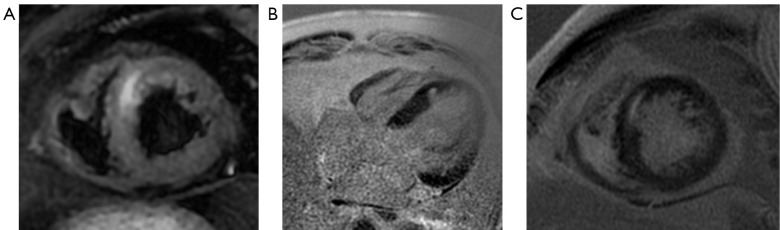
A 52-year-old male was diagnosed with kidney cancer and lung metastasis. He had hypertension for 2 years, and blood pressure was maintained stably by antihypertensive drugs (amlodipine and enalapril). He received sindilizumab for 22 months. His Tn level was elevated to 2.61 ng/mL before admission. ECG showed ST-T alterations present on part of the leads. Cardiac ultrasound findings were normal. The patient did not have any symptoms, but CMR suggested myocardial injury (Figure 3), and thus CTRCD was considered. Cardiac polarization fluid, ubiquinone Q10, and tritazidine were administered but glucocorticoids were not, and the Tn level gradually decreased after treatment.
Figure 3.
Cardiac MR imaging of case 3 at diagnosis before treatment. (A) T2W imaging in the short-axis view showed a striatal increased myocardial signal in the septal wall of the apical portion of the ventricle. (B,C) A focal apical-ventricular septal delayed enhancement lesion was apparent in the four-chamber and short-axis views. MR, magnetic resonance; T2W, T2-weighted.
Discussion
Cardiotoxicity is one of the most life-threatening adverse drug reactions in cancer treatment, especially in patients receiving immunotherapy. The means to diagnosing and treating CTRCD, reducing the risk and degree of cardiotoxicity injury, and ultimately improving the prognosis of patients has been intensively investigated. However, assessing cardiotoxicity in cancer treatment still remains challenging, especially if the test results or symptoms are atypical (1). Moreover, cardiologists and oncologists are faced with an increasing number of patients with CTRCD.
In most cases, ECG, Tn level, NT-pro-BNP level, and cardiac ultrasound serve as pragmatic approaches for the regular monitoring of cardiac toxicities (6). Significant reductions in LVEF have been reported as a late marker of impaired cardiac function, occurring only after a longer period of myocardial damage and when all compensatory mechanisms are no longer adequate (8). The 2020 European Society of Medical Oncology (ESMO) Consensus states that measuring the Tn level during baseline examination of cancer treatment may be helpful in predicting or assessing cardiovascular toxicity after treatment (9). The recent Cardio-Oncology Clinical Practice Guidelines list myocardial markers such as Tn and BNP as diagnostic indicators for CTRCD (10).
CMR is more sensitive and specific in the early stages of injury by virtue of its unique tissue characterization (11,12). CMR is helpful in detecting the pathological phenomena of edema and vacuolation in early cardiomyocytes. Importantly, cavitation in the early stage is reversible after treatment. This process manifests as early T1/T2 prolongation on CMR and normalization of T1/T2 relaxation time in postresolution CMR, further supporting its occurrence in the reversible disease stage (7). This is crucial in the early identification of CTRCD and suggests that CMR is particularly valuable in detecting subclinical myocardial injury. Because CTRCD has a narrow time window for treatment once it occurs, missed intervention is one of the main causes of the high mortality rate. In particular, CMR helps to expand the intervention time window of certain high-risk groups and to reduce the interruption of antitumor therapy caused by CTRCD. In the three cases we reported, CMR contributed significantly to diagnosing CTRCD.
In case 2, the patient had chest tightness, fatigue, nausea and loss of appetite, while ECG and cardiac ultrasound were normal, the Tn level was elevated, indicating the possible presence of CTRCD, with abnormal CMR images further supporting this diagnosis. In case 3, the patient had no symptoms and only changes in Tn level, and thus clinically concealed CTRCD could not be ruled out. CMR provides critical information for the early diagnosis and intervention in CTRCD. Moreover, early identification and early intervention of CTRCD are significant for the prevention of myocardial injury in the reversible stage of cardiotoxicity. Moreover, in our case, the dynamic changes of Tn level were consistent with the CMR manifestations. In our treatment, the collaborative evaluation of Tn level and CMR enabled the earliest possible diagnosis of CTRCD, providing better clinical outcomes in the reversible treatment of myocardial injury.
In case 1, the first MRI examination showed a slight thinning of the left ventricular wall locally and supported the diagnosis of myocardial infarction. After antimyocarditis treatment, the ventricular wall thickness returned to normal. However, this is inconsistent with our knowledge that myocarditis can lead to thickening of the ventricular wall. Through retrospective analysis of the images, we found there were artifacts in the images in the first examination of case that might have affected our judgment regarding ventricular wall thickness. Therefore, as it pertains to this case, whether myocarditis can cause thinning of the ventricular wall needs further verification.
Monitoring serum Tn level can not only facilitate the diagnosis of myocardial damage but also guide the early use of cardioprotective drugs to prevent myocardial damage from fulminant myocarditis (6), which is well demonstrated in our cases. Glucocorticoid administration is a cornerstone treatment for CTRCD, but the timing and dosage should be carefully considered according to the grading of myocardial damage (13). Occasionally, with the early diagnosis of CTRCD, glucocorticoids can be avoided altogether. As shown above, two patients (case 2 and case 3) recovered from CTRCD without glucocorticoid usage. In addition, gamma globulin and antithymocyte immunoglobulin can be administered simultaneously in severe cases, such as case 1. Because myocardial biopsies are invasive tests that are difficult to perform widely, the relatively new application of CMR could allow cardio-oncologists to quickly and accurately differentiate myocardial damage, which could aid in more personalized treatment. We hope that with the application of more advanced diagnostic methods and new therapeutic drugs, CTRCD’s early noninvasive diagnosis and therapeutic response detection could be achieved more widely and accurately.
During this study, we only scanned conventional sequences for all cases. Some advanced sequences, such as T1 and T2 mapping, which are of particular interest in the current research on myocardial injury, were not used. In future work, we are committed to applying these sequences to better reveal the imaging manifestations of myocarditis in order to help clinically identify early myocardial abnormalities. Additionally, the application of the Lake Louis criteria in myocarditis diagnosis can also be evaluated.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: This study was funded by Henan provincial Medical Science and Technology Research Project (grant No. LHGJ20210206 to Y.Z. and grant No. LHGJ20210198 to F.Z.).
Ethics Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures in this study were performed in accordance with the ethical standards of the ethics committee of Henan Cancer Hospital (No. 2021-KY-0159) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for publication of this article and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Footnotes
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-910/coif). The authors have no conflicts of interest to declare.
References
- 1.Chaganti BT, Negishi K, Okajima K. Role of Myocardial Strain Imaging in Cancer Therapy-Related Cardiac Dysfunction. Curr Cardiol Rep 2022;24:739-48. 10.1007/s11886-022-01692-7 [DOI] [PubMed] [Google Scholar]
- 2.Moslehi JJ, Salem JE, Sosman JA, Lebrun-Vignes B, Johnson DB. Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. Lancet 2018;391:933. 10.1016/S0140-6736(18)30533-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Escudier M, Cautela J, Malissen N, Ancedy Y, Orabona M, Pinto J, Monestier S, Grob JJ, Scemama U, Jacquier A, Lalevee N, Barraud J, Peyrol M, Laine M, Bonello L, Paganelli F, Cohen A, Barlesi F, Ederhy S, Thuny F. Clinical Features, Management, and Outcomes of Immune Checkpoint Inhibitor-Related Cardiotoxicity. Circulation 2017;136:2085-7. 10.1161/CIRCULATIONAHA.117.030571 [DOI] [PubMed] [Google Scholar]
- 4.Mecinaj A, Gulati G, Ree AH, Gravdehaug B, Røsjø H, Steine K, Wisløff T, Geisler J, Omland T, Heck SL. Impact of the ESC Cardio-Oncology Guidelines Biomarker Criteria on Incidence of Cancer Therapy-Related Cardiac Dysfunction. JACC CardioOncol 2024;6:83-95. 10.1016/j.jaccao.2023.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Curigliano G, Cardinale D, Dent S, Criscitiello C, Aseyev O, Lenihan D, Cipolla CM. Cardiotoxicity of anticancer treatments: Epidemiology, detection, and management. CA Cancer J Clin 2016;66:309-25. 10.3322/caac.21341 [DOI] [PubMed] [Google Scholar]
- 6.Lyon AR, López-Fernández T, Couch LS, Asteggiano R, Aznar MC, Bergler-Klein J, et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur Heart J 2022;43:4229-361. 10.1093/eurheartj/ehac244 [DOI] [PubMed] [Google Scholar]
- 7.Leo I, Vidula M, Bisaccia G, Procopio MC, Licordari R, Perotto M, La Vecchia G, Miaris N, Bravo PE, Bucciarelli-Ducci C. The Role of Advanced Cardiovascular Imaging Modalities in Cardio-Oncology: From Early Detection to Unravelling Mechanisms of Cardiotoxicity. J Clin Med 2023;12:4945. 10.3390/jcm12154945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ewer MS, Lenihan DJ. Left ventricular ejection fraction and cardiotoxicity: is our ear really to the ground? J Clin Oncol 2008;26:1201-3. 10.1200/JCO.2007.14.8742 [DOI] [PubMed] [Google Scholar]
- 9.Curigliano G, Lenihan D, Fradley M, Ganatra S, Barac A, Blaes A, et al. Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann Oncol 2020;31:171-90. 10.1016/j.annonc.2019.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leong DP, Lenihan DJ. Clinical Practice Guidelines in Cardio-Oncology. Heart Fail Clin 2022;18:489-501. 10.1016/j.hfc.2022.02.002 [DOI] [PubMed] [Google Scholar]
- 11.Park HS, Hong YJ, Han K, Kim PK, An E, Lee JY, Park CH, Lee HJ, Hur J, Kim YJ, Choi BW. Ultrahigh-field cardiovascular magnetic resonance T1 and T2 mapping for the assessment of anthracycline-induced cardiotoxicity in rat models: validation against histopathologic changes. J Cardiovasc Magn Reson 2021;23:76. 10.1186/s12968-021-00767-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Faron A, Isaak A, Mesropyan N, Reinert M, Schwab K, Sirokay J, Sprinkart AM, Bauernfeind FG, Dabir D, Pieper CC, Heine A, Kuetting D, Attenberger U, Landsberg J, Luetkens JA. Cardiac MRI Depicts Immune Checkpoint Inhibitor-induced Myocarditis: A Prospective Study. Radiology 2021;301:602-9. 10.1148/radiol.2021210814 [DOI] [PubMed] [Google Scholar]
- 13.Li X, Peng W, Wu J, Yeung SJ, Yang R. Advances in immune checkpoint inhibitors induced-cardiotoxicity. Front Immunol 2023;14:1130438. 10.3389/fimmu.2023.1130438 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as