Abstract
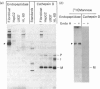
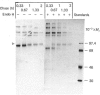
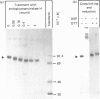
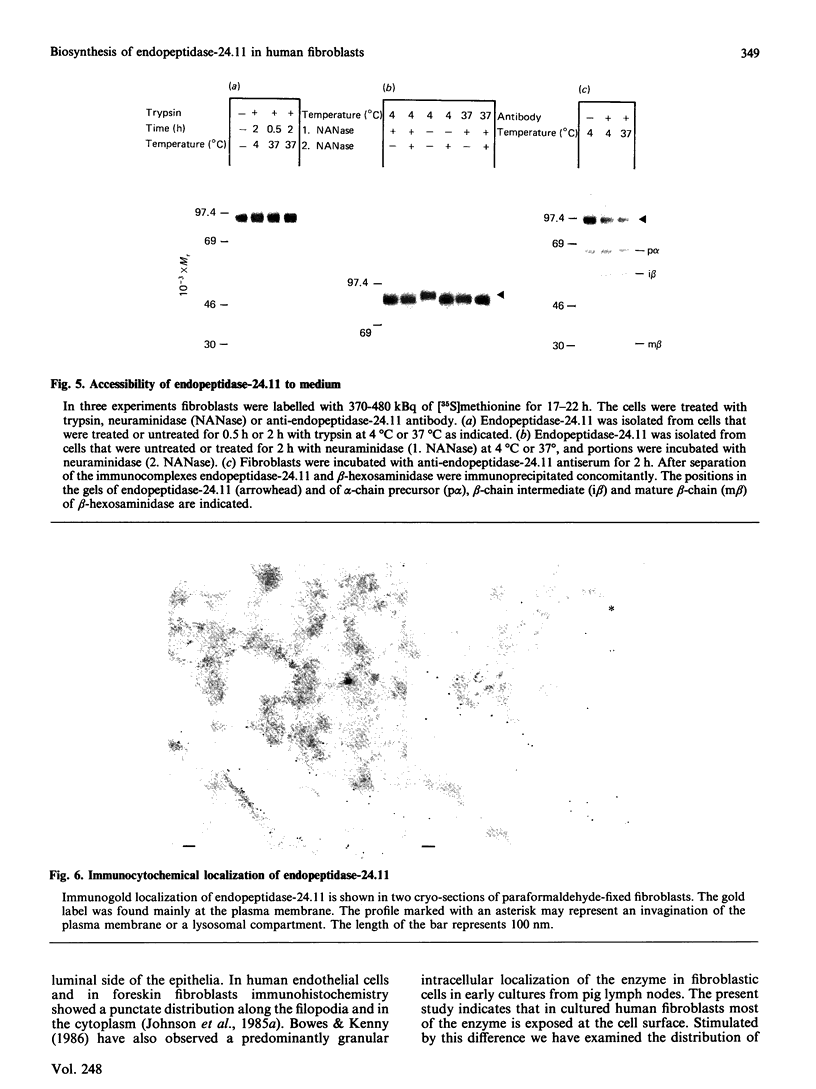
The biosynthesis, glycosylation and subcellular localization of the neutral endopeptidase-24.11 were studied in cultured human fibroblasts. The enzyme was synthesized as a precursor (Mr 88,000) containing four or five N-linked oligosaccharides. Within 1 h the synthesis-mature (Mr 94,000) endopeptidase-24.11 was formed and contained sialylated oligosaccharides. The half-life of endopeptidase-24.11 was 3.7 days and in the presence of 10 mM-NH4Cl it increased to 6 days. Mature endopeptidase-24.11 was solubilized with 0.2% saponin and partitioned into Triton X-114. In intact fibroblasts, endopeptidase-24.11 was accessible to antibodies and to neuraminidase even when the treatment was performed at 4 degrees C. The localization of endopeptidase-24.11 to the plasma membrane in cultured fibroblasts was further demonstrated by immunocytochemistry.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almenoff J., Teirstein A. S., Thornton J. C., Orlowski M. Identification of a thermolysin-like metalloendopeptidase in serum: activity in normal subjects and in patients with sarcoidosis. J Lab Clin Med. 1984 Mar;103(3):420–431. [PubMed] [Google Scholar]
- Almenoff J., Wilk S., Orlowski M. Membrane bound pituitary metalloendopeptidase: apparent identity to enkephalinase. Biochem Biophys Res Commun. 1981 Sep 16;102(1):206–214. doi: 10.1016/0006-291x(81)91508-4. [DOI] [PubMed] [Google Scholar]
- Bowes M. A., Kenny A. J. An immunohistochemical study of endopeptidase-24.11 and aminopeptidase N in lymphoid tissues. Immunology. 1987 Feb;60(2):247–253. [PMC free article] [PubMed] [Google Scholar]
- Bowes M. A., Kenny A. J. Endopeptidase-24.11 in pig lymph nodes. Purification and immunocytochemical localization in reticular cells. Biochem J. 1986 Jun 15;236(3):801–810. doi: 10.1042/bj2360801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins S. J., Gallo R. C., Gallagher R. E. Continuous growth and differentiation of human myeloid leukaemic cells in suspension culture. Nature. 1977 Nov 24;270(5635):347–349. doi: 10.1038/270347a0. [DOI] [PubMed] [Google Scholar]
- Connelly J. C., Skidgel R. A., Schulz W. W., Johnson A. R., Erdös E. G. Neutral endopeptidase 24.11 in human neutrophils: cleavage of chemotactic peptide. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8737–8741. doi: 10.1073/pnas.82.24.8737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conzelmann A., Spiazzi A., Hyman R., Bron C. Anchoring of membrane proteins via phosphatidylinositol is deficient in two classes of Thy-1 negative mutant lymphoma cells. EMBO J. 1986 Dec 1;5(12):3291–3296. doi: 10.1002/j.1460-2075.1986.tb04642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsen E. M., Vyas J. P., Kenny A. J. A neutral endopeptidase in the microvillar membrane of pig intestine. Partial purification and properties. Biochem J. 1980 Nov 1;191(2):645–648. doi: 10.1042/bj1910645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean R. T., Jessup W., Roberts C. R. Effects of exogenous amines on mammalian cells, with particular reference to membrane flow. Biochem J. 1984 Jan 1;217(1):27–40. doi: 10.1042/bj2170027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdös E. G., Schulz W. W., Gafford J. T., Defendini R. Neutral metalloendopeptidase in human male genital tract. Comparison to angiotensin I-converting enzyme. Lab Invest. 1985 Apr;52(4):437–447. [PubMed] [Google Scholar]
- Fulcher I. S., Chaplin M. F., Kenny A. J. Endopeptidase-24.11 purified from pig intestine is differently glycosylated from that in kidney. Biochem J. 1983 Nov 1;215(2):317–323. doi: 10.1042/bj2150317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulcher I. S., Kenny A. J. Proteins of the kidney microvillar membrane. The amphipathic forms of endopeptidase purified from pig kidneys. Biochem J. 1983 Jun 1;211(3):743–753. doi: 10.1042/bj2110743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulcher I. S., Pappin D. J., Kenny A. J. The N-terminal amino acid sequence of pig kidney endopeptidase-24.11 shows homology with pro-sucrase-isomaltase. Biochem J. 1986 Nov 15;240(1):305–308. doi: 10.1042/bj2400305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gafford J. T., Skidgel R. A., Erdös E. G., Hersh L. B. Human kidney "enkephalinase", a neutral metalloendopeptidase that cleaves active peptides. Biochemistry. 1983 Jun 21;22(13):3265–3271. doi: 10.1021/bi00282a035. [DOI] [PubMed] [Google Scholar]
- Gee N. S., Bowes M. A., Buck P., Kenny A. J. An immunoradiometric assay for endopeptidase-24.11 shows it to be a widely distributed enzyme in pig tissues. Biochem J. 1985 May 15;228(1):119–126. doi: 10.1042/bj2280119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee N. S., Matsas R., Kenny A. J. A monoclonal antibody to kidney endopeptidase-24.11. Its application in immunoadsorbent purification of the enzyme and immunofluorescent microscopy of kidney and intestine. Biochem J. 1983 Aug 15;214(2):377–386. doi: 10.1042/bj2140377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuze H. J., Slot J. W., Strous G. J., Hasilik A., von Figura K. Possible pathways for lysosomal enzyme delivery. J Cell Biol. 1985 Dec;101(6):2253–2262. doi: 10.1083/jcb.101.6.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gieselmann V., Pohlmann R., Hasilik A., Von Figura K. Biosynthesis and transport of cathepsin D in cultured human fibroblasts. J Cell Biol. 1983 Jul;97(1):1–5. doi: 10.1083/jcb.97.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta D. K., Schmidt A., von Figura K., Hasilik A. Processing and transport of lysosomal enzymes in human monocyte line U937. Hoppe Seylers Z Physiol Chem. 1984 Aug;365(8):867–876. doi: 10.1515/bchm2.1984.365.2.867. [DOI] [PubMed] [Google Scholar]
- Hasilik A., Neufeld E. F. Biosynthesis of lysosomal enzymes in fibroblasts. Synthesis as precursors of higher molecular weight. J Biol Chem. 1980 May 25;255(10):4937–4945. [PubMed] [Google Scholar]
- Hasilik A., Von Figura K. Oligosaccharides in lysosomal enzymes. Distribution of high-mannose and complex oligosaccharides in cathepsin D and beta-hexosaminidase. Eur J Biochem. 1981 Dec;121(1):125–129. doi: 10.1111/j.1432-1033.1981.tb06440.x. [DOI] [PubMed] [Google Scholar]
- Hersh L. B. Degradation of enkephalins: the search for an enkephalinase. Mol Cell Biochem. 1982 Aug 20;47(1):35–43. doi: 10.1007/BF00241564. [DOI] [PubMed] [Google Scholar]
- Horsthemke B., Hamprecht B., Bauer K. Heterogeneous distribution of enkephalin-degrading peptidases between neuronal and glial cells. Biochem Biophys Res Commun. 1983 Sep 15;115(2):423–429. doi: 10.1016/s0006-291x(83)80161-2. [DOI] [PubMed] [Google Scholar]
- Johnson A. R., Ashton J., Schulz W. W., Erdös E. G. Neutral metalloendopeptidase in human lung tissue and cultured cells. Am Rev Respir Dis. 1985 Sep;132(3):564–568. doi: 10.1164/arrd.1985.132.3.564. [DOI] [PubMed] [Google Scholar]
- Johnson A. R., Coalson J. J., Ashton J., Larumbide M., Erdös E. G. Neutral endopeptidase in serum samples from patients with adult respiratory distress syndrome. Comparison with angiotensin-converting enzyme. Am Rev Respir Dis. 1985 Dec;132(6):1262–1267. doi: 10.1164/arrd.1985.132.6.1262. [DOI] [PubMed] [Google Scholar]
- Kenny A. J., Fulcher I. S., McGill K. A., Kershaw D. Proteins of the kidney microvillar membrane. Reconstitution of endopeptidase in liposomes shows that it is a short-stalked protein. Biochem J. 1983 Jun 1;211(3):755–762. doi: 10.1042/bj2110755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr M. A., Kenny A. J. The molecular weight and properties of a neutral metallo-endopeptidase from rabbit kidney brush border. Biochem J. 1974 Mar;137(3):489–495. doi: 10.1042/bj1370489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Matlin K. S. Ammonium chloride slows transport of the influenza virus hemagglutinin but does not cause mis-sorting in a polarized epithelial cell line. J Biol Chem. 1986 Nov 15;261(32):15172–15178. [PubMed] [Google Scholar]
- Matsas R., Kenny A. J., Turner A. J. The metabolism of neuropeptides. The hydrolysis of peptides, including enkephalins, tachykinins and their analogues, by endopeptidase-24.11. Biochem J. 1984 Oct 15;223(2):433–440. doi: 10.1042/bj2230433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsas R., Stephenson S. L., Hryszko J., Kenny A. J., Turner A. J. The metabolism of neuropeptides. Phase separation of synaptic membrane preparations with Triton X-114 reveals the presence of aminopeptidase N. Biochem J. 1985 Oct 15;231(2):445–449. doi: 10.1042/bj2310445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer R. J., Doherty F. Intracellular protein catabolism: state of the art. FEBS Lett. 1986 Mar 31;198(2):181–193. doi: 10.1016/0014-5793(86)80403-3. [DOI] [PubMed] [Google Scholar]
- Neblock D. S., Berg R. A. Lysosomotropic agents ammonium chloride and chloroquine inhibit both the synthesis and secretion of procollagen by freshly isolated embryonic chick tendon cells. Biochem Biophys Res Commun. 1982 Apr 14;105(3):902–908. doi: 10.1016/0006-291x(82)91055-5. [DOI] [PubMed] [Google Scholar]
- Relton J. M., Gee N. S., Matsas R., Turner A. J., Kenny A. J. Purification of endopeptidase-24.11 ('enkephalinase') from pig brain by immunoadsorbent chromatography. Biochem J. 1983 Dec 1;215(3):519–523. doi: 10.1042/bj2150519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sando G. N., Titus-Dillon P., Hall C. W., Neufeld E. F. Inhibition of receptor-mediated uptake of a lysosomal enzyme into fibroblasts by chloroquine, procaine and ammonia. Exp Cell Res. 1979 Mar 15;119(2):359–364. doi: 10.1016/0014-4827(79)90364-1. [DOI] [PubMed] [Google Scholar]
- Skidgel R. A., Engelbrecht S., Johnson A. R., Erdös E. G. Hydrolysis of substance p and neurotensin by converting enzyme and neutral endopeptidase. Peptides. 1984 Jul-Aug;5(4):769–776. doi: 10.1016/0196-9781(84)90020-2. [DOI] [PubMed] [Google Scholar]
- Stephenson S. L., Kenny A. J. Metabolism of neuropeptides. Hydrolysis of the angiotensins, bradykinin, substance P and oxytocin by pig kidney microvillar membranes. Biochem J. 1987 Jan 1;241(1):237–247. doi: 10.1042/bj2410237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart J. R., Kenny A. J. Proteins of the kidney microvillar membrane. Biosynthesis of endopeptidase-24.11, dipeptidylpeptidase IV and aminopeptidases N and A in pig kidney slices. Biochem J. 1984 Dec 1;224(2):549–558. doi: 10.1042/bj2240549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundström C., Nilsson K. Establishment and characterization of a human histiocytic lymphoma cell line (U-937). Int J Cancer. 1976 May 15;17(5):565–577. doi: 10.1002/ijc.2910170504. [DOI] [PubMed] [Google Scholar]
- Waheed A., Hasilik A., von Figura K. Enhanced breakdown of arylsulfatase A in multiple sulfatase deficiency. Eur J Biochem. 1982 Apr 1;123(2):317–321. doi: 10.1111/j.1432-1033.1982.tb19770.x. [DOI] [PubMed] [Google Scholar]
- von Figura K., Gieselmann V., Hasilik A. Mannose 6-phosphate-specific receptor is a transmembrane protein with a C-terminal extension oriented towards the cytosol. Biochem J. 1985 Jan 15;225(2):543–547. doi: 10.1042/bj2250543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Figura K., Steckel F., Hasilik A. Juvenile and adult metachromatic leukodystrophy: partial restoration of arylsulfatase A (cerebroside sulfatase) activity by inhibitors of thiol proteinases. Proc Natl Acad Sci U S A. 1983 Oct;80(19):6066–6070. doi: 10.1073/pnas.80.19.6066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Figura K., Weber E. An alternative hypothesis of cellular transport of lysosomal enzymes in fibroblasts. Effect of inhibitors of lysosomal enzyme endocytosis on intra- and extra-cellular lysosomal enzyme activities. Biochem J. 1978 Dec 15;176(3):943–950. doi: 10.1042/bj1760943. [DOI] [PMC free article] [PubMed] [Google Scholar]