Abstract
Aim
We aimed to assess the role of FGF21 in metabolic dysfunction‐associated steatotic liver disease (MASLD) at a multi‐scale level.
Methods
We used human MASLD pathology samples for FGF21 gene expression analyses (qPCR and RNAseq), serum to measure circulating FGF21 levels and DNA for genotyping the FGF21 rs838133 variant in both estimation and validation cohorts. A hepatocyte‐derived cell line was exposed to free fatty acids at different timepoints. Finally, C57BL/6J mice were fed a high‐fat and choline‐deficient diet (CDA‐HFD) for 16 weeks to assess hepatic FGF21 protein expression and FGF21 levels by ELISA.
Results
A significant upregulation in FGF21 mRNA expression was observed in the liver analysed by both qPCR (fold change 5.32 ± 5.25 vs. 0.59 ± 0.66; p = 0.017) and RNA‐Seq (3.5 fold; FDR: 0.006; p < 0.0001) in MASLD patients vs. controls. Circulating levels of FGF21 were increased in patients with steatohepatitis vs. bland steatosis (386.6 ± 328.9 vs. 297.9 ± 231.5 pg/mL; p = 0.009). Besides, sex, age, A‐allele from FGF21, GG genotype from PNPLA3, ALT, type 2 diabetes mellitus and BMI were independently associated with MASH and significant fibrosis in both estimation and validation cohorts. In vitro exposure of Huh7.5 cells to high concentrations of free fatty acids (FFAs) resulted in overexpression of FGF21 (p < 0.001). Finally, Circulating FGF21 levels and hepatic FGF21 expression were found to be significantly increased (p < 0.001) in animals under CDA‐HFD.
Conclusions
Hepatic and circulating FGF21 expression was increased in MASH patients, in Huh7.5 cells under FFAs and in CDA‐HFD animals. The A‐allele from the rs838133 variant was also associated with an increased risk of steatohepatitis and significant and advanced fibrosis in MASLD patients.
Keywords: FGF21, fibroblast growth factor 21, gene expression, hepatokine, MASH, MASLD, metabolic syndrome, NAFLD, NASH, steatohepatitis
Key summary.
Summarise the established knowledge on this subject:
MASLD is the result of a complex and dynamic interplay of several factors.
FGF21 is a key metabolic regulator and has received growing attention as a therapeutic agent in MASLD.
Several SNPs have been previously related to the onset and development of MASLD.
What are the significant and/or new findings of this study?
FGF21 gene hepatic expression and circulating FGF21 protein levels are increased in both MASLD patients and a preclinical model.
FGF21 gene is increased both in vitro and in vivo and correlates with liver damage and different metabolic disturbances in the CDA‐HFD model.
A novel risk‐conferring SNP is identified and associated with increased hazard of developing MASH and significant fibrosis.
INTRODUCTION
Metabolic dysfunction‐associated steatotic liver disease (MASLD) is recognized as one of the most common liver diseases worldwide, and it is considered a key factor in the development of complications related to metabolic syndrome. 1 This entity comprises a broad spectrum of lesions ranging from simple steatosis to steatohepatitis, defined by inflammation and ballooning, accounting for a high liver‐related morbidity and mortality with extensive multi‐organ involvement. Up to twenty‐per‐cent of these patients evolve to liver fibrosis and cirrhosis, and are at risk of potentially life‐threatening liver‐related complications, such as hepatocellular carcinoma, decompensations, or orthotropic liver transplantation. 2
FGF21 is a hepatokine involved in the regulation of hepatic glucose and lipid metabolism. 3 The physiology of FGF21 is complex mainly due to its pleiotropic actions on numerous target tissues. 4 Since FGF21 is synthesized in the hepatocytes, it is rational to consider that liver disorders could modify its expression. Despite the beneficial effects derived from FGF21 exogenous administration already proven in both preclinical model 5 and human settings, 6 circulating FGF21 levels have been previously demonstrated to be elevated in different settings of liver‐related patients, such as non‐obese HIV patients 7 and obese MASH patients. 8 FGF21 analogues are currently in clinical development for the treatment of non‐alcoholic steatohepatitis.
Over the years, several studies have tried to find the implication of different SNPs (single nucleotide polymorphisms) in the onset and development of this disease. In this regard, PNPLA3 was described for the first time in 2008, 9 and this association remains significant to date. 10 On the other hand, recent studies have shown that a common allele in the FGF21 gene, the rs838133 variant, is able to influence the balance of macronutrient intake. 11 Furthermore, this synonymous change to the first exon of this FGF21 variant has been associated with higher blood pressure and altered body shape, despite lower total body‐fat percentage, 12 suggesting a potential implication in lean‐MASH development.
Although FGF21 is receiving growing attention as a novel therapeutic target for MASLD, the mechanism by which FGF21 exerts its beneficial effects is not fully understood yet. Considering this scenario, we aimed to explore the role of FGF21 on a multi‐level scale in MASLD.
MATERIAL AND METHODS
FGF21 analyses in liver tissue sections from CDA‐HFD animals
Eight weeks‐old male C57BL/6J mice were purchased from Charles Rivers (L'Arbresle Cedex, France). Mice were housed in ventilated plastic cages and maintained on a 12‐h light‐dark cycle with ad libitum access to pellet chow and water. For diet‐induced MASLD studies, 8‐week‐old male mice were housed in individual cages. One group of 7 mice was exposed to high‐fat (60% of Kcal of fat) and choline deficient diet supplemented with 0.1% methionine (CDA‐HFD; A06071302 Open Source Diets) for 16 weeks. In parallel, another group of 7 mice were fed a control diet containing 10% Kcal of fat (D12450 Research Diet Inc). After the nutritional intervention, CDA‐HFD mice displayed inflammation, ballooning and fibrosis. Then, mice were sacrificed by cervical dislocation and plasma and liver samples were collected and processed. The study was approved by the Institutional Animal Care Committee of CABIMER (permission numbers 06‐10‐14‐138 and 19/02/2016/023), and performed according to the Spanish law on animal use RD 53/2013. Liver FGF21 histological studies were performed as indicated. Liver samples were fixed in 4% paraformaldehyde in PBS at 4 ºC overnight and then processed for paraffin embedding with a Leica ASP200S tissue processor (Leica, Wetzlar, Germany). Sections with a thickness of 5 μm were cut on a Leica DM6000B microtome (Leica, Wetzlar, Germany). Mouse liver sections were treated at 121°C to retrieve antigens in a pressure steamer containing 10 mM citrate buffer (pH 6.0) for 1 h. The sections were immersed in 3% hydrogen peroxide for 15 min and incubated at 4ºC overnight with a rabbit Anti‐FGF21 antibody diluted 1:200 (Cat #ab66564, Abcam Inc, Cambridge, UK). The following day, the slices were washed and incubated with second anti‐rabbit IgG‐Biotin (Thermo Fisher Scientific). The slices were incubated with the peroxidase substrate diaminobenzidine (DAB) to develop the colour, using the Vectastain Elite ABC kit (Vector Laboratories, Burlingame, CA, USA). The sections were then counterstained with hematoxylin and mounted with DPX. FGF21 staining was observed under light microscopy (Leica, Wetzlar, Germany). Images were analysed using the open source image processing package ImageJ. Quantification was carried out at 40x magnification (n = 4 pictures each) with the Open Source Plugin IHC Profiler protocol previously optimized. 13 This plugin offers the pixel‐by‐pixel quantification, and after calculations, the results are expressed as the number of positive pixels from the total number of pixels from each image.
Huh 7.5 cell culture and free fatty acids treatment
A hepatocyte‐derived cell line (Huh7.5) was cultured and exposed to 1mM of oleic, palmitic or palmitoleic acid for 24, 48 and 72 h following the protocol previously described. 14 In brief, free fatty acids were added to the cell culture to achieve an oleic acid concentration of 1M, palmitic acid of 0.5 M, or a mixture of palmitic and oleic acids in a proportion of 2:1. Total RNA was prepared by using miRVana isolation kit following manufacturer's instructions and FGF21 mRNA expression was analysed as stated below by qRT‐PCR, using 18S RNA as the endogenous control.
Patients and study design
Patients were recruited from different Hepatology Units considering the following exclusion criteria: significant alcohol intake (≥30 g/day in men and ≥20 g/day in women), recreational drug abuse, evidence of viral or autoimmune hepatitis, HIV, drug‐induced fatty liver or other metabolic liver diseases (such as hemochromatosis or Wilson's disease), together with pregnancy and parenteral nutrition. All patients included in this study were biopsy‐proven MASLD, including control subjects who had a prior history of transaminase fluctuations that was evaluated with a liver biopsy (please, refer to flowchart in 3A). All patients underwent a screening visit including medical history, physical examination, laboratory tests and signed an informed consent to clinical investigations according to the principles embodied in the Declaration of Helsinki of 1975, as revised in 1983. The Institutional Review Board Committee from each participating hospital approved the study protocol. Study procedures agreed with the ethical standards of the responsible Committee on Human Experimentation and were approved by the Human Research Ethics Committee from each centre (C.I. 0359‐N‐15).
Clinical, anthropometric and biochemical measurements
Clinical, laboratory and anthropometric data, including body mass index (BMI) and abdominal perimeter, were collected along with the liver biopsy. An overnight fasting blood sample was drawn prior to the liver biopsy for routine biochemical analyses to rule out occult diseases. This included transaminase (ALT, AST and γGT), alkaline phosphatase, glucose, insulin, total cholesterol, high‐density lipoprotein cholesterol (HDL‐c), low‐density lipoprotein cholesterol (LDL‐c), total bilirubin, albumin, triglycerides and viral serology for hepatitis B and C viruses. Concomitant treatments at the time of biopsy were recorded, as well as comorbidities such as type 2 diabetes mellitus, arterial hypertension and dyslipidaemia.
Histological assessment and patient classification
Percutaneous liver biopsies were performed under local anaesthesia and ultrasound guidance. Liver specimens were obtained, after an overnight fast, from “tru‐cut” needle (sample length/diameter = 20/1.2 mm) using a biopsy gun. Biopsy samples were formalin‐fixed and paraffin‐embedded. A fraction was immediately shock‐frozen and stored at −80ºC for further analyses. Specimens were stained with haematoxylin‐eosin, reticulin and Masson's trichrome. An experienced pathologist blinded with respect to the provenance of the samples and unaware of clinical data, assessed the samples to determine the NAFLD Activity Score (NAS Score) and fibrosis stage by Kleiner Score 15 together with SAF Score. 16 A further 2‐level scale of fibrosis was applied: mild (F0‐F1) and significant (F2‐F3‐F4) fibrosis.
Evaluation of FGF21 liver expression
Total RNA was isolated from eighteen snap‐frozen liver specimens using the MirVana Isolation kit (Life Technologies) following manufacturer's instructions. RNA Integrity number (RIN) was measured by electrophoresis to ensure the quality of samples using an Agilent 2100 Bioanalyzer System. Total RNA quantification was determined using Qubit 2.0 (Life Technologies). FGF21 messenger RNA was evaluated using quantitative real‐time polymerase chain reactions (qRT‐PCR) carried out in triplicate using the 7500 Fast Real‐Time PCR System. RNA normalization was performed by amplification of RNA 18S as an endogenous control. The 2−ΔCT method was used for the analysis of relative gene expression, 17 and results were expressed as fold change.
Human transcriptomic analyses
FGF21 performance was further analysed in a second cohort of MASLD patients in whom the differential expression by RNA‐seq analysis was recently published (dataset GSE130970). 18 All patients underwent a liver biopsy in order to diagnose MASLD, except control subjects who were either donors for living donor transplant or had a prior history of ALT fluctuations evaluated with liver biopsy. Groups compared in this study were classified using the SAF Score as follows: (i) Controls (n = 7); (ii) MASLD patients (n = 23) and (iii) MASH patients: (n = 44). Gene counts were estimated using Tximport v0.99.8 and differential expression analysis was performed using edgeR, filtering out genes that had less than 5 counts per million (CPM) in more than 5 samples (minimal sampling group).
Evaluation of circulating human and mouse FGF21 levels
Venous blood samples were collected after overnight fasting. Samples were centrifuged 10 min at 2500 × g at 4°C right after being obtained, aliquoted and immediately stored at −80ºC until analysis. Circulating FGF21 levels were measured in 50 μL using a human commercial enzyme‐linked immunosorbent assay (ELISA) (Quantikine, R&D Systems) according to the manufacturer's instructions. On the other hand, animals were fasted overnight prior to the blood collection into heparinized tubes and immediately processed by centrifugation for 20 min at 2500 × g at 4°C, and then, plasma was separated, aliquoted and frozen at −80°C for further analysis. Circulating FGF21 levels were measured in 10 μL using a mouse Fibroblast Growth Factor 21 Elisa Kit (Millipore Corporation) according to the manufacturer's instructions.
DNA isolation, quantification and single‐nucleotide polymorphisms genotyping
In the estimation cohort, three‐hundred and eighty‐seven biopsy‐proven MASLD patients were included. The validation cohort was composed of one‐hundred and twenty‐five biopsy‐proven MASLD patients. DNA was automatically isolated from 400 μL of whole blood using Magnapure® Compact equipment (Roche Diagnostics) following the manufacturer's protocol. DNA quantification was performed using NanoDrop™ 2000® (Wilmington, USA) to avoid chemical interferences in the process. FGF21 rs838133 and PNPLA3 rs738409 variants were determined by allelic discrimination using a predesigned Taqman assay (Applied Biosystems, Foster City, CA, EEUU). Additional analyses were performed using the SNPStats statistical software. 19
Statistical analysis
The software package SPSS 22.0 (IBM, Chicago, IL, USA) was used to record data and perform the detailed statistical analysis. Graphs were generated with GraphPad Prism 6.0 (La Jolla, California). Statistical analyses using t‐tests or ANOVA were carried out for normal distributions, and U‐Mann Whitney or Kruskal‐Wallis tests were carried out for non‐normal variables, corrected by the Bonferroni test. All p‐values ≤0.05 were considered statistically significant. Categorical variables were explored by chi‐squared analysis, and finally, continuous variables were assessed by Pearson correlation coefficient. Variables showing p‐values ≤0.05 in univariate analysis were entered into backward Wald logistic regression analysis one at the time for genotyping evaluation, to escape from potentially confounding factors and identify factors related to steatohepatitis and liver fibrosis (a significance level of 0.05 was used to eliminate them from the model). Odds ratios (OR) and their 95% confidence intervals (CI) were estimated. The method used for missing data was complete‐case analysis since statistical packages exclude individuals with any missing value.
RESULTS
FGF21 is increased in both liver and serum from CDA‐HFD animals model
The main biochemical features of these mice are shown in Supplementary Figure S1. Briefly, and consistently with the literature, after 16 weeks of diet, CDA‐HFD mice displayed less body weight (20.6 ± 1.5 vs. 26.6 ± 3 g; p = 0.002), glucose (73.6 ± 20.9 vs. 107.4 ± 7.9 mg/dL; p = 0.009) and total cholesterol (49.7 ± 118.8 vs. 87.2 ± 17.4 mg/dL; p < 0.0001) than controls. On the other hand, ALT (182.5 ± 64.7 vs. 26.8 ± 7.8 IU/L; p = 0.002) and AST (479.5 ± 20.9 vs. 107.4 ± 7.9 IU/L; p < 0.0001) were found to be increased in CDA‐HFD animals. Finally, no significant differences were found regarding triglyceride levels between both groups (67.1 ± 8.8 vs. 62.9 ± 9.9 mg/dL; p = ns) (please, refer to supplementary Figure S1). As previously reported, after 16 weeks, CDA‐HFD animals displayed features of both MASH and liver fibrosis, as evaluated by the anatomopathologist (Figure 1a, supplementary Table S1). In the immunohistochemistry staining, after 16 weeks of dietary regimen, FGF21 was found to be increased in the livers of CDA‐HFD animals when compared with controls (1 × 104 ± 5.9 × 102 vs. 338.8 ± 274.2 stained pixels; p < 0.0001) (Figure 1b). Furthermore, CDA‐HFD animals also presented significantly higher circulating FGF21 levels when compared to controls (1717 ± 82.55 vs. 505.5 ± 52.2 pg/mL; p < 0.0001), accordingly to hepatic injury (Figure 1c), and there was a correlation with FGF21 levels and NAS Score (r = 0.847, n = 11, p = 0.002) (please, see supplementary Figure S2).
FIGURE 1.
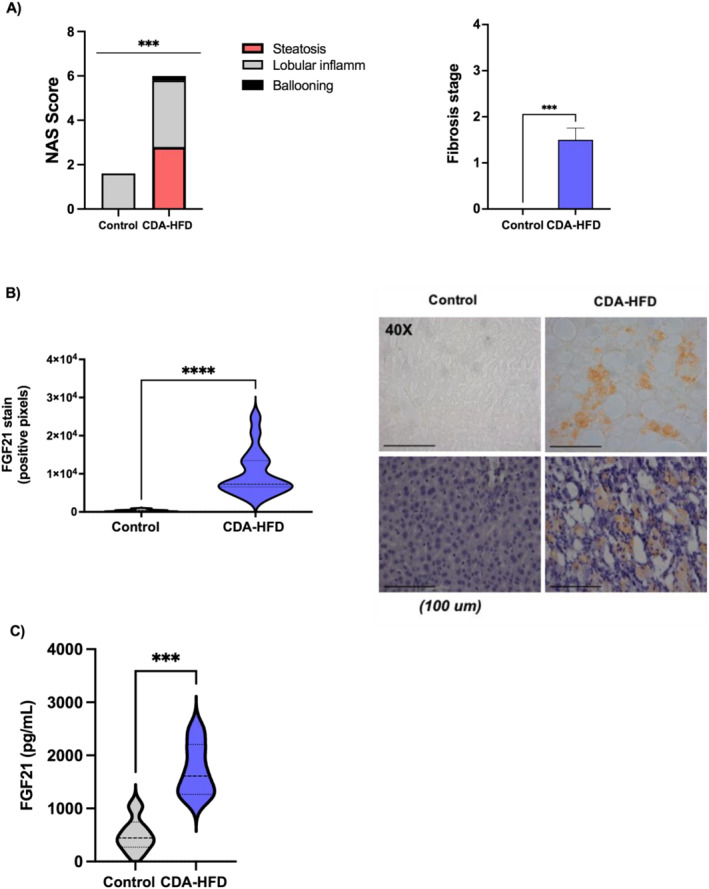
FGF21 findings in CDA‐HFD and control mice. (a) Histological findings in CDA‐HFA animals versus controls; (b) Protein expression evaluated in the livers by immunohistochemistry and (c) circulating levels in sera of mice. CDA‐HFD, choline‐deficient and high‐fat diet. ***p < 0.001.
FGF21 expression is increased in huh 7.5 cells after exposure to free fatty acids
Huh7.5 cells treated with oleic, palmitic and palmitoleic acid showed a significant increase in FGF21 mRNA expression compared with control (all p < 0.001; Figure 2). In the case of oleic acid, the higher effect on FGF21 was reached during the first 24 h of exposure; nevertheless, in the case of palmitoleic acid, this difference was found to be higher after 72 h of treatment.
FIGURE 2.
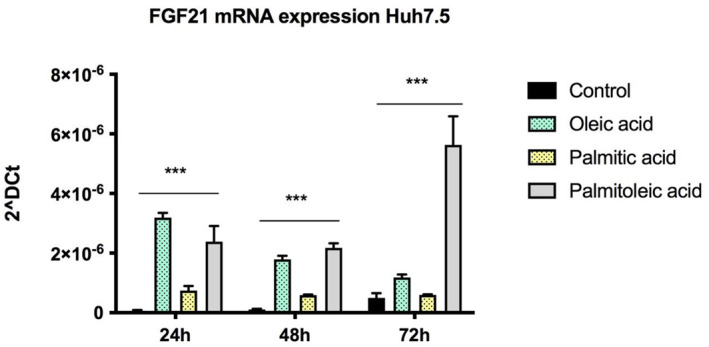
FGF21 mRNA expression is found to be increased in Huh7.5 cells treated with free fatty acids at different timepoints (24, 48 and 72 h). ***p < 0.001.
Hepatic FGF21 expression is increased in MASLD patients
Eighteen patients were included, of which 8 healthy controls, 5 with bland steatosis and 5 with steatohepatitis (Supplementary Table S2). Quantitative PCR analysis revealed that the hepatic expression of FGF21 increased in parallel with the hepatic injury (fold change controls: 0.59 ± 0.23 vs. bland steatosis: 1.14 ± 0.59 vs. fold change MASH: 5.32 ± 2.34; p = 0.029) (Figure 3b). Furthermore, RNA‐seq analysis in a cohort composed of seventy‐four biopsy‐proven MASLD patients revealed an increase in FGF21 expression in MASLD patients when compared to healthy controls (3.5 fold; FDR: 0.006; p < 0.001) (Figure 3c).
FIGURE 3.
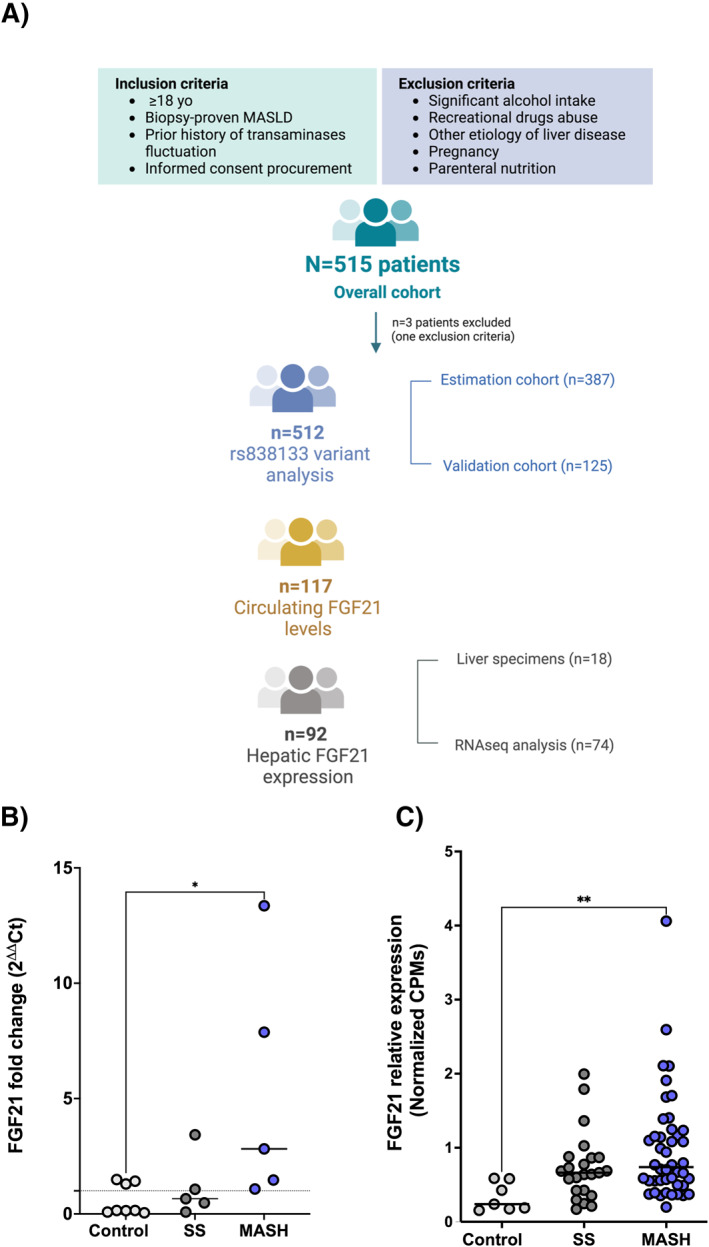
(a) Flowchart of clinical studies; (b) FGF21 relative expression in liver biopsies from healthy controls, simple steatosis and MASH patients classified according to SAF Score; (c) Transcriptomic analysis of FGF21 mRNA expression in healthy controls, bland steatosis and steatohepatitis revealed that FGF21 expression correlated with liver damage progression. Data are expressed as mean SEM. FGF21, Fibroblast Growth Factor 21; MASH, metabolic dysfunction associated steatohepatitis; SS, Simple Steatosis. *p 0.05; ***p < 0.001.
Besides, in our cohort, significant correlations were found between FGF21 mRNA hepatic levels and several histological and clinical features, such as BMI (r = 0.741, n = 14; p = 0.002; Figure 4a) and HOMA‐IR (r = 0.721, n = 15; p = 0.002; Figure 4b), NAS Score (r = 0.604; n = 18; p = 0.009; Figure 4c), hepatic steatosis (r = 0.472; n = 18; p = 0.048; Figure 4d), ballooning (r = 0.503; n = 18; p = 0.033; Figure 4e); lobular inflammation (r = 0.327; n = 17; p = 0.185); and liver fibrosis (r = 0.541; n = 18; p = 0.021; Figure 4f).
FIGURE 4.
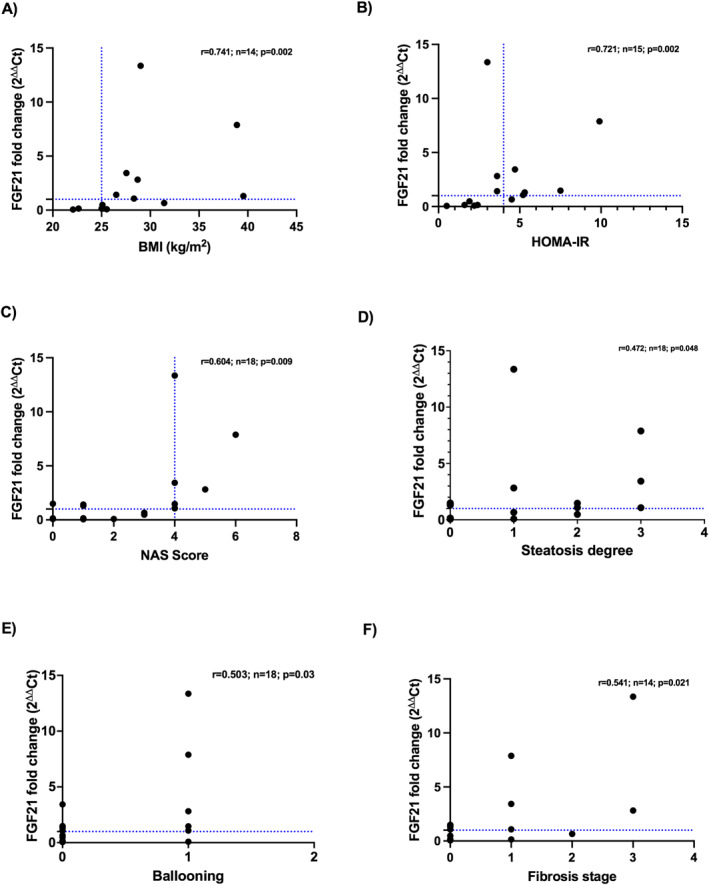
Correlations between hepatic FGF21 mRNA expression and several clinical and histopathological features: (a) BMI; (b) HOMA‐IR; (c) NAS Score; (d) Steatosis degree; (e) ballooning; and (f) fibrosis stage.
Serum FGF21 levels are increased in MASH patients
One hundred and seventeen patients were included; of them, 28/117 (24%) were healthy controls, 45/117 (39%) displayed bland steatosis and 44/117 (37%) suffered from steatohepatitis and fibrosis according to the SAF Score (Supplementary Table S3). No patient presented with serum FGF21 levels below the quantification limit.
MASLD patients showed increased FGF21 circulating levels significantly higher than controls (341.8 ± 285.7 vs. 193.4 ± 135.5 pg/mL; p = 0.002) (Figure 5a). MASH patients showed increased FGF21 levels than simple steatosis and healthy controls (386.6 ± 328.9 vs. 297.9 ± 231.5 vs. 193.4 ± 135.5 pg/mL; p = 0.003) (Figure 5b). FGF21 levels were found to be significantly increased according to steatosis degree (grade III 357.4 ± 300.9 vs. grade II 397.9 ± 345.7 vs. grade I 298.2 ± 229.7 vs. grade 0193.41 ± 135.5 pg/mL; p = 0.008) (Figure 5c) and ballooning presence (ballooning 381.1 ± 319.3 vs. non‐ballooning 252.4 ± 203.8 pg/mL; p = 0.009) (Figure 5d). Lobular inflammation followed the same trend, although statistical significance was not reached (lobular inflammation 330.6 ± 298.1 vs. 251.4 ± 158.9 pg/mL, p = ns). Similarly, FGF21 circulating levels were found to be increased in significant fibrosis patients but did not reach statistical significance (significant fibrosis 370.6 ± 399.6 vs. non‐significant fibrosis 285.1 ± 189.5 pg/mL; p = ns).
FIGURE 5.
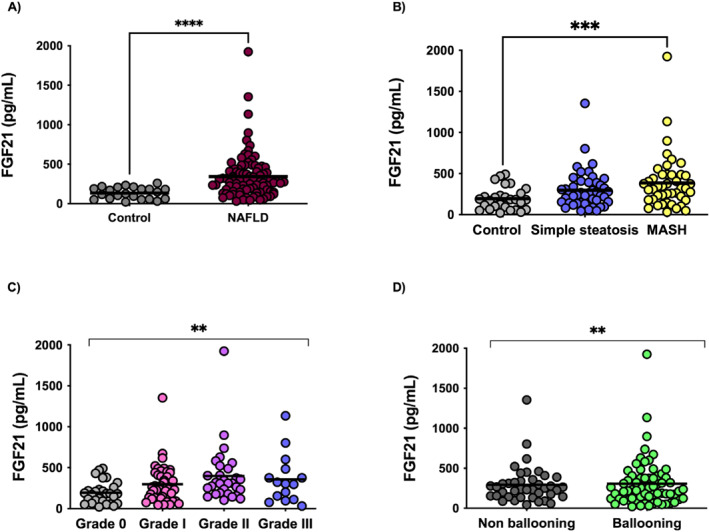
Serum FGF21 levels are raised in (a) MASLD patients (n = 89) versus healthy controls (n = 28); (b) MASH (n = 44) versus MASLD (n = 44) versus healthy controls (n = 28); (c) according to steatosis degree, 0%–5% steatosis (n = 28), 6%–33% steatosis (n = 44), 34%–66% steatosis(n = 30) and 66% steatosis (n = 15); (d) according to hepatocyte ballooning. ***p < 0.001; **p < 0.01; *p < 0.05. Mann‐Whitney and Kruskal‐Wallis tests corrected for the Bonferroni test have been used for these comparisons.
Carrying A‐allele from FGF21 rs838133 confers susceptibility to liver damage
In univariate analysis, in the estimation cohort FGF21 rs838133 A‐allele (AA/AG 36.2% (97/268) vs. GG 26.1% (31/119); p = 0.050) was associated with MASH. The PNPLA3 gene was also found associated with MASH (GG 44.4% (28/63) vs. GC/CC 30.6% (98/320); p = 0.033) (please, refer to Table 1). Further variables associated with MASH were male sex, type 2 diabetes mellitus, AST, ALT, insulin, glucose, HOMA‐IR, triglycerides and platelets. Besides, in multivariate analysis, variables independently associated with MASH were A‐allele of FGF21 rs838133 variant [OR 3.94 (95% CI 1.27–12.27); p = 0.018]; ALT [OR 1.02 (95% CI 1.01–1.103); p = 0.002]; type 2 diabetes mellitus [OR 7.12 (95% CI 2.43–20.86); p = 0.0001] and male sex [OR 2.70 (95% CI 1.12–6.51); p = 0.0027] (Table 1). GG genotype from PNPLA3 showed a p‐value close to significance [OR 2.64 (95% CI 0.96–7.26); p = 0.060]. The coexistence of the A‐allele of FGF21 rs838133 and GG genotype of PNPLA3 increased the risk of MASH [OR 11.67 (95% CI 2.50–54.54); p = 0.002] vs. the presence of just one of them [OR 5.79 (95% CI 1.53–21.97); p = 0.010] (taking the lack of these factors as reference).
TABLE 1.
Univariate and multivariate analyses according to MASH (N = 387).
| Variable | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| Steatosis (n = 259) | MASH (n = 128) | p‐value | OR [95%CI] | P‐value | |
| Gender distribution (males vs. females, %) | 46.4% | 58.3% | 0.043 | OR 2.70 [95% CI 1.12–6.51] | p = 0.0027 |
| BMI ≥25 kg/m2 | 86.4% | 97.5% | 0.001 | ||
| Abdominal perimeter (cm) | 106.4 ± 15.2 | 105.4 ± 16.3 | 0.749 | ||
| Age (years) | 44.7 ± 12.6 | 45.7 ± 12.7 | 0.478 | ||
| T2DM (diabetic vs. non‐diabetic) | 11.8% | 22.6% | 0.011 | OR 7.12 [95% CI 2.43–20.86] | p = 0.0001 |
| HTA | 24.1% | 30.6% | 0.437 | ||
| PNPLA3 (GG vs. CG/CC) | 30.6% | 44.4% | 0.033 | O.R. 2.64 [95% CI 0.96–7.26] | p = 0.060 |
| FGF21 (AA/AG vs. GG) | 66% | 75.8% | 0.05 | O.R. 3.94 [95% CI 1.27–12.27] | p = 0.018 |
| TM6SF2 (CT/TT vs. CC) | 14.3% | 15.7% | 0.815 | ||
| AST (IU/mL) | 31.6 ± 25.2 | 40.9 ± 23.9 | 0.001 | ||
| ALT (IU/mL) | 43.9 ± 37.5 | 68.1 ± 40.9 | 0.000 | O.R. 1.02 [95% CI 1.01–1.103] | p = 0.002 |
| GGT (IU/mL) | 76.3 ± 80.3 | 89.9 ± 83.4 | 0.140 | ||
| Glucose (mg/dL) | 100.3 ± 27.5 | 107.7 ± 29.1 | 0.022 | ||
| Insulin (mg/dL) | 11.9 ± 8.8 | 17.5 ± 12.7 | 0.000 | ||
| HOMA‐IR | 3.1 ± 3.07 | 4.9 ± 4.7 | 0.000 | ||
| Total cholesterol (mg/dL) | 191.2 ± 47.5 | 192.2 ± 41.5 | 0.842 | ||
| HDL‐cholesterol (mg/dL) | 60.9 ± 24.3 | 57.3 ± 21.2 | 0.186 | ||
| LDL‐cholesterol (mg/dL) | 115.6 ± 40.4 | 112.0 ± 33.9 | 0.179 | ||
| Triglycerides (mg/dL) | 119.2 ± 61.1 | 166.1 ± 107.5 | 0.006 | ||
| Albumin (mg/dL) | 4244.7 ± 288.9 | 4261.2 ± 418.5 | 0.857 | ||
| Alkaline phosphatase (IU/mL) | 77.0 ± 31.8 | 81.2 ± 38.6 | 0.470 | ||
| Platelet count (x109) | 240.1 ± 64.4 | 224.4 ± 77.6 | 0.322 | ||
| Fibrosis, stage | 0.4 ± 0.7 | 1.4 ± 1.12 | 0.000 | ||
Note: Independent variables showing p‐values ≤0.05 in univariate analysis were entered into backward Wald logistic regression analysis.
The A‐allele of FGF21 rs838133 was not associated with significant fibrosis in the overall cohort, but excluding morbid obese patients, the A‐allele of FGF21 rs838133 variant was significantly associated with significant fibrosis (19.3% (38/197) vs. 9.4% (8/85); p = 0.039). In addition, BMI, age, AST, ALT, HOMA; glucose, insulin, DM, triglycerides and platelets were associated with significant fibrosis, as well as GG genotype of PNPLA3 (31.4% (16/51) vs. 13.5% (32/237); p = 0.002). A‐allele of FGF21 rs838133 [OR 3.34 (95% CI 1.19–9.4); p = 0.022]; GG genotype of PNPLA3 [OR 2.57 (95% CI 1.05–6.30); p = 0.039]; ALT [OR 1.02 (95% CI 1.01–1.103); p = 0.0001]; type 2 diabetes mellitus [OR 2.98 (95% CI 1.25–7.12); p = 0.014], age [OR 1.07 (95% CI 1.03–1.10); p = 0.0001] and BMI [OR 1.13 (95% CI 1.04–1.23); p = 0.006] were found to be independent predictors of the presence of significant fibrosis. Again, the coexistence of the A‐allele of FGF21 rs838133 and GG genotype of PNPLA3 increased the risk of significant fibrosis [OR 7.61 (95% CI 2.00–29.00); p = 0.003]. Further, in this cohort, genotype‐protein level analysis was performed. Circulating FGF21 levels were found to be increased in the presence of the A‐allele from rs838133, but did not reach statistical significance (A‐allele (n = 85) 324.9 ± 291.7 vs. non‐A‐allele (n = 28) 264.7 ± 177.4 pg/mL, p = ns) (please see supplementary Figure S3).
Finally, in the validation cohort (please see supplementary Table S4), A‐allele from FGF21 rs838133 [OR 2.69 (95% CI 1.06–6.77); p = 0.036]; GG genotype from PNPLA3 [OR 3.47 (95% CI 1.18–10.15); p = 0.023]; ALT [OR 0.97 (95% CI 0.94–0.99); p = 0.017]; HOMA‐IR [OR 1.41 (95% CI 1.19–1.67); p < 0.0001] were found to be independent predictors of the presence of significant fibrosis.
GPNPLA3/AFGF21 haplotype increases susceptibility to liver fibrosis
SNP analyses confirmed the association of G‐allele carriers from PNPLA3 and A‐allele from FGF21 with liver fibrosis. Further, GPNPLA3/AFGF21 haplotype increased the susceptibility to both significant and advanced liver fibrosis in the estimation cohort (significant fibrosis OR 2.58 (95% CI 1.63–4.09); p < 0.0001; advanced fibrosis OR 2.96 (95% CI 1.65–5.29); p = 0.0003; Figure 6a) and the overall cohort (significant fibrosis OR 2.31(95% CI 1.60–3.33); p < 0.0001; advanced fibrosis OR 2.38 (95% CI 1.57–3.59); p < 0.0001; Figure 6c). The validation cohort showed the same trend but did not reach statistical significance (significant fibrosis OR 1.51 (95% CI 0.66–3.46); p = ns; advanced fibrosis OR 1.21 (95% CI 0.56–2.60); p = ns; Figure 6b) (For full description, please refer to supplementary Figure S4).
FIGURE 6.
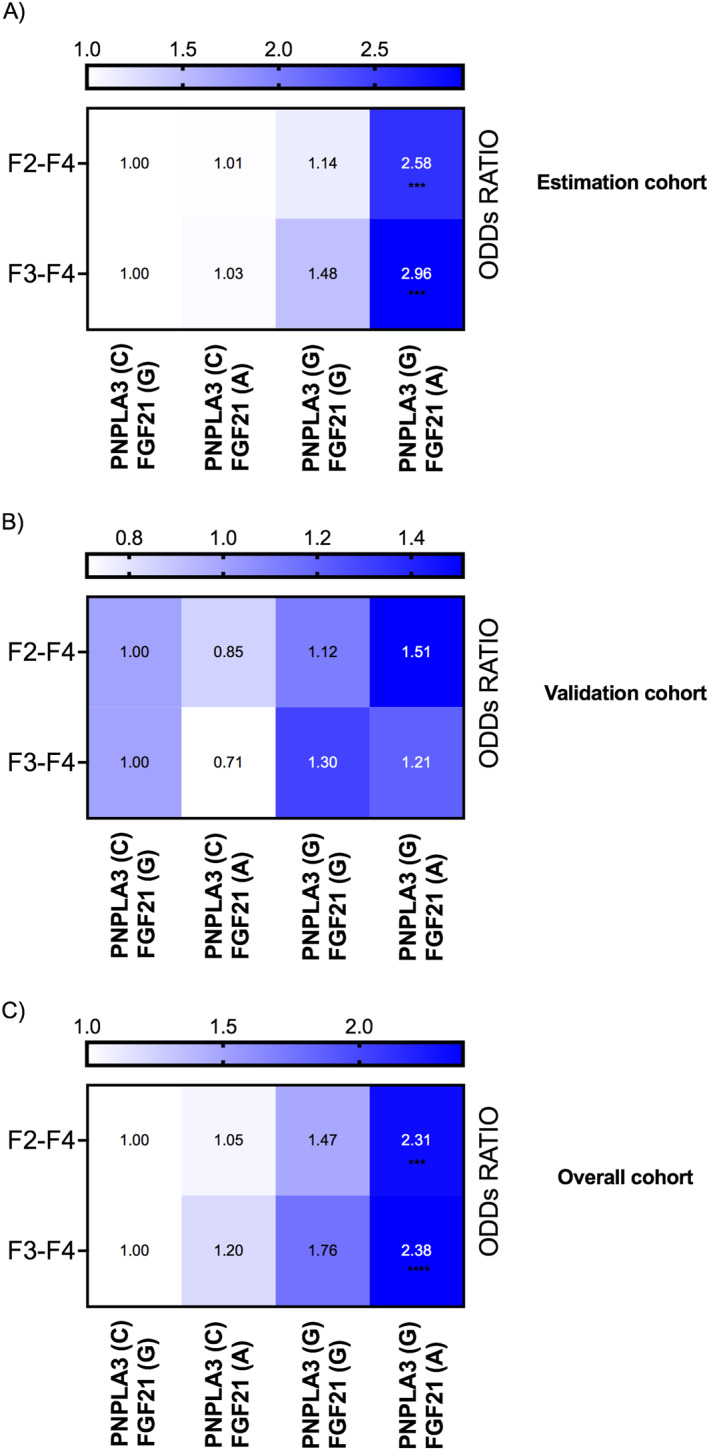
Patients bearing the GPNPLA3/AFGF21 haplotype showed an increased susceptibility to both significant and advanced liver fibrosis.
DISCUSION
In the present study, we demonstrated that FGF21 gene hepatic expression and serum FGF21 protein concentration were increased in biopsy‐proven MASLD patients. These findings were confirmed in a preclinical model mimicking MASLD, and directly correlated with liver damage and metabolic disturbances. In vitro exposure of Huh7.5 cells to high concentrations of free fatty acids resulted in an overexpression of FGF21, similar to that observed in MASH patients. Finally, we identified a novel risk‐conferring SNP, rs838133, associated with increased hazard of developing both MASH and significant fibrosis, and we proved that the GPNPLA3/AFGF21 haplotype increased the susceptibility to significant liver fibrosis.
Preclinical studies have shown that FGF21 displays anti‐inflammatory, anti‐diabetic and hypolipidemic properties. Exogenous administration of FGF21 to rodents and diabetic rhesus monkeys causes pharmacological profits on the cluster of obesity‐related metabolic disturbances. These benefits include weight loss and improvement of insulin sensitivity and hepatic steatosis and an enhancement of longevity and hepatic health more effective than caloric restriction in mice. 20 , 21 Indeed, it has been demonstrated that FGF21 administration lowered plasma triglycerides through a dual mechanism, reducing VLDL secretion and increasing CD36 levels in white and brown adipose tissue. 22 In preliminary results, FGF21 analogues, including Efruxifermin, demonstrated in preliminary results, demonstrated favourable results in steatosis, MASH resolution and fibrosis regression. 23 , 24 , 25 Further, treatment with pegozafermin, a glycoPEGylated FGF21 analogue, has been proven to reduce liver fat, circulating lipids and improved liver function. 26
Additionally, FGF21 homeostasis has received considerable attention due to its anti‐diabetic effect being paradoxically elevated in the setting of impaired glucose and lipid metabolism. Moreover, it plays a significant role in the pathogenicity of steatohepatitis, through the accumulation of inactivated fatty acids, resulting in lipotoxic damage. 27 These data suggest that raised FGF21 may occur as a compensatory response to offset metabolic disturbances in a “FGF21 resistance state” 28 or insensitivity, that may not respond to exogenous administration of FGF21 in supraphysiological concentrations. However, FGF21 could correct multiple metabolic parameters in vitro and in vivo by inducing autophagy 29 and may be part of a strategy to combat hepatic steatosis and inflammation. It has recently unveiled some of the mechanisms of action through FGF21 alleviates liver injury, such as its modulation via physiological activation of myeloid p38, that helps maintain macrophage‐liver crosstalk as well as brown adipose tissue thermogenesis. 30
There is a clear unmet need for MASLD treatment; therefore, it is mandatory to identify platforms to test novel drug compounds. The genomics of fat‐laden hepatocytes revealed lipid droplet‐associated gene regulations and perturbed metabolic pathways in lipid‐laden human primary hepatocytes and human hepatoma cell culture, including the PPAR family and FGF21, among others. 31 Moreover, reports have shown the positive effect of palmitic acid on ATF4 expression and that of oleic acid on PPARα transcriptional activity. 32 , 33 Thus, the FFA‐induced gene expression of ATF4 and the enhanced transcriptional activity of PPARα in the hepatocyte derived cell line led to the synergistically increased FGF21 expression.
In a previous genome‐wide meta‐analysis including 33,000 participants, the rs838133 variant located in the FGF21 gene was found to be associated with decreased protein intake and increased carbohydrate consumption, potentially determining dietary macronutrient intake and subsequently conditioning MASLD disorder. 9 The relationship between this variant and the selective ingestion of palatable foods including sweets was further analysed in a subsequent cohort, revealing an association between rs838133 and increased consumption of candy, as well as increased plasma FGF21 levels after oral sucrose ingestion, suggesting that the liver may secrete hormones that influence eating behaviour. 34 Besides, the A‐allele has been strongly associated with higher blood pressure and waist‐hip ratio, despite an association with lower total body‐fat percentage, 10 as well as linked to high salt intake. 35 Finally, a minor A‐allele from rs838133 was recently associated with increased hepatic inflammation in MASLD patients. 36 Altogether, these findings support a potential connection of this variant with some of the main traits of metabolic syndrome and MASLD. In this study, we demonstrated that the minor A‐allele of rs838133 is associated with liver fibrosis and an increased risk of developing steatohepatitis, in both the estimation and the validation cohorts. Despite the significant differences among them in terms of liver fibrosis and MASH, this association remains significant. Further, the presence of an additive effect of the A‐allele in this novel variant with the G‐allele of PNPLA3, the most studied SNP in MASLD, has been proven. This might be of special relevance, since there is a lack of non‐invasive tools currently available, the genotyping of these variants might help in the diagnosis of MASLD patients.
In conclusion, FGF21 plays multiple and vital roles in regulating energy homeostasis, expenditure and glucose‐lipid metabolism, and currently, the mechanisms that underlie this association with liver damage in MASLD are still being cleared. In alignment with these hypotheses, we found FGF21 upregulation at various scales, such as the hepatic expression and the circulating fraction in both humans and CDA‐HFD animals, as well as describing a novel variant located in FGF21 conferring risk of liver fibrosis. Interestingly, exogenous FGF21 analogues seem to work in MASLD at the same time the patients showed increased FGF21 expression and protein levels suggesting a FGF21‐resistant state. Nevertheless, deeper functional studies are warranted to further explore the physiological functions and regulation of FGF21.
AUTHOR CONTRIBUTIONS
Guarantors of the article: Rocío Gallego‐Durán, Manuel Romero‐Gómez. Study concept and design: Rocío Gallego‐Durán, Javier Ampuero, Douglas Maya‐Miles, Manuel Romero‐Gómez. Drafting of the manuscript: Rocío Gallego‐Durán, Manuel Romero‐Gómez. Statistical analyses and interpretations: Rocío Gallego‐Durán, Javier Ampuero, Douglas Maya‐Miles, Manuel Romero‐Gómez. Data acquisition and critical revision of the manuscript: All authors. All authors approved the final version of the article, including the authorship list.
CONFLICT OF INTEREST STATEMENT
All authors affirm that there is no conflict of interest to declare.
ETHICS APPROVAL
This study was performed in line with the principles of the Declaration of Helsinki. The Institutional Review Board Committee from each participating hospital approved the study protocol. Study procedures agreed with the ethical standards of the responsible committee on human experimentation and were approved by the human research ethics committee from each centre (C.I. 0359‐N‐15). Further, the study was approved by the Institutional Animal Care Committee of CABIMER (permission numbers 06‐10‐14‐138 and 19/02/2016/023), and performed according to the Spanish law on animal use RD 53/2013. All patients signed an informed consent for clinical investigations according to the principles embodied in the Declaration of Helsinki of 1975, as revised in 1983.
Supporting information
Supporting Information S1
ACKNOWLEDGEMENTS
The research leading to these results has received funding from the Consejería de Salud de la Junta de Andalucía under grant agreement PC‐0148‐2016‐0148 and PE‐0451‐2018, and from the Instituto de Salud Carlos III under grant agreements CD21/00095, IFI18/00041, PI19/01404, PI19/00589, PI22/01342, PI22/01345 and Ministerio de Economía y Competitividad under grant agreement AGL‐2017‐86927‐R. Rocío Gallego‐Durán has received the Andrew K Burroughs Fellowship from the European Association for the Study of the Liver (EASL), Aprendizaje de Nuevas Tecnologías fellowship from Asociación Española para el Estudio del Hígado (AEEH) and CIBERehd Grant to support researcher's mobility.
Gallego‐Durán R, Ampuero J, Maya‐Miles D, Pastor‐Ramírez H, Montero‐Vallejo R, Rivera‐Esteban J, et al. Fibroblast growth factor 21 is a hepatokine involved in MASLD progression. United European Gastroenterol J. 2024;12(8):1056–68. 10.1002/ueg2.12534
Rocío Gallego‐Durán and Manuel Romero‐Gómez share co‐senior authorship.
Rocío Gallego‐Durán, Javier Ampuero and Douglas Maya‐Miles have contributed equally to this work.
Contributor Information
Rocío Gallego‐Durán, Email: rgallego-ibis@us.es.
Manuel Romero‐Gómez, Email: mromerogomez@us.es.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding authors, RGD and MRG, upon reasonable request.
REFERENCES
- 1. Ampuero J, Aller R, Gallego‐Durán R, Crespo J, Calleja JL, García‐Monzón C, et al. Significant fibrosis predicts new‐onset diabetes mellitus and arterial hypertension in patients with NASH. J Hepatol. 2020;73(1):17–25. 10.1016/j.jhep.2020.02.028 [DOI] [PubMed] [Google Scholar]
- 2. Bhala N, Angulo P, van der Poorten D, Lee E, Hui JM, Saracco G, et al. The natural history of nonalcoholic fatty liver disease with advanced fibrosis or cirrhosis: an international collaborative study. Hepatology. 2011;54(4):1208–1216. 10.1002/hep.24491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Koliaki C, Szendroedi J, Kaul K, Jelenik T, Nowotny P, Jankowiak F, et al. Adaptation of hepatic mitochondrial function in humans with non‐alcoholic fatty liver is lost in steatohepatitis. Cell Metab. 2015;21(5):739–746. 10.1016/j.cmet.2015.04.004 [DOI] [PubMed] [Google Scholar]
- 4. Fisher FM, Maratos‐Flier E. Understanding the physiology of FGF21. Annu Rev Physiol. 2016;78(1):223–241. 10.1146/annurev-physiol-021115-105339 [DOI] [PubMed] [Google Scholar]
- 5. Xu J, Lloyd DJ, Hale C, Stanislaus S, Chen M, Sivits G, et al. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet‐induced obese mice. Diabetes. 2009;58(1):250–259. 10.2337/db08-0392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Watanabe M, Risi R, Camajani E, Contini S, Persichetti A, Tuccinardi D, et al. Baseline HOMA IR and circulating FGF21 levels predict NAFLD improvement in patients undergoing a low carbohydrate dietary intervention for weight loss: a prospective observational pilot study. Nutrients. 2020;12(7):2141. 10.3390/nu12072141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Praktiknjo M, Djayadi N, Mohr R, Schierwagen R, Bischoff J, Dold L, et al. Fibroblast growth factor 21 is independently associated with severe hepatic steatosis in non‐obese HIV‐infected patients. Liver Int. 2019;39(8):1514–1520. 10.1111/liv.14107 [DOI] [PubMed] [Google Scholar]
- 8. Barb D, Bril F, Kalavalapalli S, et al. Plasma fibroblast growth factor 21 is associated with severity of nonalcoholic steatohepatitis in patients with obesity and type 2 diabetes. J Clin Endocrinol Metab. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40(12):1461–1465. 10.1038/ng.257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Anstee QM, Darlay R, Cockell S, Meroni M, Govaere O, Tiniakos D, et al. Genome‐wide association study of non‐alcoholic fatty liver and steatohepatitis in a histologically characterised cohort. J Hepatol. 2020;73(3):505–515. 10.1016/j.jhep.2020.04.003 [DOI] [PubMed] [Google Scholar]
- 11. Chu AY, Workalemahu T, Paynter NP, Rose LM, Giulianini F, Tanaka T, et al. Novel locus including FGF21 is associated with dietary macronutrient intake. Hum Mol Genet. 2013;22(9):1895–1902. 10.1093/hmg/ddt032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Frayling TM, Beaumont RN, Jones SE, Yaghootkar H, Tuke MA, Ruth KS, et al. A common allele in FGF21 associated with sugar intake is associated with body shape, lower total body‐fat percentage, and higher blood pressure. Cell Rep. 2018;23(2):327–336. 10.1016/j.celrep.2018.03.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Varghese F, Bukhari AB, Malhotra R, De A. IHC Profiler: an open source plugin for the quantitative evaluation and automated scoring of immunohistochemistry images of human tissue samples. PLoS One. 2014;9(5):e96801. 10.1371/journal.pone.0096801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chavez‐Tapia NC, Rosso N, Tiribelli C. Effect of intracellular lipid accumulation in a new model of non‐alcoholic fatty liver disease. BMC Gastroenterol. 2012;12(1):20. 10.1186/1471-230x-12-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313–1321. 10.1002/hep.20701 [DOI] [PubMed] [Google Scholar]
- 16. Bedossa P, Poitou C, Veyrie N, Bouillot JL, Basdevant A, Paradis V, et al. Histopathological algorithm and scoring system for evaluation of liver lesions in morbidly obese patients. Hepatology. 2012;56(5):1751–1759. 10.1002/hep.25889 [DOI] [PubMed] [Google Scholar]
- 17. Ginzinger DG. Gene quantification using real‐time quantitative PCR: an emerging technology hits the mainstream. Exp Hematol. 2002;30(6):503–512. 10.1016/s0301-472x(02)00806-8 [DOI] [PubMed] [Google Scholar]
- 18. Hoang SA, Oseini A, Feaver RE, Cole BK, Asgharpour A, Vincent R, et al. Gene expression predicts histological severity and reveals distinct molecular profiles of nonalcoholic fatty liver disease. Sci Rep. 2019;9(1):12541. 10.1038/s41598-019-48746-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Solé X, Guinó E, Valls J, Iniesta R, Moreno V. SNPStats: a web tool for the analysis of association studies. Bioinformatics. 2006;22(15):1928–1929. 10.1093/bioinformatics/btl268 [DOI] [PubMed] [Google Scholar]
- 20. Coskun T, Bina HA, Schneider MA, Dunbar JD, Hu CC, Chen Y, et al. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology. 2008;149(12):6018–6027. 10.1210/en.2008-0816 [DOI] [PubMed] [Google Scholar]
- 21. Keinicke H, Sun G, Mentzel CMJ, Fredholm M, John LM, Andersen B, et al. FGF21 regulates hepatic metabolic pathways to improve steatosis and inflammation. Endocr Connect. 2020;9(8):755–768. 10.1530/ec-20-0152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schlein C, Talukdar S, Heine M, Fischer A, Krott L, Nilsson S, et al. FGF21 lowers plasma triglycerides by accelerating lipoprotein catabolism in white and Brown adipose tissues. Cell Metab. 2016;23(3):441–453. 10.1016/j.cmet.2016.01.006 [DOI] [PubMed] [Google Scholar]
- 23. Sanyal A, Charles ED, Neuschwander‐Tetri BA, Loomba R, Harrison SA, Abdelmalek MF, et al. Pegbelfermin (BMS‐986036), a PEGylated fibroblast growth factor 21 analogue, in patients with non‐alcoholic steatohepatitis: a randomised, double‐blind, placebo‐controlled, phase 2a trial. Lancet. 2019;392(10165):2705–2717. 10.1016/s0140-6736(18)31785-9 [DOI] [PubMed] [Google Scholar]
- 24. Geng L, Lam KSL, Xu A. The therapeutic potential of FGF21 in metabolic diseases: from bench to clinic. Nat Rev Endocrinol. 2020;16(11):654–667. 10.1038/s41574-020-0386-0 [DOI] [PubMed] [Google Scholar]
- 25. Harrison SA, Ruane PJ, Freilich B, Neff G, Patil R, Behling C, et al. A randomized, double‐blind, placebo‐controlled phase IIa trial of efruxifermin for patients with compensated NASH cirrhosis. JHEP Rep. 2022;5(1):100563. 10.1016/j.jhepr.2022.100563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Loomba R, Lawitz EJ, Frias JP, Ortiz‐Lasanta G, Johansson L, Franey BB, et al. Safety, pharmacokinetics, and pharmacodynamics of pegozafermin in patients with non‐alcoholic steatohepatitis: a randomised, double‐blind, placebo‐controlled, phase 1b/2a multiple‐ascending‐dose study. Lancet Gastroenterol Hepatol. 2023;8(2):120–132. 10.1016/S2468-1253(22)00347-8 [DOI] [PubMed] [Google Scholar]
- 27. Fisher FM, Chui PC, Nasser IA, Popov Y, Cunniff JC, Lundasen T, et al. Fibroblast growth factor 21 limits lipotoxicity by promoting hepatic fatty acid activation in mice on methionine and choline‐deficient diets. Gastroenterology. 2014;147(5):1073–1083. 10.1053/j.gastro.2014.07.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu J, Xu Y, Hu Y, Wang G. The role of fibroblast growth factor 21 in the pathogenesis of non‐alcoholic fatty liver disease and implications for therapy. Metabolism. 2015;64(3):380–390. 10.1016/j.metabol.2014.11.009 [DOI] [PubMed] [Google Scholar]
- 29. Zhu S, Wu Y, Ye X, Ma L, Qi J, Yu D, et al. FGF21 ameliorates nonalcoholic fatty liver disease by inducing autophagy. Mol Cell Biochem. 2016;420(1‐2):107–119. 10.1007/s11010-016-2774-2 [DOI] [PubMed] [Google Scholar]
- 30. Crespo M, Nikolic I, Mora A, Rodríguez E, Leiva‐Vega L, Pintor‐Chocano A, et al. Myeloid p38 activation maintains macrophage‐liver crosstalk and BAT thermogenesis through IL‐12‐FGF21 axis. Hepatology. 2022;77(3):874–887. 10.1002/hep.32581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Breher‐Esch S, Sahini N, Trincone A, Wallstab C, Borlak J. Genomics of lipid‐laden human hepatocyte cultures enables drug target screening for the treatment of non‐alcoholic fatty liver disease. BMC Med Genomics. 2018;11(1):111. 10.1186/s12920-018-0438-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cho H, Wu M, Zhang L, Thompson R, Nath A, Chan C. Signaling dynamics of palmitate‐induced ER stress responses mediated by ATF4 in HepG2 cells. BMC Syst Biol. 2013;7(1):9. 10.1186/1752-0509-7-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Krey G, Braissant O, L’Horset F, Kalkhoven E, Perroud M, Parker MG, et al. Fatty acids, eicosanoids, and hypolipidemic agents identified as ligands of peroxisome proliferator‐activated receptors by coactivator‐dependent receptor ligand assay. Mol Endocrinol. 1997;11(6):779–791. 10.1210/mend.11.6.0007 [DOI] [PubMed] [Google Scholar]
- 34. Søberg S, Sandholt CH, Jespersen NZ, Toft U, Madsen AL, von Holstein‐Rathlou S, et al. FGF21 is a sugar‐induced hormone associated with sweet intake and preference in humans. Cell Metab. 2017;25(5):1045–1053. 10.1016/j.cmet.2017.04.009 [DOI] [PubMed] [Google Scholar]
- 35. Saber‐Ayad M, Hammoudeh S, Radwan H, Manzoor S, Jabbar H, Wardeh R, et al. The FGF‐21 genetic variants rs838133 and rs838145 are associated with high salt intake in the Emirati population. J Adv Res. 2020;24:485–494. 10.1016/j.jare.2020.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bayoumi A, Elsayed A, Han S, Petta S, Adams LA, Aller R, et al. Mistranslation drives alterations in protein levels and the effects of a synonymous variant at the fibroblast growth factor 21 locus. Adv Sci. 2021;8(11):2004168. 10.1002/advs.202004168 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information S1
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors, RGD and MRG, upon reasonable request.


