Abstract
Background and Aims
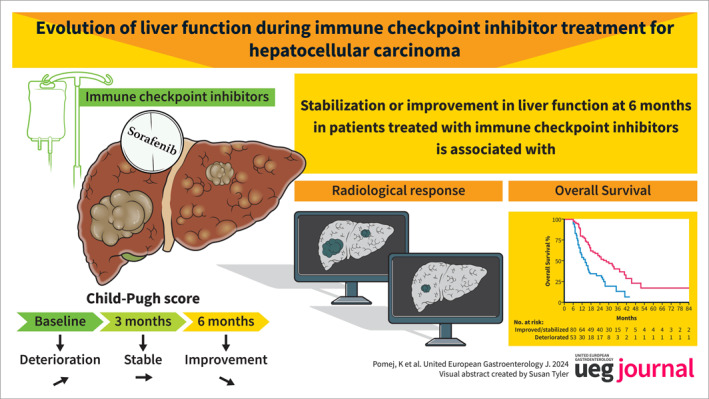
Deterioration of liver function is a leading cause of death in patients with advanced hepatocellular carcinoma (HCC). We evaluated the impact of immune checkpoint inhibitor (ICI)‐treatment on liver function and outcomes.
Method
HCC patients receiving ICIs or sorafenib between 04/2003 and 05/2024 were included. Liver function (assessed by Child‐Pugh score [CPS]) was evaluated at the start of ICI‐treatment (baseline, BL) and 3 and 6 months thereafter. A ≥1 point change in CPS was defined as deterioration (−) or improvement (+), while equal CPS points were defined as stable (=).
Results
Overall, 182 ICI‐treated patients (66.8 ± 11.8 years; cirrhosis: n = 134, 74%) were included. At BL, median CPS was 5 (IQR: 5–6; CPS‐A: 147, 81%). After 3 months, liver function improved/stabilized in 102 (56%) and deteriorated in 61 (34%) patients, while 19 (10%) patients deceased/had missing follow‐up (d/noFU). Comparable results were observed at 6 months (+/=: n = 82, 45%; −: n = 55, 30%; d/noFU: n = 45, 25%). In contrast, 54 (34%) and 33 (21%) out of 160 sorafenib patients achieved improvement/stabilization at 3 and 6 months, respectively. Radiological response was linked to CPS improvement/stabilization at 6 months (responders vs. non‐responders, 73% vs. 50%; p = 0.007). CPS improvement/stabilization at 6 months was associated with better overall survival following landmark analysis (6 months: +/=: 28.4 [95% CI: 18.7–38.1] versus −: 14.2 [95% CI: 10.3–18.2] months; p < 0.001). Of 35 ICI‐patients with CPS‐B at BL, improvement/stabilization occurred in 16 (46%) patients, while 19 (54%) patients deteriorated/d/noFU at 3 months. Comparable results were observed at 6 months (CPS +/=: 14, 40%, −: 8, 23%). Importantly, 6/35 (17%) and 9/35 (26%) patients improved from CPS‐B to CPS‐A at 3 and 6 months.
Conclusion
Radiological response to ICI‐treatment was associated with stabilization or improvement in liver function, which correlated with improved survival, even in patients with Child‐Pugh class B at baseline.
Keywords: hepatocellular carcinoma, immune checkpoint inhibitors, liver cancer, liver cirrhosis, liver function, sorafenib
Key summary.
What are the significant and/or new findings of this study?
In our retrospective study including 182 immune checkpoint inhibitor (ICI)‐treated patients, we observed an improvement/stabilization of liver function in more than half of the patients at 3 months, which was widely preserved at 6 months.
Achieving an objective response (ORR) to treatment linked to a beneficial effect on liver function and patients with improved or stabilized liver function had a significantly better survival.
The proportion of patients with a sustained stabilization or improvement of liver function was markedly higher in patients receiving ICI‐based therapies compared with patients receiving sorafenib.
INTRODUCTION
Hepatocellular carcinoma (HCC) represents the third most common cause of cancer‐related death globally 1 with a predicted increase in incidence by >50% until 2040. 2 Even though screening programs have been implemented in many countries, the majority of patients are still diagnosed at advanced stages only amenable to palliative systemic treatment. 3 While tyrosine kinase inhibitors dominated the field for more than a decade, immune checkpoint inhibitor (ICI)‐based combinations (e.g., atezolizumab plus bevacizumab, tremelimumab plus durvalumab) now represent the standard of care in systemic front‐line treatment for the majority of patients. 3 , 4 , 5
In contrast to numerous other cancer entities, prognosis does not only depend on tumor burden and tumor biology but also on the severity of the underlying liver disease, as most HCC lesions develop in patients with cirrhosis. 3 Importantly, phase III clinical trials, which established the current standard‐of‐care systemic therapies, excluded patients with Child‐Pugh class B or C to prevent interference of study results by non‐HCC related outcomes. 6 , 7 , 8 Therefore, no high‐level evidence for the treatment of patients with impaired liver function exists. Nevertheless, these patients are commonly receiving systemic therapies in real‐world clinical practice. 5
While the prognosis of patients with HCC and Child‐Pugh class B is inherently worse than that of Child‐Pugh A patients, some Child‐Pugh B patients may still derive a clinically meaningful benefit. 9 , 10 In a small prospective phase I/II clinical trial (cohort five of the Checkmate‐040 study), nivolumab monotherapy was safe and resulted in a reasonable overall response rate (ORR) in 49 patients with Child‐Pugh class B, and some patients (mostly responders) even achieved a durable improvement to Child‐Pugh class A. 11
While real‐world evidence supporting the safety and efficacy of ICI‐treatment in patients with advanced HCC is accumulating, these studies did not assess the evolution of liver function as an important determinant of overall survival (OS). 12
Therefore, we assessed changes in liver function during ICI‐treatment in patients with Child‐Pugh class A or B in a large real‐world cohort recruited from two tertiary care centers. In addition to assessing the association between stabilization of liver function and OS, we correlated radiological response to treatment with changes in liver function, and assessed the proportion of patients improving from Child‐Pugh class B to A.
METHODS
Study design and patients
Patients (≥18 years) with histologically or radiologically confirmed advanced HCC, receiving ICIs or sorafenib at the Medical University of Vienna (Austria) and the Hôpital Beaujon (France) between 04/2003 and 05/2024, were included. Patients with Child‐Pugh class C, Barcelona clinic liver cancer (BCLC) stage D, and patients with insufficient records (i.e., missing baseline data or follow‐up of <3 months after inclusion) were excluded from this study. Patient characteristics, laboratory parameters, tumor characteristics, information on previous/current treatments, and Eastern Cooperative Oncology Group performance status were collected from the clinical documentation systems. Baseline (BL) was defined as the date of start of systemic therapy.
Data analysis was approved by the local ethics committee of the Medical University of Vienna. Due to the retrospective nature of the study, the need for written informed consent was waived.
Definition and evaluation of changes in liver function
Liver function was evaluated at the start of ICI or sorafenib treatment (BL) as well as 3 and 6 months thereafter using Child‐Pugh score (CPS) and Albumin‐Bilirubin (ALBI) grades. For CPS/ALBI calculations, we considered available laboratory parameters up to 28 days before BL and within 14 days prior to or after the respective timepoints at 3 and 6 months. Changes in CPS points/ALBI grades over time were defined as deterioration (−) or improvement (+), while equal CPS points/ALBI grades were defined as stable (=). Changes in CPS points/ALBI grades at both timepoints were calculated and compared with BL. For all group comparisons, except landmark analyses, patients were included in the improved or stable (+/=) versus the deteriorated or deceased or lost to follow‐up (−/d/noFU) group. For landmark analyses, we only considered patients with ongoing follow‐up at the respective landmark time points (3 and 6 months). An equally sized control cohort of HCC patients treated with the tyrosine kinase inhibitor sorafenib was used for comparison of liver function changes.
Study endpoints
Duration of systemic therapy was defined as time from treatment initiation until the date of treatment stop or death/last follow‐up (for censored patients). OS was defined as the time from the start of systemic therapy until the date of death or last follow‐up (for censored patients). Progression free survival (PFS) was defined as the time from the start of systemic therapy until the date of radiological progression or date of death/last follow‐up, whatever came first. Overall response rate (ORR) was defined as the proportion of patients with complete response (CR) or partial response (PR) as the best overall response. The best overall response was assessed according to the modified Response Evaluation Criteria in Solid Tumors. 13
Statistical analyses
Statistical analyses were performed using IBM SPSS Statistics 27 (SPSS Inc.), R 4.3.1 (R Core Team, R Foundation for Statistical Computing) and GraphPad Prism 9 (GraphPad Software). Continuous variables were reported as mean ± standard deviation (SD) or median (IQR), and categorical variables were shown as numbers (n) and proportions (%) of patients. Comparisons of proportions and continuous variables were performed by the chi‐squared test and unpaired Student's t test, respectively. Median estimated follow‐up was calculated using the reverse Kaplan–Meier method. Landmark analyses of OS at 3 and 6 months were conducted, and comparisons were performed using log‐rank test. Survival curves were plotted using the Kaplan–Meier method. A Simon–Makuch plot was generated to graphically demonstrate the impact of liver function improvement/stabilization on OS considering the change in liver function as a time‐dependent covariable. 14 Uni‐ and multivariable cox regression analysis was calculated using death as the outcome of interest and time on active treatment and progression as time‐dependent covariable. In patients with incomplete dates, that is, available month and year but missing day, the 15th of the respective month was used for calculations. A two‐sided p‐value ≤0.05 was considered statistically significant.
RESULTS
Patient characteristics
In total, 436 HCC patients treated with palliative systemic therapies at two institutions between 04/2003 and 05/2024 were screened for this study. Patient selection after application of in‐ and exclusion criteria is shown in Figure 1. One hundred eighty‐two patients who received ICI‐treatment were included, while the control cohort comprised 160 sorafenib‐treated patients. In the ICI‐cohort, most patients were male (n = 145, 80%) with a mean age of 66.8 ± 11.8 years. Cirrhosis was present in 134 (74%) patients, predominantly with non‐viral etiology (n = 104, 57%). At BL, median CPS was 5 (IQR: 5–6, CPS A: n = 147, 81%, CPS B: 35, 19%), and the majority of patients had advanced stage HCC (BCLC C: n = 129, 71%). ICI‐treatment was initiated as systemic first‐line treatment in 148 (81%) of ICI‐treated patients and median estimated follow‐up was 33.1 (95% CI: 31.4–34.7) months. Detailed patient characteristics are shown in Table 1 and Table S1.
FIGURE 1.
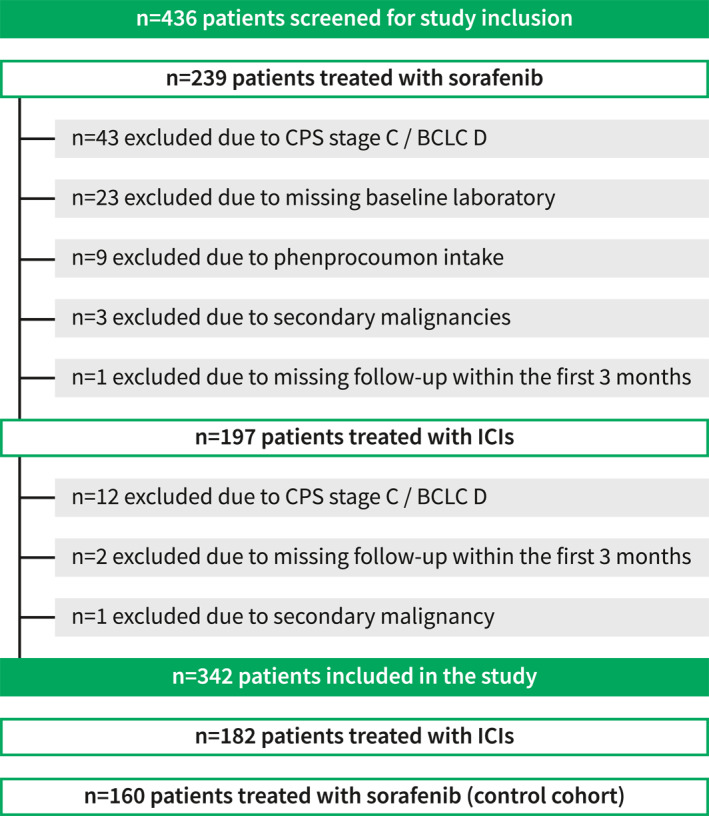
Patient flowchart. BCLC, Barcelona clinic liver cancer; CPS, Child‐Pugh score; ICIs, immune checkpoint inhibitors.
TABLE 1.
Baseline characteristics.
| ICI cohort n = 182 | Child‐Pugh A n = 147 | Child‐Pugh B n = 35 | p‐value a | |
|---|---|---|---|---|
| N (%) and median (IQR)/mean ± SD | ||||
| Age, years | 66.8 ± 11.8 | 67.3 ± 11.6 | 64.6 ± 12.5 | 0.220 |
| Range | 17.9–89.5 | 17.9–89.5 | 28.7–82.0 | |
| Sex | ||||
| Male | 145 (80%) | 117 (80%) | 28 (88%) | 0.957 |
| Female | 37 (20%) | 30 (20%) | 7 (20%) | |
| Cirrhosis | ||||
| Yes | 134 (74%) | 102 (70%) | 32 (91%) | 0.008 |
| No | 48 (26%) | 45 (30%) | 3 (9%) | |
| Etiology of liver disease | ||||
| HCV | 52 (29%) | 39 (27%) | 13 (37%) | 0.212 |
| HBV | 30 (17%) | 27 (18%) | 3 (9%) | 0.160 |
| ALD | 51 (28%) | 40 (27%) | 11 (31%) | 0.618 |
| MASLD/MASH | 54 (30%) | 47 (32%) | 7 (20%) | 0.163 |
| Others b | 10 (6%) | 7 (5%) | 3 (9%) | 0.408 |
| Unknown | 19 (10%) | 15 (10%) | 4 (11%) | 0.765 |
| Child‐Pugh score, points | 5 (5–6) | 5 (5–6) | 7 (7–8) | ‐ |
| ALBI score, points | −2.5 ([−2.8] to [2.0]) | −2.6 ([−2.8] to [−2.3]) | −1.7 ([−2.0] to [1.4]) | |
| Grade 1 | 74 (41%) | 74 (50%) | ‐ | <0.001 |
| Grade 2 | 102 (56%) | 73 (50%) | 29 (83%) | |
| Grade 3 | 6 (3%) | ‐ | 6 (17%) | |
| ECOG PS | ||||
| 0 | 128 (70%) | 110 (75%) | 18 (51%) | 0.006 |
| ≥1 | 54 (30%) | 37 (25%) | 17 (49%) | |
| MVI c | ||||
| Yes | 66 (37%) | 52 (36%) | 14 (42%) | 0.481 |
| No | 112 (63%) | 93 (64%) | 19 (58%) | |
| EHS c | ||||
| Yes | 82 (46%) | 64 (44%) | 18 (53%) | 0.371 |
| No | 96 (57%) | 80 (56%) | 16 (47%) | |
| BCLC stage | ||||
| A | 2 (1%) | 2 (1%) | ‐ | 0.569 |
| B | 51 (28%) | 43 (29%) | 8 (23%) | |
| C | 129 (71%) | 102 (70%) | 27 (77%) | |
| Line of treatment d | ||||
| 1st | 148 (81%) | 120 (82%) | 28 (80%) | 0.821 |
| 2nd e | 31 (17%) | 25 (17%) | 6 (17%) | |
| Further lines f | 3 (2%) | 2 (1%) | 1 (3%) | |
Abbreviations: ALBI, Albumin‐Bilirubin; BCLC, Barcelona Clinic Liver Cancer; CPS, Child‐Pugh score; ECOG PS, Eastern Cooperative Oncology Group performance status; EHS, extrahepatic spread; HBV, hepatitis B virus; HCV, hepatitis C virus; ICI, immune checkpoint inhibitor; IQR, interquartile range; MASLD/MASH, metabolic dysfunction‐associated steatotic liver disease/metabolic dysfunction associated steatohepatitis; MVI, macrovascular invasion; SD, standard deviation.
CPS A versus CPS B.
Others = biliary liver diseases, autoimmune hepatitis, hemochromatosis, cryptogenic liver disease.
Missing values in n = 4 patients.
n = 146 patients with Atezolizumab/Bevacizumab, n = 16 patients with Pembrolizumab, n = 15 patients with Nivolumab, n = 3 patients with Tislizumab, n = 2 patients with Atezolizumab monotherapy.
First‐line treatments: Sorafenib: n = 23, Lenvatinib: n = 5, Cabozantinib: n = 3.
Previous lines of systemic treatment: Sorafenib/Regorafenib: n = 2, Sorafenib/Lenvatinib/Cabozantinib: n = 1.
Survival and response in ICI‐ and sorafenib‐treated patients
Median OS was 14.2 (95% CI: 11–17.4) and 9.5 (95% CI: 7.7–11.4) months in the ICI and the control cohort, while median PFS was 6.9 (95% CI: 5.4–8.3) and 4.2 (95% CI: 3.2–5.2) months, and both OS and PFS were significantly longer in the ICI versus sorafenib cohort (p < 0.001 for both OS and PFS). Disease control rate and overall response rate (ORR) were 71% versus 62% (p = 0.061) and 39% versus 28% (p = 0.025) in ICI‐ and sorafenib‐treated patients, respectively.
Impact of ICI treatment on liver function and outcome
Three months after ICI initiation, liver function, as measured by CPS, improved (+, n = 13, 7%) or stabilized (=, n = 89, 49%) in 102 (56%) patients, while 61 (34%) patients presented with worse (−) CPS, and 19 (10%) patients were deceased/not evaluable for CPS. Comparable results were observed at 6 months: while 45 (25%) patients had died/were lost to follow‐up, an improvement/stabilization of CPS was achieved in 82 (45%) patients compared to 55 (30%) patients with deterioration of CPS (Table 2a). At both timepoints, significantly more ICI‐treated patients had improved/stable liver function when compared to the sorafenib cohort (3 months: +/=: ICI: 102, 56% vs. control: 54, 34%; p < 0.001; 6 months: +/=: ICI: 82, 45% vs. sorafenib: 33, 21%; p < 0.001).
TABLE 2.
Changes in liver function, measured by Child‐Pugh score, at 3 and 6 months, comparing patients treated with immune checkpoint inhibitors and sorafenib, in all patients (a) and in Child‐Pugh class B patients only (b).
| a) | ICI cohort, n = 182 | Sorafenib cohort, n = 160 | p‐value |
|---|---|---|---|
| N (%) | |||
| At 3 months | |||
| Improved/stabilized | 102 (56%) | 54 (34%) | <0.001 |
| Deteriorated/deceased/noFU | 80 (44%) | 106 (66%) | |
| At 6 months | |||
| Improved/stabilized | 82 (45%) | 33 (21%) | <0.001 |
| Deteriorated/deceased/noFU | 100 (55%) | 127 (79%) | |
| b) | ICI cohort, n = 35 | Sorafenib cohort, n = 44 | p‐value |
|---|---|---|---|
| N (%) | |||
| At 3 months | |||
| Improved/stabilized | 16 (46%) | 12 (27%) | 0.089 |
| Deteriorated/deceased/noFU | 19 (54%) | 32 (73%) | |
| At 6 months | |||
| Improved/stabilized | 14 (40%) | 5 (11%) | 0.003 |
| Deteriorated/deceased/noFU | 21 (60%) | 39 (89%) | |
Abbreviations: CPS, Child‐Pugh score; ICI, immune checkpoint inhibitor; noFU, missing follow up.
Improvement/stabilization of CPS was associated with a significantly better OS following landmark analysis at 3 months (+/=: 17.3 [95% CI: 13.2–21.3] months versus −: 11.1 [95% CI: 8.3–13.9] months; p = 0.023) and 6 months (+/=: 28.4 [95% CI: 18.7–38.1] months versus −: 14.2 [95% CI: 10.3–18.2] months; p < 0.001) (Figure 2). Improvement/stabilization of liver function was also associated with better OS (HR 0.46 [95% CI: 0.30–0.71], p < 0.001) when considering liver function changes as a time‐dependent co‐variable (Figure 2c).
FIGURE 2.
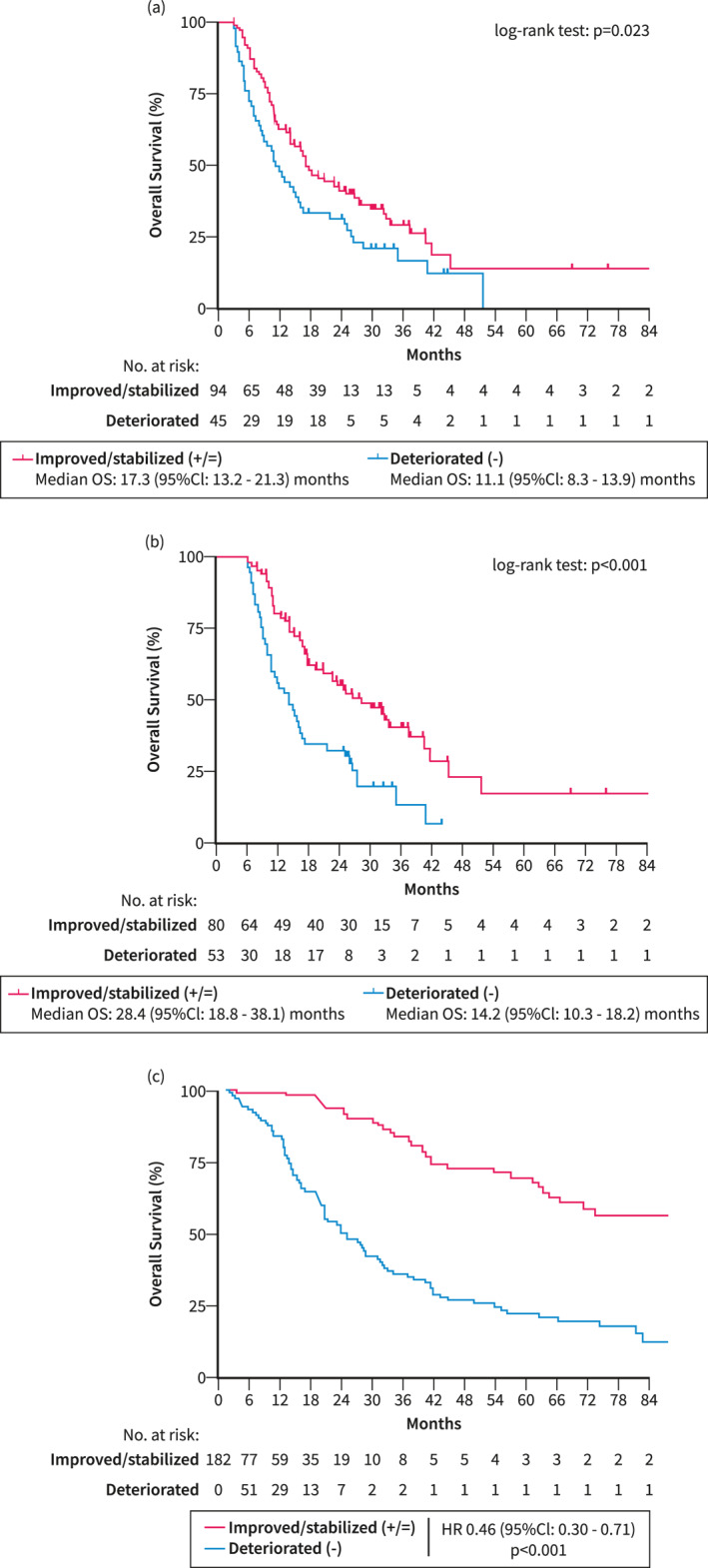
Overall survival according to evolution of liver function in patients with hepatocellular carcinoma treated with immunotherapy. Landmark analyses at (a) 3 and (b) 6 months comparing overall survival (OS) between patients who achieved an improvement/stabilization of liver function at the landmark compared to those with deterioration of liver function. (c) Graphical demonstration of the impact of changes in liver function on overall survival using a Simon–Makuch plot. −, deteriorated; +/=, improved/stabilized; CI, confidence interval; CPS, Child‐Pugh score; ICI, immune checkpoint inhibitor; OS, overall survival.
Importantly, improvement/stabilization of liver function at 6 months (aHR 0.53 [95% CI: 0.35–0.80], p = 0.002) was significantly associated with better OS, independent of etiology (non‐viral vs. viral etiology: aHR 1.83 [95% CI: 1.18–2.83], p = 0.007), Child‐Pugh class at baseline (CPS B vs. CPS A: aHR 2.81 [95% CI: 1.75–4.51], p < 0.001), α‐fetoprotein (AFP) levels (≥400 vs. <400 IU/mL: aHR 1.60 [95% CI: 1.05–2.44], p = 0.030), and active treatment (aHR 0.01 [95% CI: 0.01–0.01], p < 0.001) (Table 3).
TABLE 3.
Uni‐ and multivariable cox regression analysis of prognostic factors for overall survival including time‐dependent covariables.
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p‐value | aHR | 95% CI | p‐value | |
| Improvement/stabilization at 6 months | ||||||
| No | 1 | 1 | ||||
| Yes | 0.46 | 0.30–0.71 | <0.001 | 0.53 | 0.35–0.80 | 0.002 |
| Age | ||||||
| <65 | 1 | ‐ | ‐ | ‐ | ||
| ≥65 | 0.94 | 0.62–1.44 | 0.790 | |||
| Etiology | ||||||
| Viral | 1 | 1 | ||||
| Non‐viral | 1.96 | 1.27–3.02 | 0.003 | 1.83 | 1.18–2.83 | 0.007 |
| Child‐Pugh class | ||||||
| A | 1 | 1 | ||||
| B | 2.93 | 1.84–4.65 | <0.001 | 2.81 | 1.75–4.51 | <0.001 |
| ECOG PS | ||||||
| 0 | 1 | |||||
| ≥1 | 1.31 | 0.74–2.32 | 0.347 | ‐ | ‐ | ‐ |
| Macrovascular invasion | ||||||
| No | 1 | ‐ | ‐ | ‐ | ||
| Yes | 1.00 | 0.64–1.58 | 0.987 | |||
| Extrahepatic spread | ||||||
| No | 1 | ‐ | ‐ | ‐ | ||
| Yes | 0.71 | 0.46–1.09 | 0.122 | |||
| AFP (IU/mL) | ||||||
| <400 | 1 | 1 | ||||
| ≥400 | 1.46 | 0.93–2.26 | 0.010 | 1.60 | 1.05–2.44 | 0.030 |
| Progression | ||||||
| No | 1 | |||||
| Yes | 2.24 | 1.44–3.48 | <0.001 | ‐ | ‐ | ‐ |
| Active treatment | ||||||
| No | 1 | 1 | ||||
| Yes | 0.01 | 0.01–0.01 | <0.001 | 0.01 | 0.01–0.01 | <0.001 |
Abbreviations: AFP, α‐fetoprotein; ECOG PS, Eastern Cooperative Oncology Group performance status.
Subgroup and sensitivity analyses on the impact of ICI treatment on liver function and outcome
When assessing liver function with ALBI grade, a significantly higher amount of ICI‐treated patients had an improvement/stabilization of ALBI grade compared to the sorafenib cohort (3 months: +/=: ICI n = 111, 61% vs. sorafenib: n = 62, 39%; p < 0.001; 6 months: +/=: ICI: n = 88, 48% vs. sorafenib: n = 50, 31%; p < 0.001) (Table S2). While ALBI grade changes were associated with improved OS, differences did not attain statistical significance following landmark analyses at 3 and 6 months (at 3 months: +: 14.2 [95% CI: 4.5–24] months versus =: 17.3 [95% CI: 13.4–21.1] months versus −: 12.6 [95% CI: 5.8–19.3] months, p = 0.346; at 6 months: +: 37.3 [95% CI: 17–57.7] months versus =: 22.6 [95% CI: 15–30.2] months versus −: 16.2 [95% CI: 9.3–23.1] months, p = 0.410).
When including only patients with cirrhosis, ICI treated patients achieved significantly more frequently an improvement/stabilization of CPS at both timepoints, which was associated with better OS in landmark analyses at 3 and 6 months (Table S3, Figure S1).
Subgroup analysis in patients with Child‐Pugh class B
The subgroup of CP class B patients treated with ICIs included 35 patients, of which almost all patients had cirrhosis (n = 32, 91%). While the majority of these patients had ALBI grade 2 (n = 29, 83%), 17% (n = 6) were graded as ALBI grade 3 at baseline. Detailed patient characteristics are outlined in Table 1. Improvement or stabilization of liver function was observed in almost half of patients (n = 16, 46%) treated with ICI at 3 months, which was maintained in 40% (n = 14) at 6 months. Indeed, 6/35 (14%) and 9/35 (26%) patients improved from CP class B to CP class A at 3 and 6 months. Of these, 5 patients had a radiological response and three patients had stable disease as the best overall response. The improvement was maintained in 5/6 (83%) patients for ≥3 months. When compared to 44 CP class B patients treated with sorafenib, higher rates of improvement/stabilization of liver function were observed in the ICI group compared to the sorafenib cohort (3 months: +/=: ICI: n = 16/35, 46% vs. sorafenib: n = 12/44, 27%; p = 0.089; 6 months: +/=: ICI: n = 14/35, 40% vs. sorafenib: n = 5/44, 11%; p = 0.003) (Table 2b).
The impact of radiological response on changes in liver function during ICI treatment
Radiological response (ORR, including PR and CR) was observed in 71/182 (39%) patients. The proportion of patients with improved/stabilized CPS at 3 months (+/=: 44/62, 71% vs. 58/101, 57%; p = 0.083) was numerically and at 6 months statistically significantly higher in those achieving ORR (+/=: n = 43/59, 73% vs. n = 39/78, 50%; p = 0.007) (Table 4).
TABLE 4.
Changes in liver function at 3 and 6 months according to radiological response (ORR) versus NR in patients treated with immune checkpoint inhibitors.
| At 3 months a | At 6 months b | |||||
|---|---|---|---|---|---|---|
| ORR n = 62 | NR n = 101 | p‐value | ORR n = 59 | NR n = 78 | p‐value | |
| N (%) | ||||||
| CPS change | ||||||
| Improved/stabilized | 44 (71%) | 58 (57%) | 0.083 | 43 (73%) | 39 (50%) | 0.007 |
| Deteriorated | 18 (29%) | 43 (43%) | 16 (27%) | 39 (50%) | ||
Abbreviations: CPS, Child‐Pugh score; NR, non‐response (including stable and progressive disease); ORR, objective response rate (including complete and partial response).
Evaluable in n = 163 patients.
Evaluable in n = 137 patients.
Of 26 (14%) ICI‐treated patients with radiological disease control >18 months, 5 (19%) and 4 (15%) patients had deterioration of liver function at 3 and 6 months. Reasons for liver function impairment in 2 of 4 patients who deteriorated at 6 months included a bacterial infection of unknown origin, and acute alcoholic steatohepatitis. The other two patients developed hepatic decompensation without any obvious trigger (Table S4).
The impact of etiological cure on changes in liver function during ICI treatment
The majority (80%) of patients within the three main liver disease etiologies (alcoholic liver disease, hepatitis B virus [HBV] and hepatitis C virus [HCV]) did not experience a change in etiological driver (i.e., alcohol relapse, cessation of HBV treatment, HCV reinfection) during follow‐up. When comparing them with patients who underwent etiological treatment during the study period, we did not observe differences in CPS changes at 3 and 6 months. Details on patients without/with etiological cure can be found in Table S5.
Subgroup analysis of patients undergoing atezolizumab/bevacizumab treatment
In total, 146 (80%) patients in the ICI cohort received atezolizumab/bevacizumab (AB). Improvement/stabilization of liver function was observed in 56% (n = 82) of AB treated patients at 3% and 46% (n = 67) of patients at 6 months. In line with the results observed in the whole cohort, improvement/stabilization of liver function at 3 and 6 months was more pronounced in AB‐patients compared to patients receiving sorafenib (3 months: +/=: ICI: n = 82/146, 56% vs. sorafenib: n = 54/160, 34%; p < 0.001; 6 months: +/=: ICI: n = 67/146, 46% vs. sorafenib: n = 33/160, 21%; p < 0.001, Table S6). Radiological response was associated with a significantly higher rate of liver function improvement/stabilization at 6 months (responder vs non‐responder: n = 38/52, 73% vs. n = 29/59, 49%; p = 0.010) (Table S7). In the subgroup of 30 (21%) AB‐patients with CPS B, improvement/stabilization of CPS at 6 months was significantly more common as compared to the sorafenib cohort (ICI: n = 11/30, 37% vs. sorafenib: n = 5/44, 11%; p = 0.009) (Table S6).
DISCUSSION
In our study including 182 ICI‐treated patients, we observed an improvement/stabilization of liver function in more than half of the patients at 3 months, which was widely preserved at 6 months. Achieving an objective response to treatment linked to a beneficial effect on liver function and patients with improved or stabilized liver function had a significantly better survival. The proportion of patients with a sustained stabilization or improvement of liver function was markedly higher in patients receiving ICI‐based therapies compared with patients receiving sorafenib.
Mounting evidence suggests that radiological response is an important surrogate of OS in patients receiving systemic therapies. In a post hoc analysis of the REFLECT phase III trial stratifying patients by response status, achieving an objective response was a strong and independent predictor of OS in patients with unresectable HCC treated with lenvatinib or sorafenib. 15 Similar observations were made in the phase III randomized controlled trials IMbrave150 16 and HIMALAYA, 17 where participants who achieved disease control with the combinations of atezolizumab plus bevacizumab and tremelimumab plus durvalumab, respectively, had favorable long‐term survival rates. We hypothesize, that the survival benefit observed in responders could be at least partly related to a stabilization or improvement of liver function.
Inspired by the analysis of cohort five of the Checkmate‐040 study including patients with CP class B cirrhosis, 11 we performed a subgroup analysis in 35 patients with CP class B liver function. While patient characteristics were comparable between our and their study, the majority (80%) of our patients were treatment‐naïve, while around half of Checkmate‐040 cohort five patients were sorafenib‐experienced. Furthermore, an objective response was much more common in our study, most likely due to the fact that the majority of our patients received combination immunotherapy as compared to nivolumab monotherapy in the Checkmate‐040 study. Initial improvement of CP class B to class A was achieved in five (10%) patients in the Checkmate‐040 study compared to six (17%) patients after 3 months and nine (26%) patients after 6 months in our study. This effect could be maintained for ≥3 months in five of our CP class B patients and for ≥6 months in all five patients of the Checkmate‐040 subgroup analysis. 11
Mechanistically, a stabilization or improvement of liver function due to treatment‐induced tumor control may result from the reversal of the space‐occupying effect and an improved energy metabolism. 11 Indeed, HCC is considered an immunogenic tumor, which arises in chronically inflamed livers and creates an immunosuppressive microenvironment by attracting immunosuppressive cell populations. 18 Reduction of cancer‐associated inflammation, caused by effective ICI‐treatment, may also promote improvement of liver function, and thus lead to prolonged OS. We recently hypothesized that patients with tumor‐associated decompensation of liver function may benefit from ICI‐treatment, while patients with a small tumor burden but decompensation due to severe cirrhosis and portal hypertension may not derive a clinical benefit from anti‐cancer treatment, even if responding. 5
Limitations of our study include the retrospective character with its known potential shortcomings. The radiological response was evaluated locally and not via an independent centralized review. However, previous studies have shown that a centralized radiological review hardly ever significantly changes results generated by local radiologists. 6 , 8 Moreover, we only included patients with a minimum follow‐up of 3 months and cannot be sure that censoring due to loss to follow‐up is non‐informative, as patients may have died very early after starting treatment. Finally, although being the largest cohort of ICI‐treated patients published on the evolution of liver function so far, the sample size is still limited. Thus, confirmation of these results in large prospective trials would be highly desirable.
In conclusion, ICI‐treatment has the potential to improve or stabilize liver function in patients with well‐preserved as well as mild‐to‐moderately impaired liver function. Improvement or stabilization of liver function was associated with better OS and was commonly observed in patients with a response to ICI treatment. Large prospective studies in the Child‐Pugh B subgroup are warranted to assess safety and efficacy as well as impact on changes in liver function over time.
AUTHOR CONTRIBUTIONS
Concept of the study (Katharina Pomej, Lorenz Balcar, Matthias Pinter, Bernhard Scheiner), extraction of data (Katharina Pomej, Sabrina Sidali, Riccardo Sartoris, Maxime Ronot, Mohamed Bouattour, Matthias Pinter) drafting of the manuscript (Katharina Pomej, Bernhard Scheiner), writing of the manuscript (Katharina Pomej, Bernhard Scheiner), revision for important intellectual content (all authors). Katharina Pomej acts as the guarantor of the article and all authors approved the final version of the manuscript.
CONFLICT OF INTEREST STATEMENT
KP has nothing to disclose. LB has nothing to disclose. SS has nothing to disclose. RS has nothing to disclose.
TM speaker's honoraria from Astra Zeneca, Chiesi and Janssen‐Cilag, consulting fees from CSL Behring and travel support from Astra Zeneca, CSL Behring, Chiesi, Jazz Pharmaceuticals, Janssen‐Cilag, Novartis and teva‐ratiopharm.
MT received speaker fees from Bristol‐Myers Squibb (BMS), Falk Foundation, Gilead, Madrigal, Intercept and Merck Sharp & Dohme (MSD); advisory board fees from Abbvie, Albireo, Boehringer Ingelheim, BiomX, Falk Pharma GmbH, GENFIT, Gilead, Hightide, Intercept, Janssen, MSD, Novartis, Phenex, Bureau, Pliant, Regulus and Shire; travel grants from AbbVie, Falk, Gilead, and Intercept; and research grants from Albireo, Alnylam, CymaBay, Falk, Gilead, Intercept, MSD, Takeda and Ultragenyx. He is also a co‐inventor of patents on the medical use of norUDCA filed by the Medical University of Graz.
MM served as a speaker and/or consultant and/or advisory board member for AbbVie, Echosens, Gilead, Ipsen, and W. L. Gore & Associates and received travel support from AbbVie and Gilead as well as grants/research support from Echosens.
TR served as a speaker and/or consultant and/or advisory board member for AbbVie, Bayer, Boehringer Ingelheim, Gilead, Intercept, MSD, Roche, Siemens, and W. L. Gore & Associates and received grants/research support from AbbVie, Boehringer Ingelheim, Gilead, MSD, Philips, and W. L. Gore & Associates as well as travel support from Boehringer Ingelheim and Gilead.
MR served as consultant for Quantum Surgical (fees paid to the institution), and received speaker fees from General Electrics, Guerbet, Servier, Terumo, Angiodynamics, AstraZeneca.
MB received speaker fees from Bayer, MSD, Sirtex Medical and Roche; advisory board fees from Bayer, MSD, Sirtex Medical, Eisai, AstraZeneca, Ipsen, Servier, Taiho and BMS.
MP received speaker honoraria from Bayer, BMS, Eisai, Lilly, MSD, and Roche; he is a consultant for AstraZeneca, Bayer, BMS, Eisai, Ipsen, Lilly, MSD, and Roche; and he received travel support from Bayer, BMS, Ipsen, and Roche.
BS received grant support from AstraZeneca and Eisai, speaker honoraria from Eisai as well as travel support from AbbVie, AstraZeneca, Ipsen and Gilead.
Supporting information
Supporting Information S1
ACKNOWLEDGMENTS
The authors declare that no financial support specific to this study was received.
Pomej K, Balcar L, Sidali S, Sartoris R, Meischl T, Trauner M, et al. Evolution of liver function during immune checkpoint inhibitor treatment for hepatocellular carcinoma. United European Gastroenterol J. 2024;12(8):1034–43. 10.1002/ueg2.12626
Mohamed Bouattour, Matthias Pinter, and Bernhard Scheiner contributed equally.
Contributor Information
Matthias Pinter, Email: matthias.pinter@meduniwien.ac.at.
Bernhard Scheiner, Email: bernhard.scheiner@meduniwien.ac.at.
DATA AVAILABILITY STATEMENT
Supporting data are available from the corresponding author upon request.
REFERENCES
- 1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2. Rumgay H, Arnold M, Ferlay J, Lesi O, Cabasag CJ, Vignat J, et al. Global burden of primary liver cancer in 2020 and predictions to 2040. J Hepatol. 2022;77(6):1598–1606. 10.1016/j.jhep.2022.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Reig M, Forner A, Rimola J, Ferrer‐Fàbrega J, Burrel M, Garcia‐Criado Á, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76(3):681–693. 10.1016/j.jhep.2021.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pinter M, Scheiner B, Peck‐Radosavljevic M. Immunotherapy for advanced hepatocellular carcinoma: a focus on special subgroups. Gut. 2021;70(1):204–214. 10.1136/gutjnl-2020-321702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pinter M, Scheiner B, Pinato DJ. Immune checkpoint inhibitors in hepatocellular carcinoma: emerging challenges in clinical practice. Lancet Gastroenterol Hepatol. 2023;8(8):760–770. 10.1016/s2468-1253(23)00147-4 [DOI] [PubMed] [Google Scholar]
- 6. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–1905. 10.1056/nejmoa1915745 [DOI] [PubMed] [Google Scholar]
- 7. Abou‐Alfa GK, Lau G, Kudo M, Chan SL, Kelley RK, Furuse J, et al. Tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. NEJM Evid. 2022;1(8):EVIDoa2100070. 10.1056/evidoa2100070 [DOI] [PubMed] [Google Scholar]
- 8. Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first‐line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non‐inferiority trial. Lancet. 2018;391(10126):1163–1173. 10.1016/s0140-6736(18)30207-1 [DOI] [PubMed] [Google Scholar]
- 9. Marrero JA, Kudo M, Venook AP, Ye SL, Bronowicki JP, Chen XP, et al. Observational registry of sorafenib use in clinical practice across Child‐Pugh subgroups: the GIDEON study. J Hepatol. 2016;65(6):1140–1147. 10.1016/j.jhep.2016.07.020 [DOI] [PubMed] [Google Scholar]
- 10. D'Alessio A, Fulgenzi CAM, Nishida N, Schönlein M, von Felden J, Schulze K, et al. Preliminary evidence of safety and tolerability of atezolizumab plus bevacizumab in patients with hepatocellular carcinoma and Child‐Pugh A and B cirrhosis: a real‐world study. Hepatology. 2022;76(4):1000–1012. 10.1002/hep.32468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kudo M, Matilla A, Santoro A, Melero I, Gracián AC, Acosta‐Rivera M, et al. CheckMate 040 cohort 5: a phase I/II study of nivolumab in patients with advanced hepatocellular carcinoma and Child‐Pugh B cirrhosis. J Hepatol. 2021;75(3):600–609. 10.1016/j.jhep.2021.04.047 [DOI] [PubMed] [Google Scholar]
- 12. Scheiner B, Kirstein MM, Hucke F, Finkelmeier F, Schulze K, von Felden J, et al. Programmed cell death protein‐1 (PD‐1)‐targeted immunotherapy in advanced hepatocellular carcinoma: efficacy and safety data from an international multicentre real‐world cohort. Aliment Pharmacol Ther. 2019;49(10):1323–1333. 10.1111/apt.15245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52–60. 10.1055/s-0030-1247132 [DOI] [PubMed] [Google Scholar]
- 14. Schultz LR, Peterson EL, Breslau N. Graphing survival curve estimates for time‐dependent covariates. Int J Methods Psychiatr Res. 2002;11(2):68–74. 10.1002/mpr.124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Cheng AL, et al. Overall survival and objective response in advanced unresectable hepatocellular carcinoma: a subanalysis of the REFLECT study. J Hepatol. 2023;78(1):133–141. 10.1016/j.jhep.2022.09.006 [DOI] [PubMed] [Google Scholar]
- 16. Ducreux M, Zhu AX, Cheng AL, Galle PR, Ikeda M, Nicholas A, et al. IMbrave150: exploratory analysis to examine the association between treatment response and overall survival (OS) in patients (pts) with unresectable hepatocellular carcinoma (HCC) treated with atezolizumab (atezo) + bevacizumab (bev) versus sorafenib (sor). J Clin Oncol. 2021;39(15_Suppl):4071. [Google Scholar]
- 17. Sangro B, Chan S, Kelley R, Lau G, Kudo M, Sukeepaisarnjaroen W, et al. SO‐15 Four‐year overall survival update from the phase 3 HIMALAYA study of tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. Ann Oncol. 2023;34:S168. 10.1016/j.annonc.2023.04.487 [DOI] [PubMed] [Google Scholar]
- 18. Llovet JM, Castet F, Heikenwalder M, Maini MK, Mazzaferro V, Pinato DJ, et al. Immunotherapies for hepatocellular carcinoma. Nat Rev Clin Oncol. 2022;19(3):151–172. 10.1038/s41571-021-00573-2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information S1
Data Availability Statement
Supporting data are available from the corresponding author upon request.


