Abstract
Introduction
Accurate diagnosis of multiple myeloma (MM) and related disorders depends on imaging studies for lesion detection, which is crucial for treatment planning. The 18F-fluorodeoxyglucose (FDG) PET imaging system is well-established, with high sensitivity and specificity in identifying myeloma lesions. Additionally, 11C-acetate serves as an effective radiotracer for detecting MM lesions. Metabolic tumor volume (MTV) measured with 18F-FDG PET has been suggested as a prognostic factor in MM patients. This study aimed to compare the feasibility of measuring MTV in patients with myeloma-related diseases using 11C-acetate or 18F-FDG PET/CT.
Methods
We retrospectively reviewed six patients with MM - three with symptomatic MM and three with smoldering MM - and one patient with a myeloma-related disorder, all of whom underwent both 11C-acetate and 18F-FDG PET/CT scans. Using a dedicated workstation (PET-STAT; AdIn Research, Inc., Tokyo, Japan) equipped with a standardized uptake value (SUV)-based automated contouring program, we calculated the SUV for MTV. Bone areas with an SUV above thresholds of 2.0 or 2.5 were grouped accordingly.
Results
MTV detection in the whole body was significantly higher with 11C-acetate compared to 18F-FDG at both thresholds. For the 2.5 threshold, MTV was 129 ± 109 mL versus 3.93 ± 9.38 mL (p = 0.016), and for the 2.0 threshold, it was 316 ± 221 mL versus 12.1 ± 23.7 mL (p = 0.016).
Conclusions
MTV measurements were significantly higher in PET/CT using 11C-acetate compared to 18F-FDG. This finding highlights the potential superiority of 11C-acetate over 18F-FDG for assessing the MTV of lesions in patients with MM.
Keywords: 11c-acetate, 18f-fluorodeoxyglucose, metabolic tumor volume, multiple myeloma, positron emission tomography
Introduction
Multiple myeloma (MM) is the second most common hematologic malignancy, characterized by the infiltration of abnormal plasma cells in the bone marrow and the production of monoclonal immunoglobulins (M-proteins) [1,2]. Diagnosis of MM and related disorders is typically guided by the International Myeloma Working Group (IMWG) criteria, with MM always being preceded by monoclonal gammopathy of undetermined significance or smoldering MM [3,4]. According to the IMWG criteria, patients with MM exhibit one or more calcium elevation, renal failure, anemia, bone lesions (CRAB) symptoms or meet specific biomarker thresholds (≥60% clonal bone marrow plasma cells, serum free light chain ratio ≥100, or >1 focal lesion on MRI) [3]. Imaging plays a critical role in determining treatment strategies, as active MM is the primary treatment target.
Related plasma cell disorders, such as POEMS syndrome, share overlapping clinical features with MM but differ in disease characteristics. POEMS syndrome, a rare plasma cell disorder, is marked by polyneuropathy, organomegaly, endocrinopathy, monoclonal plasma cell disorder, and skin changes. While POEMS syndrome presents with CRAB-like symptoms, it typically has lower levels of bone marrow plasmacytosis than MM. Imaging studies are equally important in managing POEMS syndrome, especially for detecting plasmacytomas that may require radiation and systemic chemotherapy [5].
The conventional whole-body skeletal survey, considered the gold standard for screening bone lesions, has relatively poor sensitivity. Advanced imaging modalities, including CT, MRI, bone scintigraphy, and 18F-fluorodeoxyglucose (FDG) PET, have been introduced to improve lesion detection [6]. Among these, 18F-FDG PET is widely used for identifying osteolytic and other myelomatous lesions, with a sensitivity of approximately 80-90% and a specificity of 80-100% [7-10]. This technique is also valuable for differentiating active from precursor lesions and evaluating treatment response in MM patients [11-13]. However, about 30% of lesions detected by MRI are not visible on 18F-FDG PET/CT, and MRI is superior in identifying early-stage diffuse disease [14,15].
To address the limitations of 18F-FDG PET/CT, several other radiopharmaceutical tracers have been tested for MM diagnosis, including 18F-fluorodeoxy-L-thymidine, 18F-alpha-methyltyrosine, 11C-acetate, and others [1,16-18]. Of these, 11C-acetate is particularly promising due to its role in lipid and cholesterol synthesis, with increased uptake reflecting the heightened demand for membrane lipids in malignancies [19]. This tracer has been found to be upregulated in various cancers, including hepatocellular carcinoma, prostate cancer, and indolent lymphoma [20-22], and has demonstrated increased uptake in MM tumor cells [23].
Comparative studies have evaluated 11C-acetate PET/CT and 18F-FDG for detecting MM lesions. One study involving 35 patients found that 11C-acetate had higher sensitivity and specificity than 18F-FDG for symptomatic MM diagnosis [24]. Another study with 15 patients revealed a higher detection rate of diffuse plasma cell infiltrates using 11C-acetate. In 50 control subjects, the average maximum standardized uptake values (SUVmax) for the bilateral posterior iliac crests were 2.3 ± 0.7 for 11C-acetate and 2.0 ± 0.6 for 18F-FDG, suggesting a diagnostic advantage for 11C-acetate [25].
Metabolic tumor volume (MTV), a parameter for assessing tumor burden through PET imaging, is a useful prognostic tool for various malignancies. While MTV measured by 18F-FDG PET is proposed as a prognostic factor for MM, the feasibility of measuring MTV using 11C-acetate remains unexplored [26,27]. This study aimed to investigate the potential of 11C-acetate PET to measure MTV in patients with MM and POEMS syndrome compared to 18F-FDG PET.
Materials and methods
Patients
The present study received approval from the Ethics Committee at Tokyo Medical and Dental University. All patients provided written informed consent prior to enrollment. Between October 2016 and February 2018, six patients who met the diagnostic criteria for MM [28] and one patient with POEMS syndrome were enrolled, all of whom underwent both 11C-acetate PET/CT and 18F-FDG PET/CT scans. Patients under 20 years of age, those who were pregnant or breastfeeding, and individuals with blood sugar levels exceeding 200 mg/dL at the time of the scan were excluded. Additionally, patients with malignancies unrelated to myeloma were also excluded from the study.
Data acquisition
The synthesis of 11C-acetate was achieved through the carbonation of the Grignard reagent. Specifically, 11C-carbon dioxide was reacted with 0.3 M methyl magnesium bromide, hydrolyzed using 1 M hydrochloric acid, and subsequently converted into 11C-acetic acid. This synthesis procedure adhered to the method established by Kruijer et al. [29,30].
Patients fasted for a minimum of six hours prior to the injection of 11C-acetate, and serum blood glucose levels were confirmed to be below 200 mg/dL. An intravenous administration of 3.7 MBq/kg of 11C-acetate was performed 20 minutes before the imaging scan. Imaging was conducted using an integrated PET/CT scanner (Celesteion®, Canon Medical Systems Corporation, Tokyo, Japan). CT data were acquired at 120 kVp and 40 mA, with a 550 mm field of view, a pitch factor of 16.0, a slice thickness of 2.0 mm, and a rotation time of 1.0 s. PET data were collected for 240 s per bed position with a 3 mm Gaussian filter and were reconstructed using time-of-flight, three-dimensional ordered subset expectation maximization (TOF 3D OSEM), covering the area from the top of the skull to the pelvis.
Image analysis
We utilized specialized software (PETSTAT; AdIn Research, Inc., Tokyo, Japan) to calculate SUV and MTV. Bone areas with SUVs exceeding thresholds of 2.0 or 2.5 were grouped, and the total volume within these tumor regions was automatically calculated. Calculations were performed separately using both thresholds of 2.0 and 2.5. Focal lesions were defined as areas of increased tracer uptake, with an SUVmax >2.0 or >2.5, correlating with CT abnormalities not attributable to other bone pathologies. Lesions were categorized into 12 anatomical regions: cervical, thoracic, and lumbar vertebrae; cranial; humerus; forearm bones; scapula; clavicle; ribs; pelvis; and femur. When a lesion extended into two or more bone regions, or when it could be automatically segmented from extra-skeletal uptake, the demarcation line was manually established by placing regions of interest. Measurements were conducted by two trained radiologists, who reached a consensus through discussion when necessary.
Statistical analysis
The Wilcoxon signed-rank test was employed to compare the MTVs measured using 11C-acetate and 18F-FDG PET. A p-value of less than 0.05 (two-tailed) was considered statistically significant. All statistical analyses were conducted using R software (version 3.5.1) and EZR (version 3.5.2) [31].
Results
Seven consecutive patients were included in this study, consisting of two men and five women, aged between 40 and 69 years. The cohort included three patients with symptomatic MM - one classified as stage II and two as stage I, according to the revised International Staging System [28] - three patients with smoldering MM (Figure 1), and one patient with POEMS syndrome (Figure 2). A summary of the cases and their respective MTVs is presented in Table 1.
Table 1. List of cases and MTVs measured with 11C-acetate and 18F-FDG.
FDG, fluorodeoxyglucose; MM, multiple myeloma; MTV, metabolic tumor volume; R-ISS, revised international staging system; sMM, smoldering multiple myeloma; SUV, standardized uptake value
| Patient | Age (years) | Diagnosis | R-ISS stage | Threshold of SUV = 2.5 | Threshold of SUV = 2.0 | ||
| 11C-acetate (mL) | 18F-FDG (mL) | 11C-acetate (mL) | 18F-FDG (mL) | ||||
| 1 | 68 | MM | II | 345.44 | 26.9 | 728.44 | 69.83 |
| 2 | 62 | sMM | NA | 174.77 | 0 | 468.24 | 5.45 |
| 3 | 40 | sMM | NA | 2.27 | 0 | 64.97 | 0 |
| 4 | 59 | sMM | NA | 142.46 | 0 | 311.16 | 0 |
| 5 | 69 | MM | I | 43.55 | 0 | 92.39 | 6.02 |
| 6 | 63 | MM | I | 28.55 | 0 | 145.05 | 0 |
| 7 | 69 | POEMS | NA | 166.59 | 0 | 400.25 | 2.03 |
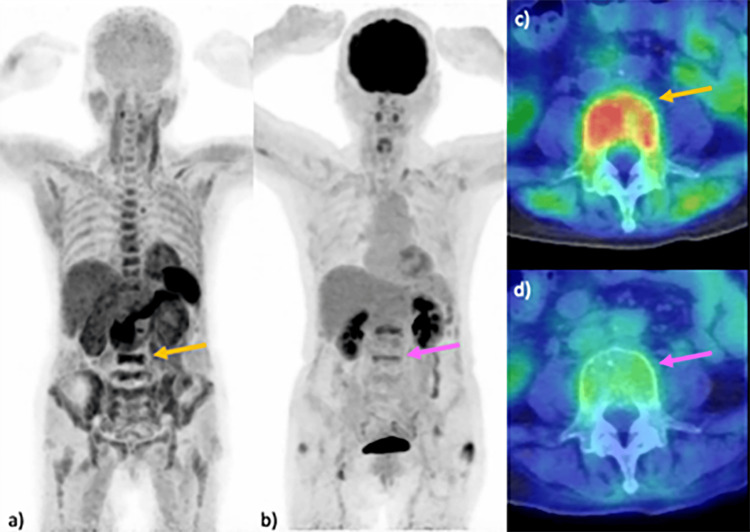
Figure 1. Comparison of PET imaging in MM: 11C-acetate versus 18F-FDG.
Imaging results from a 68-year-old woman with MM are presented. MIP images reveal multiple lesions in the vertebrae, including the L5 vertebral body (arrow) and pelvic bones, identified using 11C-acetate PET (a). Lesions in the lumbar vertebrae, including the L5 vertebral body (arrow), are detected by 18F-FDG PET (b). An axial fused PET/CT image utilizing 11C-acetate at the L5 vertebra demonstrates increased uptake in the vertebral body (SUVmax 4.0) (arrow) (c), in contrast to the relatively weak uptake observed in the 18F-FDG PET/CT image (SUVmax 2.0) (arrow) (d).
FDG, fluorodeoxyglucose; MIP, maximum intensity projection; MM, multiple myeloma; SUV, standardized uptake value
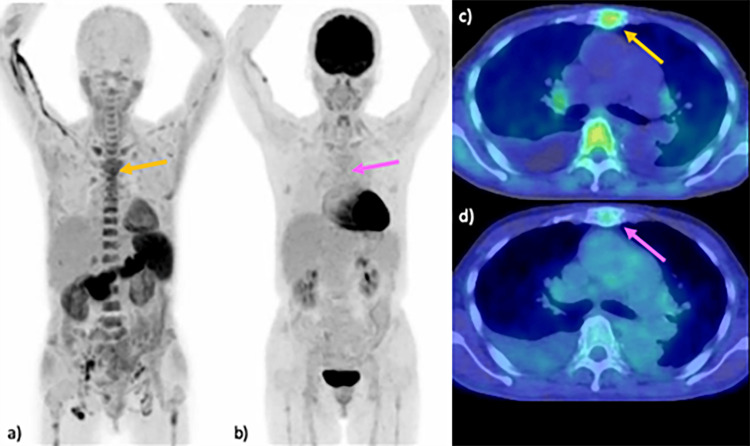
Figure 2. Comparison of PET imaging in POEMS syndrome: 11C-acetate versus 18F-FDG.
Imaging results of a 69-year-old woman with POEMS syndrome are presented. MIP images from the 11C-acetate PET scan revealed multiple lesions in the vertebrae and sternum (arrow) (a). Lesions in the thoracic vertebrae and sternum are detected using 18F-FDG PET (arrow) (b). An axial fused PET/CT image at the level of the thoracic vertebra demonstrates increased uptake of 11C-acetate in the sternal lesion (SUVmax 6.4) (arrow) (c), in contrast to minimal uptake observed with 18F-FDG (SUVmax 2.0) (arrow) (d).
FDG, fluorodeoxyglucose; MIP, maximum intensity projection; SUV, standardized uptake value
The whole-body MTV measured using 11C-acetate was significantly greater than that measured with 18F-FDG, with values of 129 ± 109 mL compared to 3.93 ± 9.38 mL, respectively (p = 0.016) at the SUV threshold of 2.5 (Table 2).
Table 2. MTVs of skeletal lesions with the SUV threshold of 2.5 measured in PET using 11C-acetate and 18F-FDG.
Data are presented as mean ± SD and range unless otherwise specified.
FDG, fluorodeoxyglucose; MTV, metabolic tumor volume; SUV, standardized uptake value
| Lesion location | 11C-acetate (mL) | 18F-FDG (mL) | p-value |
| Cervical | 0.56 ± 0.68(0-1.91) | 0 ± 0 | 0.1 |
| Thoracic | 36.2 ± 27.4 (1.00-73.1) | 0 ± 0 | 0.016 |
| Lumbar | 37.2 ± 30.4 (0-77.11) | 3.84 ± 9.41 (0-26.9) | 0.036 |
| Cranial | 0.12 ± 0.21 (0-0.57) | 0 ± 0 | 0.371 |
| Humerus | 0.48 ± 1.14 (0-3.26) | 0 ± 0 | 0.371 |
| Forearm | 0.04 ± 0.07 (0-0.20) | 0 ± 0 | 0.371 |
| Scapula | 0.08 ± 0.19 (0-0.53) | 0 ± 0 | 1 |
| Clavicle | 0.68 ± 1.21 (0-3.39) | 0 ± 0 | 0.371 |
| Ribs | 0.56 ± 1.15 (0-3.36) | 0 ± 0 | 0.098 |
| Sternum | 3.44 ± 6.29 (0-18.2) | 0 ± 0 | 0.181 |
| Pelvis | 47.8 ± 66.9 (0-207) | 0.09 ± 0.21 (0-0.60) | 0.036 |
| Femur | 1.88 ± 2.14 (0-5.62) | 0 ± 0 | 0.1 |
| Total | 129 ± 109 (2.27-345) | 3.93 ± 9.38 (0-26.90) | 0.016 |
Lesions were detected in all seven patients using 11C-acetate PET, while only two patients had detectable lesions with 18F-FDG PET. At an SUV threshold of 2.0, the whole-body MTV was also significantly greater with 11C-acetate compared to 18F-FDG, measuring 316 ± 221 mL versus 12.1 ± 23.7 mL, respectively (p = 0.016) (Table 3).
Table 3. MTVs of skeletal lesions with the SUV threshold of 2.0 measured in PET using 11C-acetate and 18F-FDG.
Data are presented as mean ± SD and range unless otherwise specified.
FDG, fluorodeoxyglucose; MTV, metabolic tumor volume; SUV, standardized uptake value
| Lesion location | 11C-acetate (mL) | 18F-FDG (mL) | p-value |
| Cervical | 4.66 ± 4.62 (0.10-13.1) | 0 ± 0 | 0.016 |
| Thoracic | 89.62 ± 51.61 (11.1-160) | 0.42 ± 0.73 (0-2.16) | 0.016 |
| Lumbar | 81.78 ± 48.41 (2.26-137) | 10.6 ± 24.1 (0-69.4) | 0.016 |
| Cranial | 0.74 ± 1.22 (0-3.40) | 0 ± 0 | 0.181 |
| Humerus | 1.37 ± 3.07 (0-8.88) | 0 ± 0 | 0.371 |
| Forearm | 0.12 ± 0.26 (0-0.76) | 0 ± 0 | 0.371 |
| Scapula | 0.4 ± 0.68 (0-1.90) | 0 ± 0 | 0.181 |
| Clavicle | 1.63 ± 2.62 (0-6.85) | 0.14 ± 0.35 (0-1.00) | 0.181 |
| Ribs | 3 ± 3.31 (0-9.35) | 0 ± 0 | 0.1 |
| Sternum | 6.66 ± 10.1 (0-29.8) | 0.07 ± 0.17 (0-0.50) | 0.059 |
| Pelvis | 120 ± 135 (8.97-424) | 0.82 ± 1.99 (0-5.69) | 0.016 |
| Femur | 6.13 ± 7.82(0-23.9) | 0.05 ± 0.12 (0-0.33) | 0.1 |
| Total | 316 ± 221 (65.0-728) | 12.1 ± 23.7 | 0.016 |
Lesions were detected in all seven patients using 11C-acetate PET, while lesions were identified in only five patients with 18F-FDG PET at the SUV threshold of 2.0.
Discussion
In the present study, MTV was significantly greater with 11C-acetate PET compared to 18F-FDG PET at SUV thresholds of 2.0 and 2.5. These findings underscore the superiority of 11C-acetate PET for measuring MTV in patients with myeloma-related diseases. This conclusion is consistent with previous reports highlighting 11C-acetate PET’s advantages over 18F-FDG PET in detecting lesions in myeloma patients [24,25]. In a prior study, a mouse model demonstrated that myeloma cells utilize extracellular acetate for anabolic metabolism, including the synthesis of cell membrane lipids [23]. Furthermore, this study indicated that the accumulation of 11C-acetate in bone corresponds with the concentration of myeloma cells in those areas. Given that prior research has established MTV measured by 18F-FDG PET/CT as a predictor of MM prognosis, the results of this study suggest that MTV measured with 11C-acetate PET/CT could also serve as a potential prognostic factor.
The optimal SUV threshold that accurately reflects tumor volume remains undetermined for both 18F-FDG PET and 11C-acetate PET. Previous studies employed an SUV threshold of ≥40% of the SUVmax in focal lesions with an SUVmax >2.5, demonstrating a significant correlation between MTV and diffuse bone marrow infiltration and disease progression [26]. At an absolute SUV threshold of 2.5, some lesions went undetected that were visible when the threshold was lowered to 2.0. However, automatic lesion demarcation proved challenging at this lower threshold, as lesions in different body areas became inseparable, potentially affecting measurement precision. Several prior studies using 18F-FDG PET/CT adopted a threshold of 2.5 to define myeloma lesions [26,32]. One study found no significant difference in average SUVmax values between 11C-acetate and 18F-FDG in 50 control subjects with normal bone marrow, with values of 2.3 ± 0.7 (range: 1.5 to 3.8) and 2.0 ± 0.6 (range: 1.2-3.2), respectively [25]. Therefore, a threshold of 2.5 or higher is deemed acceptable for detecting myeloma lesions with 11C-acetate PET/CT, leading us to define the optimal SUV threshold as 2.5 for measurements using this modality.
In this study, MTVs measured with 18F-FDG PET/CT were notably smaller compared to those reported in previous studies [26,27], likely because we did not include patients with advanced MM. Previous research has demonstrated the usefulness of 18F-FDG PET/CT in assessing POEMS syndrome [32-35]. In our cases, 11C-acetate PET/CT exhibited greater sensitivity in detecting osseous lesions associated with POEMS syndrome compared to 18F-FDG PET/CT, suggesting the potential value of 11C-acetate in evaluating this condition.
The present study has several limitations. First, the small number of patients included may limit the generalizability of the results. Second, the absence of a definitive lesion-based reference standard, such as biopsies, restricts the reliability of lesion evaluations. Due to the nature of this study conducted within routine clinical practice, performing invasive procedures was not feasible, resulting in no definitive lesion-based reference standard being established. Although we conducted patient-based assessments combining 18F-FDG PET/CT, MRI, and blood tests to evaluate active lesions, the accuracy of these assessments remains a limitation. Future studies that incorporate biopsies, MRI, and 18F-FDG PET/CT for lesion comparison will be essential to establish a more robust reference standard.
Third, while comparing visual and quantitative assessments is crucial, the lack of a definitive reference standard complicates the determination of one method’s superiority over another. SUV and MTV serve as objective measures to quantify visual information. Historically, visual assessments have classified lesions based on uptake patterns, while quantitative measures like SUVmax often attract more attention. We believe that MTV, which integrates uptake intensity and volume, offers a more comprehensive representation of lesion burden and aligns closely with radiologists’ visual assessments. Thus, we employed quantitative measures, including SUV and MTV, in this study to provide an objective representation of visual evaluations.
Lastly, manual demarcation of bone lesions may impair reproducibility. We utilized software to automatically extract areas with SUV above the threshold for MTV measurements, expecting minimal error for most lesions [36]. While there remains a possibility of overestimation in MTV measurements due to physiological uptake, we contend that this is more likely to occur with 18F-FDG than with 11C-acetate. To mitigate demarcation errors, we established consensus-based divisions of lesions among two observers. Further studies involving larger patient populations are needed to clarify the utility of MTV measurements as a prognostic factor for MM.
Conclusions
11C-acetate PET/CT shows promise for measuring MTV in lesions of patients with MM and POEMS syndrome. This finding underscores the potential superiority of 11C-acetate over 18F-FDG in assessing MTV in these patient populations. Given 11C-acetate’s higher sensitivity for detecting bone lesions, it not only serves as a diagnostic tool but also holds promise for monitoring disease progression and treatment response. More accurate measurement of metabolic tumor burden with 11C-acetate may offer new prognostic insights, especially for patients with early or less aggressive disease, where 18F-FDG may be less effective.
Future studies involving larger cohorts are crucial for further validating these findings and establishing 11C-acetate PET/CT as a reliable imaging modality in clinical practice for MM and related plasma cell dyscrasias. Additionally, comparisons with other emerging radiotracers could refine the role of 11C-acetate in the comprehensive management of MM and associated conditions.
Disclosures
Human subjects: Consent was obtained or waived by all participants in this study. Institutional Review Board of Tokyo Medical and Dental University issued approval M2016-088. The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Tokyo Medical and Dental University.
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following:
Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work.
Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work.
Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Author Contributions
Acquisition, analysis, or interpretation of data: Kota Yokoyama, Momo Wakui, Junichi Tsuchiya, Chikara Kase, Ukihide Tateishi, Masahide Yamamoto
Drafting of the manuscript: Kota Yokoyama, Momo Wakui, Junichi Tsuchiya
Critical review of the manuscript for important intellectual content: Kota Yokoyama, Junichi Tsuchiya, Chikara Kase, Ukihide Tateishi, Masahide Yamamoto
Concept and design: Junichi Tsuchiya, Ukihide Tateishi
Supervision: Ukihide Tateishi
References
- 1.¹⁸F-FDG PET/CT: a review of diagnostic and prognostic features in multiple myeloma and related disorders. Dammacco F, Rubini G, Ferrari C, Vacca A, Racanelli V. Clin Exp Med. 2015;15:1–18. doi: 10.1007/s10238-014-0308-3. [DOI] [PubMed] [Google Scholar]
- 2.Multiple myeloma: diagnosis and treatment. Rajkumar SV, Kumar S. Mayo Clin Proc. 2016;91:101–119. doi: 10.1016/j.mayocp.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Rajkumar SV, Dimopoulos MA, Palumbo A, et al. Lancet Oncol. 2014;15:538–548. doi: 10.1016/S1470-2045(14)70442-5. [DOI] [PubMed] [Google Scholar]
- 4.Monoclonal gammopathy of undetermined significance (MGUS) consistently precedes multiple myeloma: a prospective study. Landgren O, Kyle RA, Pfeiffer RM, et al. Blood. 2009;113:5412–5417. doi: 10.1182/blood-2008-12-194241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.POEMS syndrome: 2017 update on diagnosis, risk stratification, and management. Dispenzieri A. Am J Hematol. 2017;92:814–829. doi: 10.1002/ajh.24802. [DOI] [PubMed] [Google Scholar]
- 6.Role of radiography, MRI and FDG-PET/CT in diagnosing, staging and therapeutical evaluation of patients with multiple myeloma. Lütje S, de Rooy JW, Croockewit S, Koedam E, Oyen WJ, Raymakers RA. Ann Hematol. 2009;88:1161–1168. doi: 10.1007/s00277-009-0829-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diagnostic utility of FDG PET in multiple myeloma. Jadvar H, Conti PS. Skeletal Radiol. 2002;31:690–694. doi: 10.1007/s00256-002-0580-2. [DOI] [PubMed] [Google Scholar]
- 8.Whole-body 18F-FDG PET identifies high-risk myeloma. Durie BG, Waxman AD, D'Agnolo A, et al. https://jnm.snmjournals.org/content/43/11/1457/tab-article-info. J Nucl Med. 2002;43:1457–1463. [PubMed] [Google Scholar]
- 9.Initial results in the assessment of multiple myeloma using 18F-FDG PET. Schirrmeister H, Bommer M, Buck AK, et al. Eur J Nucl Med Mol Imaging. 2002;29:361–366. doi: 10.1007/s00259-001-0711-3. [DOI] [PubMed] [Google Scholar]
- 10.Value of FDG PET in the assessment of patients with multiple myeloma. Bredella MA, Steinbach L, Caputo G, Segall G, Hawkins R. AJR Am J Roentgenol. 2005;184:1199–1204. doi: 10.2214/ajr.184.4.01841199. [DOI] [PubMed] [Google Scholar]
- 11.Multiple myeloma: a review of imaging features and radiological techniques. Healy CF, Murray JG, Eustace SJ, Madewell J, O'Gorman PJ, O'Sullivan P. Bone Marrow Res. 2011;2011:583439. doi: 10.1155/2011/583439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Current and future imaging modalities for multiple myeloma and its precursor states. Tan E, Weiss BM, Mena E, Korde N, Choyke PL, Landgren O. Leuk Lymphoma. 2011;52:1630–1640. doi: 10.3109/10428194.2011.573036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The role of fluorine-18-fluorodeoxyglucose positron emission tomography in evaluating the response to treatment in patients with multiple myeloma. Caldarella C, Treglia G, Isgrò MA, Treglia I, Giordano A. Int J Mol Imaging. 2012;2012:175803. doi: 10.1155/2012/175803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.A prospective comparison of 18F-fluorodeoxyglucose positron emission tomography-computed tomography, magnetic resonance imaging and whole-body planar radiographs in the assessment of bone disease in newly diagnosed multiple myeloma. Zamagni E, Nanni C, Patriarca F, et al. Haematologica. 2007;92:50–55. doi: 10.3324/haematol.10554. [DOI] [PubMed] [Google Scholar]
- 15.Whole-body MRI versus PET in assessment of multiple myeloma disease activity. Shortt CP, Gleeson TG, Breen KA, McHugh J, O'Connell MJ, O'Gorman PJ, Eustace SJ. AJR Am J Roentgenol. 2009;192:980–986. doi: 10.2214/AJR.08.1633. [DOI] [PubMed] [Google Scholar]
- 16.11C-choline vs. 18F-FDG PET/CT in assessing bone involvement in patients with multiple myeloma. Nanni C, Zamagni E, Cavo M, et al. World J Surg Oncol. 2007;5:68. doi: 10.1186/1477-7819-5-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clinical value of ¹¹C-methionine PET/CT in patients with plasma cell malignancy: comparison with ¹⁸F-FDG PET/CT. Nakamoto Y, Kurihara K, Nishizawa M, et al. Eur J Nucl Med Mol Imaging. 2013;40:708–715. doi: 10.1007/s00259-012-2333-3. [DOI] [PubMed] [Google Scholar]
- 18.11C-Methionine-PET: a novel and sensitive tool for monitoring of early response to treatment in multiple myeloma. Lückerath K, Lapa C, Albert C, et al. Oncotarget. 2015;6:8418–8429. doi: 10.18632/oncotarget.3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Evaluation of prostate cancer with 11C-acetate PET/CT. Spick C, Herrmann K, Czernin J. J Nucl Med. 2016;57:30–37. doi: 10.2967/jnumed.115.169599. [DOI] [PubMed] [Google Scholar]
- 20.Stimulation of tumor-associated fatty acid synthase expression by growth factor activation of the sterol regulatory element-binding protein pathway. Swinnen JV, Heemers H, Deboel L, et al. Oncogene. 2000;19:5173–5181. doi: 10.1038/sj.onc.1203889. [DOI] [PubMed] [Google Scholar]
- 21.The importance of acetyl coenzyme A synthetase for 11C-acetate uptake and cell survival in hepatocellular carcinoma. Yun M, Bang SH, Kim JW, Park JY, Kim KS, Lee JD. J Nucl Med. 2009;50:1222–1228. doi: 10.2967/jnumed.109.062703. [DOI] [PubMed] [Google Scholar]
- 22.Tumor identification of less aggressive or indolent lymphoma with whole-body 11C-acetate PET/CT. Tsuchiya J, Yamamoto M, Bae H, Oshima T, Yoneyama T, Miura O, Tateishi U. Clin Nucl Med. 2019;44:276–281. doi: 10.1097/RLU.0000000000002464. [DOI] [PubMed] [Google Scholar]
- 23.Evaluating acetate metabolism for imaging and targeting in multiple myeloma. Fontana F, Ge X, Su X, et al. Clin Cancer Res. 2017;23:416–429. doi: 10.1158/1078-0432.CCR-15-2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.11C-acetate PET/CT for metabolic characterization of multiple myeloma: a comparative study with 18F-FDG PET/CT. Ho CL, Chen S, Leung YL, et al. J Nucl Med. 2014;55:749–752. doi: 10.2967/jnumed.113.131169. [DOI] [PubMed] [Google Scholar]
- 25.11C-acetate as a new biomarker for PET/CT in patients with multiple myeloma: initial staging and postinduction response assessment. Lin C, Ho CL, Ng SH, et al. Eur J Nucl Med Mol Imaging. 2014;41:41–49. doi: 10.1007/s00259-013-2520-x. [DOI] [PubMed] [Google Scholar]
- 26.Metabolic tumor volume assessed by 18F-FDG PET/CT for the prediction of outcome in patients with multiple myeloma. Fonti R, Larobina M, Del Vecchio S, et al. J Nucl Med. 2012;53:1829–1835. doi: 10.2967/jnumed.112.106500. [DOI] [PubMed] [Google Scholar]
- 27.Assessment of total lesion glycolysis by 18F FDG PET/CT significantly improves prognostic value of GEP and ISS in myeloma. McDonald JE, Kessler MM, Gardner MW, et al. Clin Cancer Res. 2017;23:1981–1987. doi: 10.1158/1078-0432.CCR-16-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Revised international staging system for multiple myeloma: a report from International Myeloma Working Group. Palumbo A, Avet-Loiseau H, Oliva S, et al. J Clin Oncol. 2015;33:2863–2869. doi: 10.1200/JCO.2015.61.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Preparation of [1−11C]acetate—an agent for the study of myocardial metabolism by positron emission tomography. Pike VW, Eakins MN, Allan RM, et al. Int J Appl Radiat Isot. 1982;33:505–512. doi: 10.1016/0020-708x(82)90003-5. [DOI] [PubMed] [Google Scholar]
- 30.A practical method for the preparation of [11C]acetate. Kruijer PS, Linden TT, Mooij R, et al. Appl Radiat Isot. 1995;317:321. [Google Scholar]
- 31.Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Kanda Y. Bone Marrow Transplant. 2013;48:452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prognostic relevance of 18-F FDG PET/CT in newly diagnosed multiple myeloma patients treated with up-front autologous transplantation. Zamagni E, Patriarca F, Nanni C, et al. Blood. 2011;118:5989–5995. doi: 10.1182/blood-2011-06-361386. [DOI] [PubMed] [Google Scholar]
- 33.Characterizing POEMS syndrome with 18F-FDG PET/CT. Pan Q, Li J, Li F, et al. J Nucl Med. 2015;56:1334–1337. doi: 10.2967/jnumed.115.160507. [DOI] [PubMed] [Google Scholar]
- 34.Usefulness of 18F-FDG PET/CT for detecting lesions in patients with POEMS syndrome. Tanaka N, Yoshinaga K, Asano C, et al. Rinsho Ketsueki. 2012;53:2013–2017. [PubMed] [Google Scholar]
- 35.18F-FDG PET/CT in the evaluation of POEMS syndrome. Albertí MA, Martinez-Yélamos S, Fernandez A, et al. Eur J Radiol. 2010;76:180–182. doi: 10.1016/j.ejrad.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 36.Metabolic tumor volume metrics in lymphoma. Kostakoglu L, Chauvie S. Semin Nucl Med. 2018;48:50–66. doi: 10.1053/j.semnuclmed.2017.09.005. [DOI] [PubMed] [Google Scholar]