Abstract
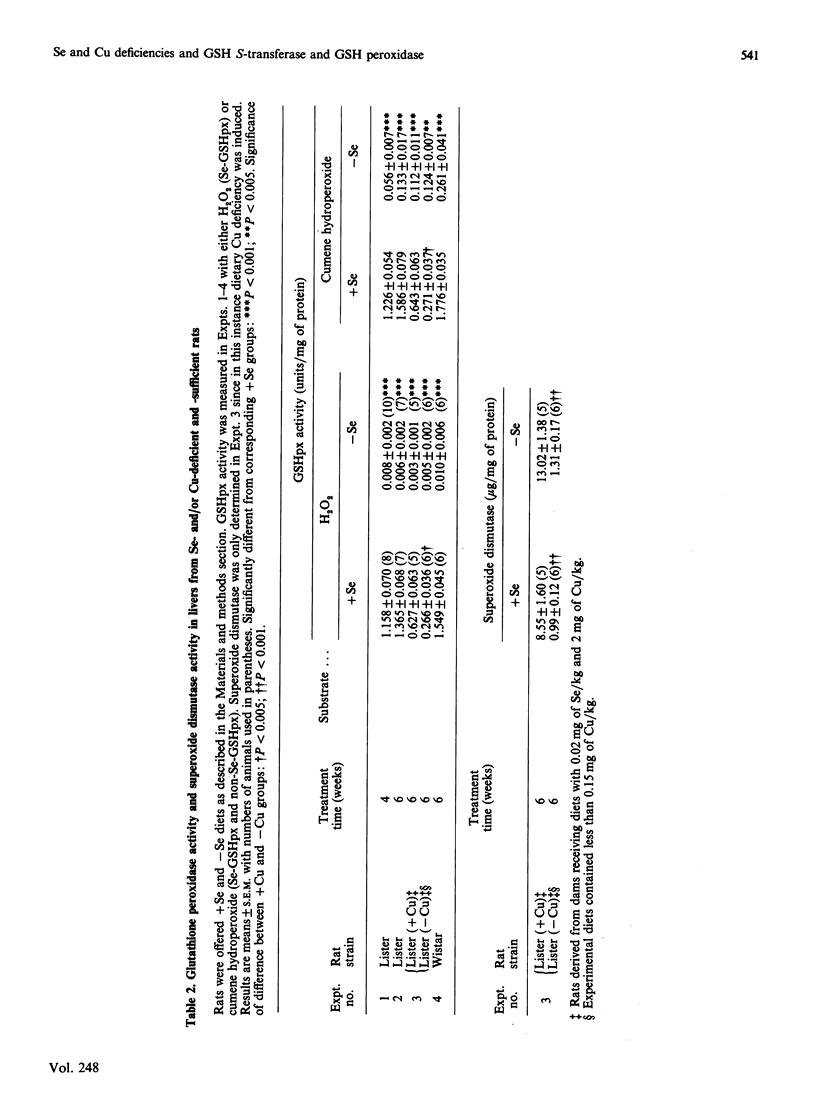
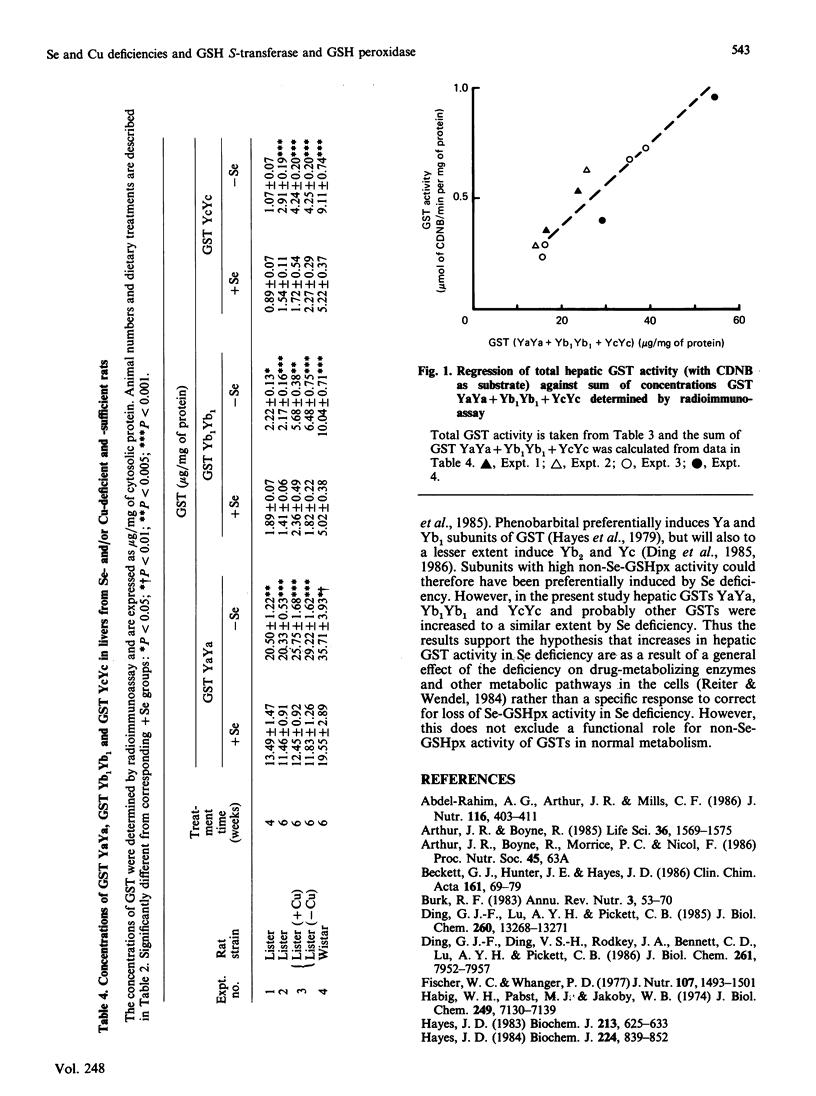
Selenium (Se) deficiency in rats produced significant increases in the activity of hepatic glutathione S-transferase (GST) with 1-chloro-2,4-dinitrobenzene as substrate and in various GST isoenzymes when determined by radioimmunoassay. These changes is GST activity and concentration were associated with Se deficiency that was severe enough to provoke decreases of over 98% in hepatic Se-containing glutathione peroxidase activity (Se-GSHpx). However, decreases in hepatic Se-GSHpx of 60% induced by copper (Cu) deficiency had no effect on GST activity or concentration. Increased GST activity in Se deficiency has previously been postulated to be a compensatory response to loss of Se-GSHpx, since some GSTs have a non-Se-glutathione peroxidase (non-Se-GSHpx) activity. However, the GST isoenzymes determined in this study, GST Yb1Yb1, GST YcYc and GST YaYa, are known to have up to 30-fold differences in non-Se-GSHpx activity, but they were all significantly increased to a similar extent in the Se-deficient rats.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdel Rahim A. G., Arthur J. R., Mills C. F. Effects of dietary copper, cadmium, iron, molybdenum and manganese on selenium utilization by the rat. J Nutr. 1986 Mar;116(3):403–411. doi: 10.1093/jn/116.3.403. [DOI] [PubMed] [Google Scholar]
- Arthur J. R., Boyne R. Superoxide dismutase and glutathione peroxidase activities in neutrophils from selenium deficient and copper deficient cattle. Life Sci. 1985 Apr 22;36(16):1569–1575. doi: 10.1016/0024-3205(85)90381-9. [DOI] [PubMed] [Google Scholar]
- Beckett G. J., Hunter J. E., Hayes J. D. Hepatic damage in the rat following administration of thyroxine or triiodothyronine, assessed by measurement of plasma glutathione S-transferase YaYa concentrations. Clin Chim Acta. 1986 Nov 30;161(1):69–79. doi: 10.1016/0009-8981(86)90264-0. [DOI] [PubMed] [Google Scholar]
- Burk R. F. Biological activity of selenium. Annu Rev Nutr. 1983;3:53–70. doi: 10.1146/annurev.nu.03.070183.000413. [DOI] [PubMed] [Google Scholar]
- Ding G. J., Ding V. D., Rodkey J. A., Bennett C. D., Lu A. Y., Pickett C. B. Rat liver glutathione S-transferases. DNA sequence analysis of a Yb2 cDNA clone and regulation of the Yb1 and Yb2 mRNAs by phenobarbital. J Biol Chem. 1986 Jun 15;261(17):7952–7957. [PubMed] [Google Scholar]
- Ding G. J., Lu A. Y., Pickett C. B. Rat liver glutathione S-transferases. Nucleotide sequence analysis of a Yb1 cDNA clone and prediction of the complete amino acid sequence of the Yb1 subunit. J Biol Chem. 1985 Oct 25;260(24):13268–13271. [PubMed] [Google Scholar]
- Fischer W. C., Whanger P. D. Fatty acid and glucose metabolism in selenium deficient rats and lambs. J Nutr. 1977 Aug;107(8):1493–1501. doi: 10.1093/jn/107.8.1493. [DOI] [PubMed] [Google Scholar]
- Habig W. H., Pabst M. J., Jakoby W. B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974 Nov 25;249(22):7130–7139. [PubMed] [Google Scholar]
- Hayes J. D., Mantle T. J. Anomalous electrophoretic behaviour of the glutathione S-transferase Ya and Yk subunits isolated from man and rodents. A potential pitfall for nomenclature. Biochem J. 1986 Aug 1;237(3):731–740. doi: 10.1042/bj2370731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes J. D. Purification and characterization of glutathione S-transferases P, S and N. Isolation from rat liver of Yb1 Yn protein, the existence of which was predicted by subunit hybridization in vitro. Biochem J. 1984 Dec 15;224(3):839–852. doi: 10.1042/bj2240839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes J. D. Rat liver glutathione S-transferases. A study of the structure of the basic YbYb-containing enzymes. Biochem J. 1983 Sep 1;213(3):625–633. doi: 10.1042/bj2130625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes J. D., Strange R. C., Percy-Robb I. W. Identification of two lithocholic acid-binding proteins. Separation of ligandin from glutathione S-transferase B. Biochem J. 1979 Sep 1;181(3):699–708. doi: 10.1042/bj1810699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekstra W. G. Biochemical function of selenium and its relation to vitamin E. Fed Proc. 1975 Oct;34(11):2083–2089. [PubMed] [Google Scholar]
- Jenkinson S. G., Lawrence R. A., Burk R. F., Williams D. M. Effects of copper deficiency on the activity of the selenoenzyme glutathione peroxidase and on excretion and tissue retention of 75SeO3(2-). J Nutr. 1982 Jan;112(1):197–204. doi: 10.1093/jn/112.1.197. [DOI] [PubMed] [Google Scholar]
- Joseph R. L., Griffiths T. W. Animal meat products, processing and the consumer. Proc Nutr Soc. 1986 Feb;45(1):63–69. doi: 10.1079/pns19860036. [DOI] [PubMed] [Google Scholar]
- Ketterer B., Beale D., Taylor J. B., Meyer D. J. The genetic relationships and inducibility of soluble glutathione transferases of the rat liver. Biochem Soc Trans. 1983 Aug;11(4):466–467. doi: 10.1042/bst0110466. [DOI] [PubMed] [Google Scholar]
- Lawrence R. A., Parkhill L. K., Burk R. F. Hepatic cytosolic non selenium-dependent glutathione peroxidase activity: its nature and the effect of selenium deficiency. J Nutr. 1978 Jun;108(6):981–987. doi: 10.1093/jn/108.6.981. [DOI] [PubMed] [Google Scholar]
- Mannervik B., Jensson H. Binary combinations of four protein subunits with different catalytic specificities explain the relationship between six basic glutathione S-transferases in rat liver cytosol. J Biol Chem. 1982 Sep 10;257(17):9909–9912. [PubMed] [Google Scholar]
- Mannervik B. The isoenzymes of glutathione transferase. Adv Enzymol Relat Areas Mol Biol. 1985;57:357–417. doi: 10.1002/9780470123034.ch5. [DOI] [PubMed] [Google Scholar]
- Masukawa T., Nishimura T., Iwata H. Differential changes of glutathione S-transferase activity by dietary selenium. Biochem Pharmacol. 1984 Aug 15;33(16):2635–2639. doi: 10.1016/0006-2952(84)90637-3. [DOI] [PubMed] [Google Scholar]
- Mehlert A., Diplock A. T. The glutathione S-transferases in selenium and vitamin E deficiency. Biochem J. 1985 May 1;227(3):823–831. doi: 10.1042/bj2270823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer D. J., Beale D., Tan K. H., Coles B., Ketterer B. Glutathione transferases in primary rat hepatomas: the isolation of a form with GSH peroxidase activity. FEBS Lett. 1985 May 6;184(1):139–143. doi: 10.1016/0014-5793(85)80670-0. [DOI] [PubMed] [Google Scholar]
- Prohaska J. R., Ganther H. E. Glutathione peroxidase activity of glutathione-s-transferases purified from rat liver. Biochem Biophys Res Commun. 1976 May 23;76(2):437–445. doi: 10.1016/0006-291x(77)90744-6. [DOI] [PubMed] [Google Scholar]
- Reiter R., Wendel A. Selenium and drug metabolism--I. Multiple modulations of mouse liver enzymes. Biochem Pharmacol. 1983 Oct 15;32(20):3063–3067. doi: 10.1016/0006-2952(83)90250-2. [DOI] [PubMed] [Google Scholar]
- Reiter R., Wendel A. Selenium and drug metabolism--II. Independence of glutathione peroxidase and reversibility of hepatic enzyme modulations in deficient mice. Biochem Pharmacol. 1984 Jun 15;33(12):1923–1928. doi: 10.1016/0006-2952(84)90548-3. [DOI] [PubMed] [Google Scholar]
- Reiter R., Wendel A. Selenium and drug metabolism--III. Relation of glutathione-peroxidase and other hepatic enzyme modulations to dietary supplements. Biochem Pharmacol. 1985 Jul 1;34(13):2287–2290. doi: 10.1016/0006-2952(85)90783-x. [DOI] [PubMed] [Google Scholar]
- Schramm H., Robertson L. W., Oesch F. Differential regulation of hepatic glutathione transferase and glutathione peroxidase activities in the rat. Biochem Pharmacol. 1985 Oct 15;34(20):3735–3739. doi: 10.1016/0006-2952(85)90239-4. [DOI] [PubMed] [Google Scholar]


