Abstract
INTRODUCTION
Geographic variation in diagnosed cases of Alzheimer's disease and related dementias (ADRD) could be due to underlying population risk or differences in intensity of new case identification. Areas with low ADRD diagnostic intensity could be targeted for additional surveillance efforts.
METHODS
Medicare claims were used for a cohort of older adults across hospital referral regions (HRRs). ADRD‐specific regional diagnosis intensity was measured as the ratio of expected new ADRD cases (estimated using population demographics, risk factors, and practice intensity) compared to observed ADRD‐diagnosed cases.
RESULTS
Crude new ADRD diagnosis rate ranged from 1.7 to 5.4 per 100 across HRRs. ADRD‐specific diagnosis intensity ranged from 0.69 to 1.47 and varied most for Black, Hispanic, and the youngest (66–74) subgroups. Across all subgroups, ADRD diagnosis intensity was associated with 2‐fold difference in receiving an ADRD diagnosis.
DISCUSSION
Where one resides influences the likelihood of receiving an ADRD diagnosis, particularly among those 66–74 years of age and minoritized groups.
Highlights
Rate of new Alzheimer's disease and related dementias (ADRD) case identification varies geographically across the United States.
Variation in case identification is greatest in Black, Hispanic, and young‐old groups.
Intensity of diagnosis (ie, case identification) unrelated to population risk differs across place.
Likelihood of receiving an ADRD diagnosis varies 2‐fold based on place of residence.
Keywords: Alzheimer's disease, dementia, diagnosis, Medicare, regional variation
1. INTRODUCTION
As the population ages, the number of Americans with Alzheimer's disease and related dementias (ADRD) is expected to grow from 6.7 million in 2023 to nearly 13.9 million by 2060. 1 A major goal of the 2011 National Plan to Address Alzheimer's Disease is to improve quality of care by ensuring early and accurate clinical diagnosis. 2 Robust evidence supports that many people living with dementia, whether due to AD or other pathological processes, have not obtained a clinical diagnosis, perhaps as many as 60% of people living with dementia. 3 , 4 Potential reasons abound for underdiagnosis including patient concerns about stigma or misunderstanding symptoms as normal aging, or clinician lack of skill in making or communicating the diagnosis. 5 , 6 , 7 , 8 Yet case‐finding, that is, identifying people with symptomatic disease as opposed to screening for people at risk, provides the opportunity for advanced care planning and care coordination and access to disease‐modifying treatment for early disease that, in turn, may help preserve independence and reduce the financial or care consequences for the patient and family. 9 , 10 , 11
One approach to making inroads in connecting older adults living with ADRD to the services and care they need is to target case‐finding to the places and populations where there are the largest gaps between what we expect and observe for diagnosed case rate. If these differences are related to practice variation, that is, how intensively clinicians identify new cases, then strategies to improve the diagnostic processes could be pursued. Prior research that demonstrates regional variation in prevalent diagnosed ADRD cases 12 and the rise in use of ADRD diagnoses in the last 2 years of life 13 provide evidence that variation in diagnosis exists. However, variation in diagnosed cases represents both the variation in true prevalence of disease, related to differences in demographic and known risk factors, and the likelihood of obtaining a clinical diagnosis. It remains to be shown whether there is practice variation in the efforts to identify new ADRD cases, after considering underlying population risk. It is important to note that underdiagnosis is a particular issue for subgroups in whom the challenges of getting an accurate diagnosis are greater, specifically due to uncertainty in early‐stage disease (i.e., younger age 3 ) or differences in care‐seeking and stigma (i.e., Black and Hispanic populations 14 , 15 ). Furthermore, in the context of newly approved AD disease‐modifying medications, equity in access to a diagnosis and incentives for more aggressive case‐finding with potential overdiagnosis warrant a better understanding of the drivers of differences in case‐finding across the health system.
RESEARCH IN CONTEXT
Systematic review: Variation across the United States in diagnosed cases of Alzheimer's disease and related dementias (ADRD) could be due to underlying population risk factors (e.g., age, education, and health) or differences in clinical diagnosis.
Interpretation: We used Medicare Fee‐For‐Service claims to measure observed new ADRD diagnosed cases and data from epidemiological population data to estimate regional ADRD new cases across the United States. The diagnosis intensity across regions, ratio of observed versus expected ADRD new cases, ranged from 0.69 to 1.47 and varied most for Black, Hispanic, and the youngest (66–74) subgroups. Across all subgroups, the likelihood of receiving an ADRD diagnosis varied 2‐fold, related to the ADRD diagnosis intensity where one lives.
Future directions: Variation in ADRD diagnosis raises important questions regarding differences in access and health care practices that may drive excess variability in ADRD detection and whether such differences translate into meaningful differences in outcomes.
To address this knowledge gap, we conducted an observational study to examine variation in newly identified ADRD cases (diagnosis rate) across the United States using national Medicare claims data. The objective of our study was to: (1) estimate the degree to which new ADRD diagnosis rates vary across health care markets after accounting for regional differences in demographics, education, health, and general diagnosis intensity; and (2) determine if ADRD new diagnosis rates differ across key subgroups. To do so, we constructed a measure of ADRD‐specific diagnostic intensity using observed to expected number of new ADRD cases in 2019. We hypothesized that there would be regional variation in ADRD‐specific diagnostic intensity beyond that expected based on population risk for disease and general diagnosis intensity, and that there would be greater regional variation for younger age (66–74) and minoritized racial and ethnic groups.
2. METHODS
Using Medicare claims for 2018–2019, we conducted an observational study to examine variation in the new diagnosis of ADRD across U.S. hospital referral regions (HRRs). We estimated the expected number of new ADRD cases using statistical models and created an ADRD‐specific diagnosis intensity measure—calculated as the ratio of the observed to expected number of new ADRD cases in the region. We examined variation in ADRD diagnosis intensity by age category and by race/ethnicity and estimated the probability of ADRD diagnosis among fee‐for‐service (FFS) Medicare beneficiaries by regional ADRD diagnosis intensity. This study received an expedited review from the Dartmouth College Institutional Review Board and abides by STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) guidelines.
2.1. Study population
Using the 20% Master Beneficiary Summary File (MBSF), we identified 9,654,691 adults who were 66 or older on January 1, 2019, and resided in a U.S. HRR (Figure S1). Among those, we identified beneficiaries enrolled in FFS Medicare (Parts A & B) in 2018 and 2019 or until death and restricted to age 66 years or older on January 1, 2019, to accommodate a 1‐year look back period to differentiate a new from existing diagnosis in 2019. Our final sample consisted of 4,842,034 older Medicare FFS beneficiaries.
2.2. Identification of new ADRD diagnosis
We used inpatient (Medicare Provider Analysis and Review), professional outpatient services (Carrier file and selected Outpatient Hospital files for clinician visits in underserved settings), home health, and hospice claims from January 1, 2018, to December 31, 2019. Using these files we applied a validated algorithm using International Classification of Diseases, Tenth Revision, Clinical Modification (ICD‐10‐CM) codes to identify individuals with an ADRD diagnosis. 16 , 17 Among our final sample of 4,842,034 Medicare FFS beneficiaries in the 20% Medicare sample, a total of 419,646 beneficiaries had an ADRD diagnosis of which 143,029 were a new ADRD diagnosis in 2019 (Figure S1).
We used U.S. HRRs, which represent regional health care markets for tertiary care, as our geographic level of analysis because we were interested in regional differences in health care for a condition that often uses specialty referral services. 18 We calculated the regional total percent of the older adult FFS population with an ADRD diagnosis (including prevalent cases either existing before 2019 and new cases in 2019) and new ADRD diagnosis cases across HRRs.
2.3. Individual and regional characteristics
For each beneficiary, we used data on age, sex, and race from the MBSF. To account for regional differences across HRRs, we obtained population characteristics from several sources. For each HRR we calculated the percent of older adults in FFS Medicare that were 66–74, 75–84, versus ≥85 years of age. Sex composition was calculated as the percentage of older adults in FFS Medicare that were female; likewise, race and ethnicity were expressed as the percentage of White/Other categories, Black, and Hispanic using the Research Triangle Institute categories in the MBSF. 19 “Other” included all categories besides White, Black, and Hispanic and was combined with White because sample size by geography would require suppression by data use guidelines and we preferred to combine them with the largest group rather than exclude them.
Based on a large body of work on risks for dementia most succinctly summarized in The Lancet Commission Report, the number of ADRD cases would be influenced by regional factors beyond demographic characteristics and population size, including population level of education, and cardiovascular and other health risk factors. 20 We used data sources that included the entire United States to create regional measures of risk factors. We chose to include education, obesity, smoking, and diabetes because of their availability, inclusion in The Lancet Commission report, and presence of regional variability. Regional educational level (proportion with < High School) was obtained from the 2014–18 American Community Survey 21 at the county level. County‐level measures of smoking were obtained from the 2018 Behavioral Risk Factor Surveillance System 22 and measures of obesity and diabetes prevalence were obtained from the 2017 U.S. Diabetes Surveillance System. 23 County‐level measures were weighted and aggregated to U.S. HRRs based on the estimated distribution of older FFS Medicare beneficiaries residing within and across HRRs boundaries. 24 To assess visually whether higher ADRD diagnosis rates were associated with proximity to National Institute on Aging–funded Alzheimer's Disease Research Centers (ADRCs), we collected address data from the institute's website and geocoded U.S. ADRCs on our maps. 25 In addition to demographic and health factors, clinical practice varies across areas in how intensely diagnoses are sought, leading to differences in observed diagnosed disease rates beyond differences in true underlying disease rates. 26 To adjust for differences in regional medical diagnosis of chronic illnesses we used an established HRR‐level measure of general diagnostic intensity. 27
2.4. Statistical analysis
Regional rates of new ADRD diagnosis (no. per 100) in 2019 were calculated for each HRR overall, by age category (66–74, 75–84, and ≥85), and by race/ethnicity (White/Other, Black, and Hispanic). Among subgroups, HRRs with denominators of fewer than 100 FFS beneficiaries were excluded from our analyses. Coefficient of variation (CV) was used to estimate variability in new ADRD diagnoses. McFadden's pseudo‐R 2 penalized for number of predictors was used to estimate the proportion of variation of new diagnosed cases explained by regional factors.
Poisson regression was used to estimate the expected number of new ADRD cases across HRRs and to adjust for regional characteristics. Sandwich variance estimators, computed by applying the generalized estimating equations procedure to models, were used to generate robust standard error estimates with new case count as the dependent variable and HRR FFS population size as an offset. In these models, the expected number of new ADRD cases was adjusted for population characteristics including population age (percent ≥80 years old), sex composition, race and ethnicity, level of education (percent < high school education), population health (percent with obesity, diabetes, and smoking), and general diagnostic intensity. We then calculated the ADRD‐specific diagnostic intensity measure as the ratio of the observed to expected number of new ADRD cases in each HRR given its size and FFS Medicare population case mix and demographic characteristics.
We measured how much regional differences in ADRD diagnostic intensity influenced the likelihood of receiving an ADRD diagnosis at the individual level. Mixed‐effect models (including a random intercept for HRR to account for clustering by region) were used to estimate the probability of ADRD diagnosis by regional ADRD diagnostic intensity adjusted for individual demographic differences. One may be concerned that diagnostic intensity as an HRR‐level measure could present a mechanical association to an individual's likelihood of diagnosis, since these same individuals contributed to the diagnostic intensity measure. However, with the large number of cases, this association has minimal impact on the result. These analyses were restricted to a simple random sample of 1 million FFS beneficiaries. We examined the association separately by age category and by race/ethnicity.
We used ArcGIS version 10.8.1 (ESRI, Redlands, CA) to create the maps for geocoding ADRCs and to calculate Global Moran's I that was used to estimate spatial autocorrelation across HRRs. 28 All claims and statistical analyses were completed using SAS version 9.4 (SAS Institute Inc., Cary, NC). Analyses were based on complete case analysis, and we set the critical alpha level to 0.05 (two‐sided). Due to the large sample size, note that even small differences can reach statistical significance.
3. RESULTS
3.1. Regional variation in total ADRD diagnosed cases
Of older adults in FFS Medicare, 7.1% (356, 656/5,041,387) were identified as having an ADRD diagnosis (either new or existing) in 2019, with substantial variation across the United States. The highest unadjusted concentration of ADRD cases was in the South and the lowest was in the West/Northwest (Figure 1). Across HRRs shown in Figure SA.2, the unadjusted mean total ADRD diagnosis rate was 6.8 per 100 (SD = 1.6). The diagnosis rate varied considerably by age category (e.g., the mean total diagnosis rate across HRRs was 2.1 per 100 among those 66–74 vs 23.2 per 100 among those ≥85) and by race/ethnicity (e.g., the mean total diagnosis rate across HRRs was 6.5 per 100 among White/Other older adults vs 9.0 per 100 among Black older Adults).
FIGURE 1.
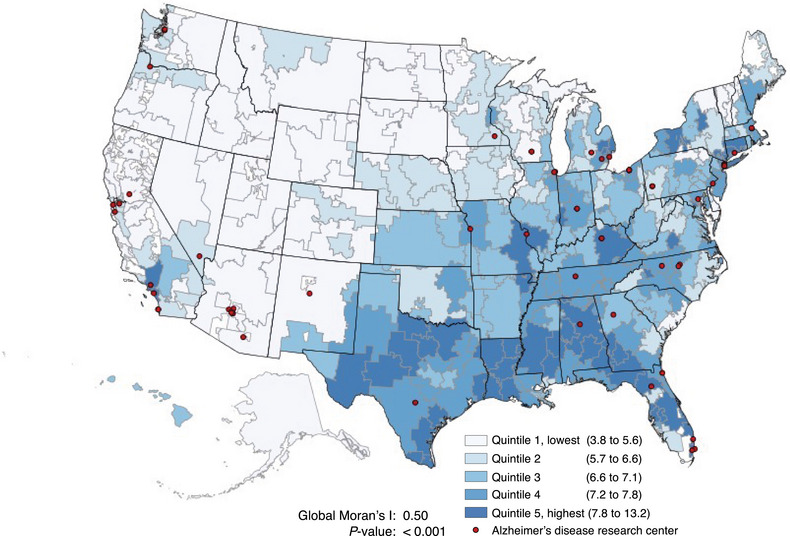
Geographic distribution of total older adults (≥66) ADRD‐diagnosed population (no. per 100) in FFS Medicare by U.S. hospital referral regions, 2019. ADRD, Alzheimer's disease and other related dementias; FFS, Fee‐For‐Service.
3.2. Unadjusted regional variation in newly diagnosed ADRD cases
Of older adults nationally, 3.0% (143,029 of 4,842,034) were identified as receiving a new ADRD diagnosis in 2019. Older adults who received a new ADRD diagnosis were older than those who did not receive a diagnosis (mean age 82.7 [SD = 8.0] vs 74.9 [SD = 7.2]) and were more likely to be female (61.1% vs 55.3%, respectively) with no other meaningful differences (Table SA.1). The unadjusted HRR ADRD new diagnosis rate ranged from 1.7 to 5.4 per 100 and varied by age category (e.g., the mean HRR diagnosis rate was 8.8 per 100 among those ≥85 vs 1.0 per 100 among those 66–74) (Figure 2). In unadjusted analyses, the variability in new ADRD diagnosis rate across U.S. HRRs was largest among the youngest age category (66–74), Black, and Hispanic groups (Figure 2).
FIGURE 2.
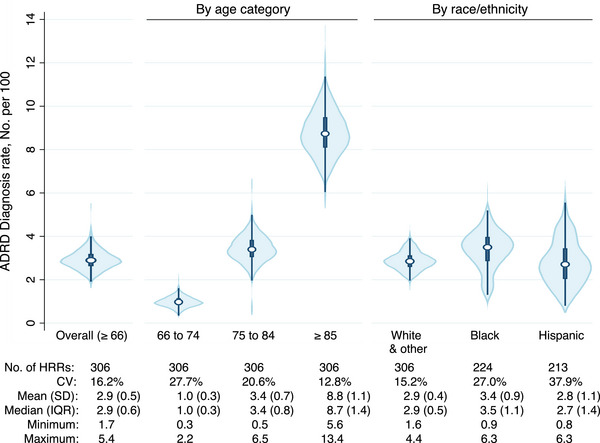
Variation in ADRD new diagnosis rate across U.S. hospital referral regions among older adults (≥66) in FFS Medicare by age category and race/ethnicity. Note: Fewer than 306 HRRs among subgroups due to exclusion of HRRs with fewer than 100 FFS beneficiaries. ADRD, Alzheimer's disease and other related dementias; CV, coefficient of variation; FFS, Fee‐For‐Service; HRR, hospital referral region; IQR, interquartile range; SD, standard deviation.
3.3. ADRD‐specific diagnosis intensity
Using the model presented in Table SA.2 to estimate expected ADRD cases, the ADRD‐specific diagnosis intensity was measured by calculating the observed to expected ratio in ADRD new diagnosis rate for each HRR. Among the regional characteristics used to estimate the expected new ADRD diagnosis rate, population age and sex explained the largest amount of variation (21%) (Table 1). Race/ethnicity, level of education, health status, and regional diagnosis intensity increased the percentage of variation of new ADRD diagnosis explained to 33%. The remaining variation is unexplained and can represent unmeasured factors including practice variation as well as statistical noise.
TABLE 1.
Percent of hospital referral regional variation (Estimated by R‐squared) in new ADRD diagnosis explained with progressive adjustment for population characteristics.
| Population characteristic | R‐squared a | % Explained by characteristic(s) |
|---|---|---|
| Age and sex | 0.21 | 21.0 |
| + Race/ethnicity | 0.27 | 5.7 |
| + Level of education | 0.31 | 4.3 |
| + Health status (smoking, obesity, diabetes) | 0.33 | 1.9 |
| + General diagnosis intensity b | 0.33 | 0.4 |
Note: Independent variables in all statistical models included age (% ≥80 years), sex (% female), race (% Black race), Hispanic ethnicity (% Hispanic), level of education (% < high school), smoking status (% smokers), obesity status (% obese), and diabetes status (% diabetes).
Abbreviation: ADRD, Alzheimer's disease and related dementias.
Estimated based on McFadden's pseudo‐R2 (penalized for no. of independent variables).
General diagnosis intensity measure obtained from: Finkelstein A, Gentzkow M, Hull P, Williams H. Adjusting Risk Adjustment—Accounting for Variation in Diagnostic Intensity. N Engl J Med. 2017;376(7):608–610.
Although the concentration of ADRD cases was highest in the South (Figure 1), ADRD diagnosis intensity across the United States exhibited a different spatial pattern from the ADRD diagnosed case regional pattern, with less global clustering: Global Moran's I = 0.27, p‐value < 0.001 for ADRD diagnosis intensity (Figure 3) versus Global Moran's I = 0.50, p‐value < 0.001 for the prevalence of diagnosed ADRD (Figure 1). Across HRRs, ADRD‐specific diagnosis intensity varied from a low of 0.69 (in Minot North, Dakota) to a high of 1.47 (in Wichita Falls, Texas). By age category and race/ethnicity, the largest variability in ADRD‐diagnosis intensity was observed among the youngest age category (66–74), Black, and Hispanic groups; CV = 20.2%, 26.2%, and 37.0%, respectively (Figure 4).
FIGURE 3.
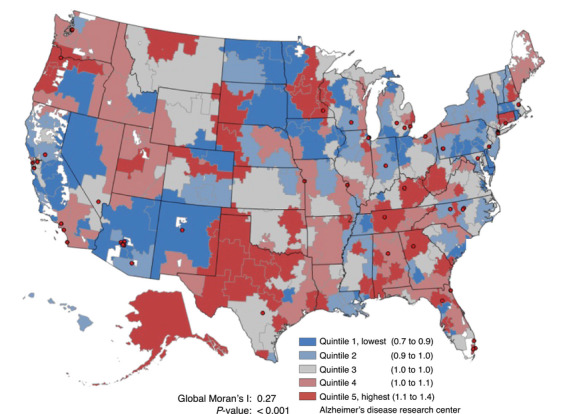
Geographic distribution of ADRD diagnosis intensity* by U.S. hospital referral regions among older adults (≥66) in FFS Medicare, 2019. ADRD, Alzheimer's disease and other related dementias; FFS, Fee‐For‐Service. * Defined as the ratio of observed to expected new ADRD cases; all analyses adjusted for population size, population age (percent ≥80 years old), sex composition, race and ethnicity, level of education (percent < high school education), population health (percent obese, diabetes, and smokers), and general diagnosis intensity.
FIGURE 4.
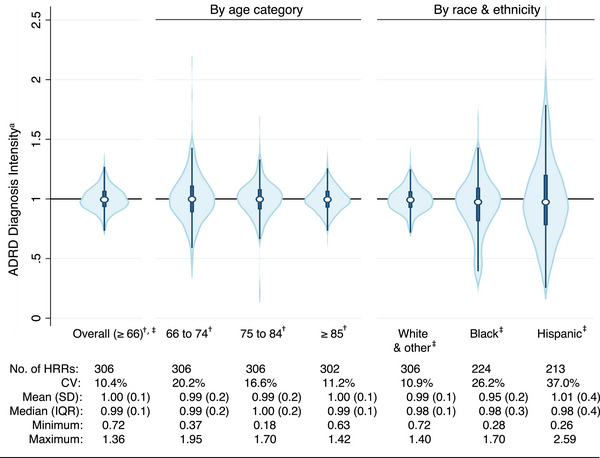
Variation in ADRD diagnosis intensity across U.S. hospital referral regions among older adults (≥66) in FFS Medicare by age category and race/ethnicity. Note: Fewer than 306 HRRs among subgroups due to exclusion of those with fewer than 100 FFS beneficiaries. ADRD, Alzheimer's disease and other related dementias; CV, coefficient of variation; FFS, Fee‐For‐Service; HRR, hospital referral region; IQR, interquartile range; SD, standard deviation. * Defined as the ratio of observed to expected ratio of new ADRD cases, all analyses adjusted for population size, sex composition, level of education (percent < high school education), population heath (percent obese, diabetes, and smokers), and general diagnosis intensity. † Further adjusted for race and ethnicity. ‡ Further adjusted for population age (percent ≥80 years old).
3.4. Individual probability of a new ADRD diagnosis
Although the regional measures inform population diagnosis rates, we tested the implication for an individual receiving an ADRD diagnosis within a year related to living in an area with higher or lower ADRD diagnosis intensity. Subgroups with the overall highest probability of ADRD diagnosis included those ≥85 years of age and Black older adults. Among all groups, the probability of receiving an ADRD diagnosis approximately doubled across regions from low to high ADRD diagnosis intensity (Figure 5). For instance, the probability of receiving an ADRD diagnosis increased from 8.2 (95% confidence interval [CI]: 8.0, 8.3) to 15.3 (95% CI: 15.0, 15.6) per 100 among those ≥85 years of age.
FIGURE 5.
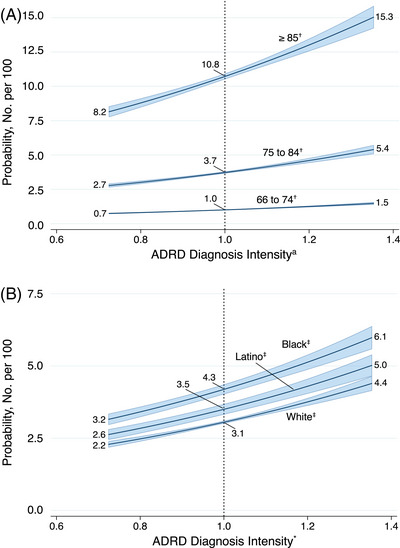
Probability of new ADRD diagnosis (No. per 100) among older adults (≥66) in FFS Medicare according to ADRD diagnosis intensity* by age category (A) and race/ethnicity (B). ADRD, Alzheimer's disease and other related dementias. * Defined as the ratio of observed to expected new ADRD cases; all analyses adjusted for sex and a random intercept included for hospital referral region. † Further adjusted for race and ethnicity. ‡ Further adjusted for population age (continuous).
4. DISCUSSION
In 2019, 8.7% of older adults in the United States in FFS Medicare had a diagnosis of ADRD, with 3% having been newly diagnosed with ADRD. The number of new diagnoses per population varies across regions related to the population age and race/ethnicity distributions as well as its underlying dementia risk factors including the prevalence of obesity, diabetes, and smoking and differences in how aggressively diagnoses across regions are sought out. The ADRD new diagnosis rate demonstrates substantial variation across areas, ranging from 1.7 to 5.4 per 100 among older adults, with the aforementioned factors accounting for 33% of that variation. The ADRD‐specific diagnosis intensity measure (i.e., the observed to expected ratio of newly diagnosed cases) exposes potentially unwarranted variation that is not explained by observable factors, which may include differences in practice norms or patient care seeking behavior that reduce an individual's opportunity to be diagnosed. 29 , 30 , 31 That variation is greatest for groups in whom prior research suggests greater challenges of underdiagnosis exist, specifically younger, Black, or Hispanic individuals. The implication of these results for individuals is that care in some health systems or areas may be more inclined toward recognition and diagnosis of ADRD. And the differences across place are greatest for younger, Black, or Hispanic older adults.
Prior research using Census geographic areas (e.g., states and counties) has demonstrated that the prevalence of dementia across the United States exhibits geographic variation that mirrors the “stroke belt,” where the population has higher cardiovascular risk factors and a greater proportion of Black residents. 12 , 32 Specifically, studies using objectively measured dementia, 32 diagnosed dementia in Medicare claims, 12 , 33 and estimated dementia prevalence 34 have shown in general that the Southeast through the Midwest industrial belt has higher dementia prevalence and/or incidence. No prior study, however, has used health care–specific regions to measure the likelihood of obtaining an ADRD diagnosis after adjusting for underlying sociodemographic and population dementia risk factors simultaneously, in addition to accounting for general diagnostic intensity for disease. Our study demonstrates that taking all these factors into account, there remains substantial geographic difference in the likelihood of being diagnosed with ADRD, and that the variation does not follow the “stroke belt,” thereby strengthening the conclusion that the ADRD diagnosis intensity measure is not merely capturing underlying differences in population risk. In addition, spatial auto‐correlation, a measure of relatedness across neighboring areas, is relatively low for ADRD diagnosis intensity, suggesting that factors related to diagnosis are localized to each area. Residing in areas with the highest ADRD diagnosis intensity is associated with a 2‐fold higher likelihood of obtaining an ADRD diagnosis. Our “expected” diagnosis rates are referenced to the national average rather than an epidemiologically determined incidence rate. As such, it would be inaccurate to label the rates as over‐ or underdiagnosis at this stage of investigation. However, in the context of the extensive literature supporting pervasive underdiagnosis, 3 places with substantially lower diagnosis rates may represent areas with the greatest barriers toward diagnosis. Overdiagnosis could be at play, particularly if there is a high degree of erroneous case identification and may also become more of an issue as commercially available biomarkers come into clinical practice.
Addressing barriers to diagnosis would necessitate further understanding of the causes of variation beyond that caused by population disease risk, especially for the subgroups in whom variation is greatest (younger, Black, and Hispanic older adults). Among the only prior studies that sought to compare observed to expected dementia cases, Mattke and colleagues used the Health and Retirement Study (HRS) to measure dementia detection rate (ratio of diagnosed cases in claims to estimated cases based on HRS assessments) and found that detection increases across age categories from 0.83 (65–69) to 1.22 (≥85) and differed by race/ethnicity (e.g., 1.37 among non‐Hispanic White older adults vs 0.70 among non‐Hispanic Black older adults). 35 These results are consistent with our findings of less diagnosis among younger and minoritized groups, although are limited to presenting only a national measure. Our study adds that there is substantial variation across smaller areas, even beyond that related to demographic factors.
Prior studies have implicated professional uncertainty, physician behaviors and beliefs, organizational design and capacity, patient demand, and quality or evidence adherence. 29 , 30 , 31 , 36 Our study design does not permit conclusions on the cause(s) but demonstrates the need for further inquiry. It is important to note that one cannot assume that the reasons for low or high diagnostic intensity across population subgroups are the same. For instance, differences in ADRD diagnosis among those age 66–74 may be driven by differences in practice or diagnosis‐seeking behavior, whereas, among minoritized racial and ethnic groups, they may be due to regional barriers to access. There is a rich literature on race and ADRD diagnosis, 37 , 38 , 39 , 40 , 41 , 42 , 43 the majority of which demonstrate delays in the time from dementia onset to identification; however, due largely to elevated risk factors, ADRD is also more common among Black older adults. To the degree that localized health care delivery features—such as access to diagnostic expertise, organization, quality, or physician beliefs—may be contributing to lower‐than‐expected new diagnosis rates, avenues exist for remediating underdiagnosis particularly for minoritized racial/ethnic groups.
4.1. Limitations
Several limitations must be acknowledged. First, although our analyses demonstrate regional variation in new ADRD diagnosis among Medicare FFS beneficiaries, these results may not be generalizable to other groups, such as those enrolled in Medicare Advantage programs. However, one previous study found comparable differences in observed to expected dementia cases in FFS and Medicare Advantage. 35 Second, our study is an observational design, so we cannot completely rule out the potential of residual confounding affecting our results despite adjustment for a variety of regional characteristics. And regionally available measures of population risks, especially education, are coarser than measures used in well‐characterized epidemiological studies; so our modeled population disease risk may be imprecise, but that imprecision of measurement should be similar across regions. In addition, administrative and large survey data have inherent limitations, and we were unable to account for all potentially important clinical factors in our analyses, although we note the health risk factors accounted for only a small portion of the variation across regions. As an example, we did not include hypertension, which was available only as self‐report. Third, we were limited in examining diagnosis intensity for only a subset of minoritized groups due to data limitations. Finally, this study was not designed to determine whether the regional differences in the likelihood of ADRD diagnosis we observed led to differences in population health outcomes.
5. CONCLUSION
We conducted an observational study to examine variation in newly identified cases of ADRD diagnosis across the United States using national data, and we found that the likelihood of receiving an ADRD diagnosis varies across the United States—particularly among those 66–74 years of age and in Black or Hispanic groups. Despite the limitations of our observational design, these findings have important implications for future strategies aimed at improving case‐finding among these groups. Furthermore, these findings raise important questions regarding the degree to which differences in access and health care practices may drive excess variability in ADRD detection.
CONFLICT OF INTEREST STATEMENT
We declare there are no conflicts of interest. Author disclosures are available in the supporting information.
CONSENT STATEMENT
The institutional review board at Dartmouth College determined that this study, which uses research data files from the Centers for Medicare & Medicaid Services, is exempt from requiring individual consent.
Supporting information
Supporting information
Supporting information
ACKNOWLEDGMENTS
This study was funded by the National Institute on Aging (NIA; grant P01 AG019783). The NIA had no role in the design of the study; the collection, analysis, and interpretation of the data; or the decision to approve publication of the finished manuscript.
Bynum JPW, Benloucif S, Martindale J, O'Malley AJ, Davis MA. Regional variation in diagnostic intensity of dementia among older U.S. adults: An observational study. Alzheimer's Dement. 2024;20:6755–6764. 10.1002/alz.14092
REFERENCES
- 1. Rajan KB, Weuve J, Barnes LL, McAninch EA, Wilson RS, Evans DA. Population estimate of people with clinical Alzheimer's disease and mild cognitive impairment in the United States (2020‐2060). Alzheimers Dement. 2021;17(12):1966‐1975. doi: 10.1002/alz.12362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Office of the Assistant Secretary for Planning and Evaluation. U.S. Department of Health and Human Services National Plan to Address Alzheimer's disease. (https://aspe.hhs.gov/report/national‐plan‐address‐alzheimers‐disease‐2020‐update)
- 3. Lang L, Clifford A, Wei L, et al. Prevalence and determinants of undetected dementia in the community: a systematic literature review and a meta‐analysis. BMJ Open. 2017;7(2):e011146. doi: 10.1136/bmjopen-2016-011146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Thoits T, Dutkiewicz A, Raguckas S, et al. Association between dementia severity and recommended lifestyle changes: a retrospective cohort study. Am J Alzheimers Dis Other Demen. 2018;33(4):242‐246. doi: 10.1177/1533317518758785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boustani M, Peterson B, Hanson L, Harris R, Lohr KN, Force USPST. Screening for dementia in primary care: a summary of the evidence for the United States Preventive Services Task Force. Ann Intern Med. 2003;138(11):927‐937. doi: 10.7326/0003-4819-138-11-200306030-00015 [DOI] [PubMed] [Google Scholar]
- 6. Boustani M, Schubert C, Sennour Y. The challenge of supporting care for dementia in primary care. Clin Interv Aging. 2007;2(4):631‐636. doi: 10.2147/cia.s1802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Holston EC. Stigmatization in Alzheimer's disease research on African American elders. Issues Ment Health Nurs. 2005;26(10):1103‐1127. (In eng). doi: 10.1080/01612840500280760 [DOI] [PubMed] [Google Scholar]
- 8. Justiss MD, Boustani M, Fox C, et al. Patients' attitudes of dementia screening across the Atlantic. Int J Geriatr Psychiatry. 2009;24(6):632‐637. doi: 10.1002/gps.2173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dubois B, Padovani A, Scheltens P, Rossi A, Dell'Agnello G. Timely diagnosis for Alzheimer's disease: a literature review on benefits and challenges. J Alzheimers Dis. 2015;49(3):617‐631. doi: 10.3233/jad-150692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. White L, Fishman P, Basu A, Crane PK, Larson EB, Coe NB. Medicare expenditures attributable to dementia. BMC Health Serv Res. 2019;54(4):773‐781. doi: 10.1111/1475-6773.13134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Getsios D, Blume S, Ishak KJ, Maclaine G, Hernández L. An economic evaluation of early assessment for Alzheimer's disease in the United Kingdom. Alzheimers Dement. 2012;8(1):22‐30. doi: 10.1016/j.jalz.2010.07.001 [DOI] [PubMed] [Google Scholar]
- 12. Koller D, Bynum JP. Dementia in the USA: state variation in prevalence. J Public Health (Oxf). 2015;37(4):597‐604. doi: 10.1093/pubmed/fdu080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Davis MA, Chang CH, Simonton S, Bynum JPW. Trends in US medicare decedents' diagnosis of dementia from 2004 to 2017. JAMA Health Forum. 2022;3(4):e220346. doi: 10.1001/jamahealthforum.2022.0346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Barnes LL, Bennett DA. Alzheimer's Disease in African Americans: risk factors and challenges for the future. Health Aff. 2014;33(4):580‐586. doi: 10.1377/hlthaff.2013.1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hinton L, Tran D, Peak K, Meyer OL, Quiñones AR. Mapping racial and ethnic healthcare disparities for persons living with dementia: A scoping review. (1552‐5279 (Electronic)) (In eng). [DOI] [PMC free article] [PubMed]
- 16. Grodstein F, Chang CH, Capuano AW, et al. Identification of dementia in recent medicare claims data, compared to rigorous clinical assessments. J Gerontol A Biol Sci Med Sci. 2021;77(6):1272‐1278. doi: 10.1093/gerona/glab377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McCarthy EP, Chang CH, Tilton N, Kabeto MU, Langa KM, Bynum JPW. Validation of claims algorithms to identify Alzheimer's disease and related dementias. J Gerontol A Biol Sci Med Sci. 2021;77(6):1261‐1271. doi: 10.1093/gerona/glab373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. The Dartmouth Atlas of Health Care. ( https://www.dartmouthatlas.org/)
- 19. Jarrin OF, Nyandege AN, Grafova IB, Dong X, Lin H. Validity of race and ethnicity codes in Medicare administrative data compared with gold‐standard self‐reported race collected during routine home health care visits. Med Care. 2020;58(1):e1‐e8. doi: 10.1097/MLR.0000000000001216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet North Am Ed. 2020;396(10248):413‐446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. U.S. Census Bureau. American Community Survey . ( https://www.census.gov/programs‐surveys/acs)
- 22. Centers for Disease Control and Prevention. Behavioral Risk Factor Surveillance System . ( https://www.cdc.gov/brfss/)
- 23. Centers for Disease Control and Prevention. U.S. Diabetes Surveillance System . ( https://gis.cdc.gov/grasp/diabetes/diabetesatlas.html)
- 24. Nanda APS, Skinner J, Fisher E. Dartmouth Atlas Project: Mapping COVID‐19. (https://www.dartmouthatlas.org/covid‐19/hrr‐mapping/#methods)
- 25. National Institute on Aging . Alzheimer's Disease Research Centers. (https://www.nia.nih.gov/health/alzheimers‐disease‐research‐centers)
- 26. Song Y, Skinner J, Bynum J, Sutherland J, Wennberg JE, Fisher ES. Regional variations in diagnostic practices. N Engl J Med. 2010;363(1):45‐53. doi: 10.1056/NEJMsa0910881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Finkelstein A, Gentzkow M, Hull P, Williams H. Adjusting risk adjustment—Accounting for variation in diagnostic intensity. N Engl J Med. 2017;376(7):608‐610. doi: 10.1056/NEJMp1613238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Anselin L. Local indicators of spacial association—LISA. Geographical Analysis. 1995;27(2):93‐115. [Google Scholar]
- 29. Cutler D, Skinner JS, Stern AD, Wennberg D. Physician beliefs and patient preferences: a new look at regional variation in health care spending. Am Econ J Econ Policy. 2019;11(1):192‐221. doi: 10.1257/pol.20150421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sutherland K, Levesque JF. Unwarranted clinical variation in health care: definitions and proposal of an analytic framework. J Eval Clin Pract. 2020;26(3):687‐696. doi: 10.1111/jep.13181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wennberg J, Gittelsohn A. Small area variations in health care delivery. Science. 1973;182(4117):1102‐1108. doi: 10.1126/science.182.4117.1102 [DOI] [PubMed] [Google Scholar]
- 32. Ailshire JA, Walsemann KM, Fisk CE. Regional variation in U.S dementia trends from 2000‐2012. SSM Popul Health. 2022;19:101164. doi: 10.1016/j.ssmph.2022.101164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kirson NY, Meadows ES, Desai U, et al. Temporal and geographic variation in the incidence of Alzheimer's disease diagnosis in the US between 2007 and 2014. J Am Geriatr Soc. 2020;68(2):346‐353. doi: 10.1111/jgs.16262 [DOI] [PubMed] [Google Scholar]
- 34. Dhana K, Beck T, Desai P, Wilson RS, Evans DA, Rajan KB. Prevalence of Alzheimer's disease dementia in the 50 US states and 3142 counties: a population estimate using the 2020 bridged‐race postcensal from the National Center for Health Statistics. Alzheimers Dement. 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mattke S, Jun H, Chen E, Liu Y, Becker A, Wallick C. Expected and diagnosed rates of mild cognitive impairment and dementia in the US Medicare population: observational analysis. Alzheimers Res Ther. 2023;15(1):128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Atsma F, Elwyn G, Westert G. Understanding unwarranted variation in clinical practice: a focus on network effects, reflective medicine and learning health systems. Int J Qual Health Care. 2020;32(4):271‐274. doi: 10.1093/intqhc/mzaa023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Davis MA, Lee KA, Harris M, et al. Time to dementia diagnosis by race: a retrospective cohort study. J Am Geriatr Soc. 2022;70(11):3250‐3259. doi: 10.1111/jgs.18078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chen Y, Tysinger B, Crimmins E, Zissimopoulos JM. Analysis of dementia in the US population using medicare claims: insights from linked survey and administrative claims data. Alzheimers Dement (N Y). 2019;5:197‐207. doi: 10.1016/j.trci.2019.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhu Y, Chen Y, Crimmins E, Zissimopoulos JM. Sex, race, and age differences in prevalence of dementia in Medicare claims and survey data. J Gerontol Ser B. 2021;76(3):596‐606. doi: 10.1093/geronb/gbaa083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lin PJ, Daly A, Olchanski N, et al. Dementia diagnosis disparities by race and ethnicity. Alzheimers Dement. 2020;16(S10). doi: 10.1002/alz.043183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lin PJ, Emerson J, Faul JD, et al. Racial and ethnic differences in knowledge about one's dementia status. J Am Geriatr Soc. 2020;68(8):1763‐1770. doi: 10.1111/jgs.16442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Amjad H, Roth DL, Sheehan OC, Lyketsos CG, Wolff JL, Samus QM. Underdiagnosis of dementia: an observational study of patterns in diagnosis and awareness in US Older Adults. J Gen Intern MEd. 2018;33(7):1131‐1138. doi: 10.1007/s11606-018-4377-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tsoy E, Kiekhofer RE, Guterman EL, et al. Assessment of racial/ethnic disparities in timeliness and comprehensiveness of dementia diagnosis in California. JAMA Neurol. 2021;78(6):657‐665. doi: 10.1001/jamaneurol.2021.0399 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information
Supporting information


