Abstract
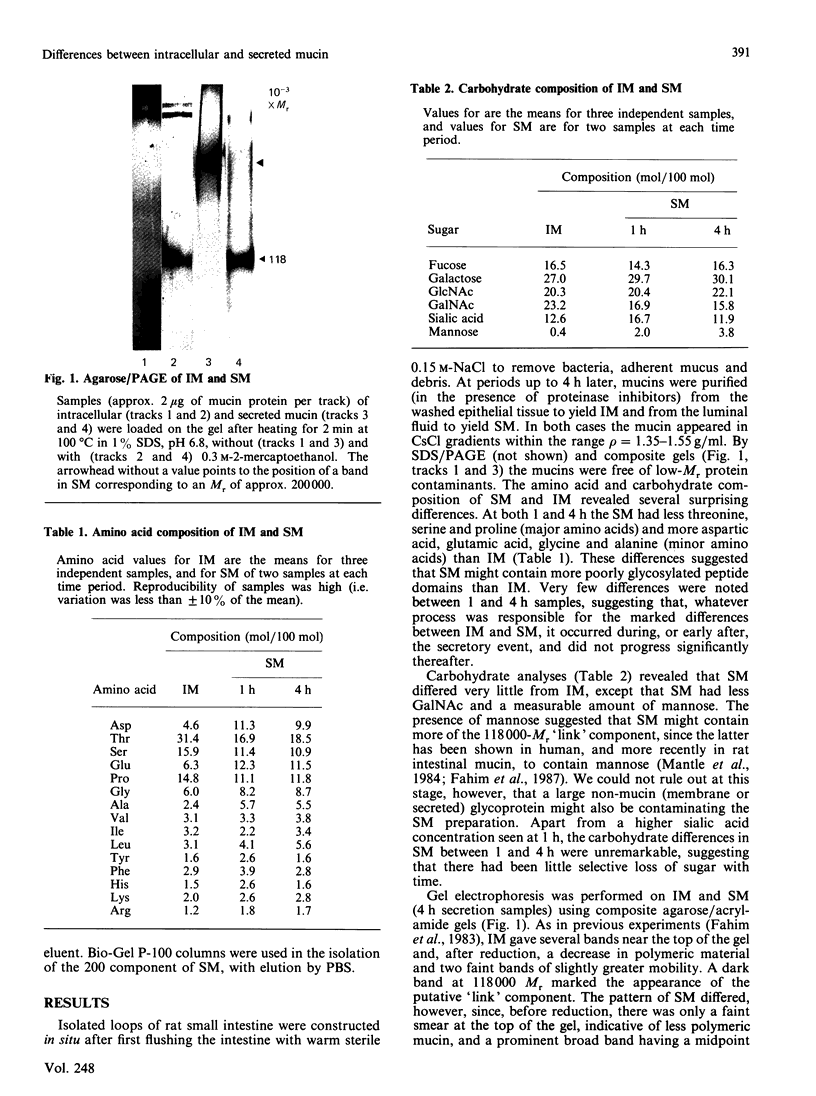
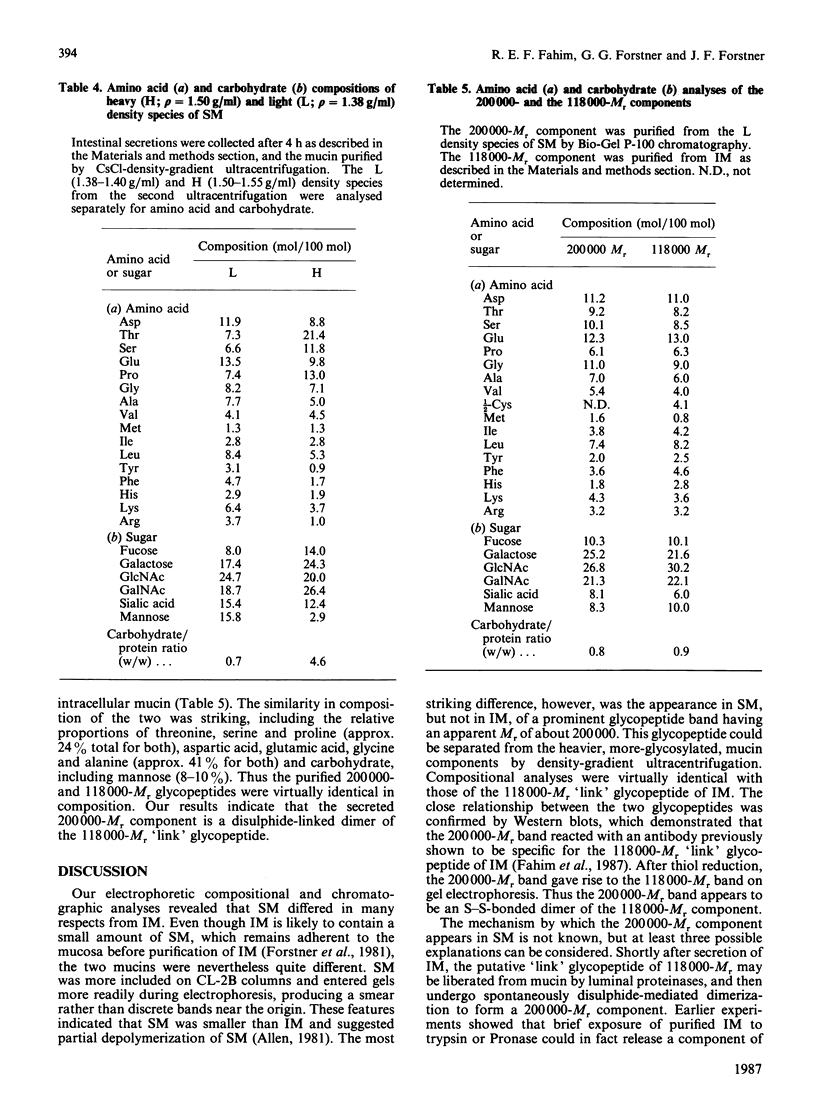
An investigation was undertaken to discover whether mucin purified from secretions in the lumen of rat small intestine differed in structure or composition from intracellular mucin purified from rat intestinal tissue. To do this, ligated loops were constructed in situ from previously washed intestinal segments and mucin purified separately from tissue homogenates or loop fluid. Secreted mucin (SM) differed from intracellular mucin (IM) by having a higher proportion of 'minor' mucin amino acids (aspartic acid, glutamic acid, glycine and alanine) and a lower proportion of 'major' amino acids (serine, proline and threonine). SM also contained less N-acetylgalactosamine and a small, but measureable, amount of mannose. Gel electrophoresis showed that SM penetrated the gel more readily and, unlike IM, gave a rather prominent, but diffuse, band having a midpoint position of Mr 200,000. After reduction both IM and SM gave rise to the putative 'link' component of Mr 118,000 and the 200,000-Mr band of SM disappeared. SM was included to a greater extent than IM on Sepharose CL-2B chromatography, suggesting a smaller size. With the use of CsCl-density-gradient ultracentrifugation of SM, a lighter species [buoyant density (rho) = 1.38 g/ml] enriched in the 200,000-Mr component, was separated from a heavier, more glycosylated, species (rho = 1.50 g/ml). Purified 200,000-Mr component had a composition identical with that of the 118,000-Mr 'link' component of IM, reacted in Western blots with an antibody specific for the 118,000-Mr 'link' component, and after reduction gave rise to a 118,000-Mr component on gel electrophoresis. Thus secreted mucin contains a 200,000-Mr component which appears to represent a disulphide-linked dimer of the previously described 118,000-Mr 'link' component of intracellular mucin.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clamp J. R., Creeth J. M. Some non-mucin components of mucus and their possible biological roles. Ciba Found Symp. 1984;109:121–136. doi: 10.1002/9780470720905.ch9. [DOI] [PubMed] [Google Scholar]
- Clamp J. R., Creeth J. M. Some non-mucin components of mucus and their possible biological roles. Ciba Found Symp. 1984;109:121–136. doi: 10.1002/9780470720905.ch9. [DOI] [PubMed] [Google Scholar]
- Fahim R. E., Forstner G. G., Forstner J. F. Heterogeneity of rat goblet-cell mucin before and after reduction. Biochem J. 1983 Jan 1;209(1):117–124. doi: 10.1042/bj2090117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahim R. E., Specian R. D., Forstner G. G., Forstner J. F. Characterization and localization of the putative 'link' component in rat small-intestinal mucin. Biochem J. 1987 May 1;243(3):631–640. doi: 10.1042/bj2430631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstner J. F., Ofosu F., Forstner G. G. Radioimmunoassay of intestinal goblet cell mucin. Anal Biochem. 1977 Dec;83(2):657–665. doi: 10.1016/0003-2697(77)90070-7. [DOI] [PubMed] [Google Scholar]
- Fouad F. M., Waldron-Edward D. Isolation and characterization of human and canine gastric mucosal glycoproteins and their degradation by proteases and acid hydrolases. Hoppe Seylers Z Physiol Chem. 1980 May;361(5):703–713. doi: 10.1515/bchm2.1980.361.1.703. [DOI] [PubMed] [Google Scholar]
- Henderson L. E., Oroszlan S., Konigsberg W. A micromethod for complete removal of dodecyl sulfate from proteins by ion-pair extraction. Anal Biochem. 1979 Feb;93(1):153–157. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mantle M., Allen A. A colorimetric assay for glycoproteins based on the periodic acid/Schiff stain [proceedings]. Biochem Soc Trans. 1978;6(3):607–609. doi: 10.1042/bst0060607. [DOI] [PubMed] [Google Scholar]
- Mantle M., Forstner G. G., Forstner J. F. Biochemical characterization of the component parts of intestinal mucin from patients with cystic fibrosis. Biochem J. 1984 Dec 1;224(2):345–354. doi: 10.1042/bj2240345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- Pearson J. P., Ward R., Allen A., Roberts N. B., Taylor W. H. Mucus degradation by pepsin: comparison of mucolytic activity of human pepsin 1 and pepsin 3: implications in peptic ulceration. Gut. 1986 Mar;27(3):243–248. doi: 10.1136/gut.27.3.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podolsky D. K., Fournier D. A., Lynch K. E. Human colonic goblet cells. Demonstration of distinct subpopulations defined by mucin-specific monoclonal antibodies. J Clin Invest. 1986 Apr;77(4):1263–1271. doi: 10.1172/JCI112429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podolsky D. K. Oligosaccharide structures of human colonic mucin. J Biol Chem. 1985 Jul 15;260(14):8262–8271. [PubMed] [Google Scholar]
- Podolsky D. K. Oligosaccharide structures of isolated human colonic mucin species. J Biol Chem. 1985 Dec 15;260(29):15510–15515. [PubMed] [Google Scholar]
- Roomi N., Laburthe M., Fleming N., Crowther R., Forstner J. Cholera-induced mucin secretion from rat intestine: lack of effect of cAMP, cycloheximide, VIP, and colchicine. Am J Physiol. 1984 Aug;247(2 Pt 1):G140–G148. doi: 10.1152/ajpgi.1984.247.2.G140. [DOI] [PubMed] [Google Scholar]
- Schrager J., Oates M. D. The isolation and partial characterisation of the principal gastric glycoprotein of 'visible' mucus. Digestion. 1971;4(1):1–12. doi: 10.1159/000197091. [DOI] [PubMed] [Google Scholar]
- Specian R. D., Neutra M. R. Cytoskeleton of intestinal goblet cells in rabbit and monkey. The theca. Gastroenterology. 1984 Dec;87(6):1313–1325. [PubMed] [Google Scholar]
- Switzer R. C., 3rd, Merril C. R., Shifrin S. A highly sensitive silver stain for detecting proteins and peptides in polyacrylamide gels. Anal Biochem. 1979 Sep 15;98(1):231–237. doi: 10.1016/0003-2697(79)90732-2. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woussen-Colle M. C., Rasinski C., de Graef J. Les glycoprotéines du mucus gastrique du chien. II.--Etude comparée des glycoprotéines des muqueuses et des sécrétions fundiques et antrales. Biol Gastroenterol (Paris) 1975 Oct-Nov;8(4):283–289. [PubMed] [Google Scholar]
- Zanetta J. P., Breckenridge W. C., Vincendon G. Analysis of monosaccharides by gas-liquid chromatography of the O-methyl glycosides as trifluoroacetate derivatives. Application to glycoproteins and glycolipids. J Chromatogr. 1972 Jul 5;69(2):291–304. doi: 10.1016/s0021-9673(00)92897-8. [DOI] [PubMed] [Google Scholar]







