Abstract
INTRODUCTION:
Public health efforts to reduce opioid overdose fatalities include educating people at risk and expanding access to naloxone, a medication that reverses opioid-induced respiratory depression. People receiving long-term opioid therapy (LTOT) are at increased risk for overdose, yet naloxone uptake in this population remains low. The objective of this study was to determine if a targeted, digital health intervention changed patient risk behavior, increased naloxone uptake, and increased knowledge about opioid overdose prevention and naloxone.
METHODS:
We conducted a pragmatic randomized clinical trial among patients prescribed LTOT in a health care delivery system in Colorado. Participants were randomly assigned to receive an animated overdose prevention and naloxone educational video (intervention arm) or usual care (control arm). The 6-minute video was designed to educate patients about opioid overdose and naloxone, increase overdose risk perception, and prompt them to purchase naloxone from the pharmacy. Over an 8-month follow-up, opioid risk behavior was assessed with the Opioid-Related Behaviors in Treatment survey instrument, and overdose and naloxone knowledge was measured with the Prescription Opioid Overdose Knowledge Scale after viewing the video at baseline. Naloxone dispensations were evaluated using pharmacy data over a 12-month period. Data were analyzed with generalized linear mixed effects and log-binomial regression models.
RESULTS:
There were 519 participants in the intervention arm and 485 participants in the usual care arm. Opioid risk behavior did not differ between the study arms over time (study arm by time interaction P=0.93). There was no difference in naloxone uptake between the arms (RR = 1.13, 95% CI: 0.77—1.66). Knowledge was significantly greater in the intervention arm compared to usual care (P<0.001).
CONCLUSIONS:
A targeted, digital health intervention video effectively increased opioid overdose and naloxone knowledge, without increasing opioid risk behavior. Naloxone uptake did not differ between the intervention and usual care arms.
TRIAL REGISTRATION:
ClinicalTrials.gov number NCT03337009.
Keywords: pragmatic randomized clinical trial, overdose, naloxone, opioids
INTRODUCTION
From 2017 to 2021, more than 78,000 people in the United States died from a drug overdose involving prescription opioids [1, 2]. Approximately 5% of adults in the US are prescribed long-term opioid therapy (LTOT) [3], and patients receiving opioid doses of greater than 20 mg per day are at increased risk for overdose compared to patients receiving lower doses [4–6].
Efforts to reduce overdose fatalities include educating people at risk and expanding access to naloxone, a medication that reverses opioid-induced respiratory depression. Naloxone has been widely used by emergency medical personnel for decades; since the 1990’s, community-based programs have provided overdose prevention education, overdose management training, and take home naloxone to laypersons, including people who use drugs and potential bystanders [7, 8]. Although such programs are effective at preventing opioid overdose deaths [9], rapidly increasing overdose fatality rates attributed to prescription and high potency synthetic opioids have prompted practices, policies, and laws to increase access to naloxone, such as standing orders, protocol orders, collaborative practice agreements [10, 11], co-prescribing naloxone with opioids, and over-the-counter status for certain naloxone products [12–14]. Despite these efforts, however, naloxone uptake remains low in the United States and other countries [15–18].
Patients prescribed LTOT experience numerous barriers to naloxone access. Clinicians tend to recognize the benefits of prescribing naloxone for take home use, but they may be reluctant to prescribe it because of competing clinical demands and fears that prescribing naloxone may stigmatize patients [19]. Clinicians have also expressed concerns about risk compensation (also referred to as “moral hazard”) [19–21], a theory that suggests individuals may be more likely to engage in risky opioid use behaviors because they are aware of and have access to naloxone. Patients may not request naloxone directly from clinicians because of knowledge gaps about overdose and naloxone, beliefs that they are not at risk of overdose because they take their opioid medications as prescribed, or concerns that requesting naloxone will imply they are misusing prescribed opioids [22]. Standing order laws, which allow individuals at risk of overdose to obtain naloxone from a community [23] or pharmacy setting [24] without an individual prescription [25], could overcome some of these barriers; however, naloxone dispensing remains suboptimal despite standing order laws, possibly because of stigma, low overdose risk perception, naloxone’s cost, and limited awareness that naloxone is available under a standing order [26]. Studies have also cited pharmacist confusion about how laws pertain to naloxone dispensing and lack of clarity for how to bill for naloxone as factors contributing to poor naloxone uptake [27–29]. Under a standing order, co-dispensing naloxone with opioid medications is an effective approach to increase access [31, 32], but implementing co-dispensing requires significant resources to educate staff, establish consistent workflows, and stock the medication [33, 17]. The Food and Drug Administration approved naloxone for over-the-counter status in March 2023 to facilitate access and potentially mitigate stigma; but cost, low risk perception, and limited knowledge about naloxone among people prescribed LTOT will likely remain barriers to uptake [34, 35].
To address barriers to expanding access to naloxone, we developed and tested an intervention by directly outreaching to patients prescribed LTOT and providing them with an animated overdose and naloxone educational video in narrative form called the Naloxone Navigator. The video was designed to educate patients about opioid overdose and naloxone, increase their overdose risk perception, and prompt them to purchase naloxone under a system-wide naloxone standing order. We conducted a pragmatic randomized clinical trial (RCT) to determine whether this intervention changed patient risk behavior, naloxone uptake, and patient knowledge about opioid overdose prevention and naloxone.
METHODS
Study design
We conducted a pragmatic RCT to evaluate the effectiveness of the Naloxone Navigator intervention among patients receiving LTOT. Participants were randomized to receive either the intervention or usual care. Participants in both arms could access naloxone by prescription from their physicians or directly from pharmacies without an individual prescription under a standing order. Participants were administered surveys at time 0 (T0), 4 months, and 8 months to assess overdose risk behavior and knowledge; electronic pharmacy records were used to measure naloxone uptake over a 12-month period from T0. We hypothesized that the intervention would not increase opioid risk behavior, but that it would increase overdose prevention and naloxone knowledge, and increase naloxone uptake.
The Kaiser Permanente Colorado Institutional Review Board approved the study (1224275), and the trial was registered with ClinicalTrials.gov (NCT03337009) on November 7, 2017, before the first patient was enrolled. We used the Consolidated Standards of Reporting Trials guideline.
Study setting, participants, and randomization
All participants were members of Kaiser Permanente Colorado (KPCO), an integrated insurance and health care delivery system that serves more than 550,000 members across Colorado, with 29 outpatient pharmacies located within KPCO ambulatory medical offices. The KPCO patient population is demographically representative of Colorado [36]. Under statewide standing order legislation [37], the study team collaborated with KPCO pharmacy operations to implement a naloxone standing order in January 2017, allowing pharmacists to dispense naloxone for take home use without patients having to obtain individual prescriptions from their physicians.
Patients were recruited in 2-month waves starting December 21, 2017. At the beginning of each wave, electronic pharmacy records were used to identify patients receiving LTOT, defined as 3 or more opioid dispensations in the previous 90 days with no more than a 5-day gap in opioid coverage. This included short- and long-acting opioid medications except for tramadol or medications used for opioid use disorder treatment. Patients also had to be 18 years or older, English-speaking, and have internet access. Patients were ineligible if they were enrolled in hospice or had a do-not-resuscitate order since the focus of this trial was the safety of chronic pain opioid management rather than end-of-life care. In each wave, a random sample of 500 to 1000 eligible patients were invited to participate first by mail, followed by email and telephone. Patients were directed to a study enrollment website where they could provide informed consent. After consent (T0), participants were randomized to the intervention or usual care arm at a 1:1 allocation ratio using the SAS/STAT (SAS Institute, Inc, Cary, NC) procedure Proc Plan. The statistician and investigators were blinded to study arm assignment. Participants received a $20 gift card for completing surveys at each time point, and all survey data was stored in a Research Electronic Data Capture (REDCap) database [38, 39].
Patient and Public Involvement
Patients and the public were not directly involved in the design of the study.
Usual Care
Under usual care, patients could be prescribed naloxone by their physicians or request it from a pharmacist under a naloxone standing order, which made naloxone available without an individual prescription in all pharmacies in KPCO. Pharmacists were trained on standing order naloxone dispensing process, counselling points, and cost quotes. Opioid prescribers were encouraged to prescribe naloxone to patients in continuing medical education sessions and made aware of the naloxone standing order in system-wide pharmacy communications. Member-facing system-wide communications informed members when naloxone was made available in system pharmacies under a standing order. The costs of naloxone ranged from $0 to $140, depending on the patient’s insurance plan. Patients in the usual care arm were not required to view a sham video intervention.
Intervention
Intervention participants received usual care and a web-based, 6-minute animated educational video. The video presented standardized messages on how to prevent, recognize, and respond to an opioid overdose through a first-person narrative of a patient prescribed opioids. It aimed to heighten patients’ overdose risk perceptions and increase their self-efficacy to acquire and use naloxone in an overdose emergency. Specifically, the video informed viewers about the overdose risk associated with opioid treatment for pain and encouraged them to purchase naloxone from the pharmacy under the standing order.
The intervention was iteratively developed using the integrate, design, assess, and share (IDEAS) framework [40]. Guided by the Theory of Planned Behavior [41] and the Health Belief Model [43], qualitative data were collected from semi-structured interviews with patients prescribed high-dose opioid therapy and from focus groups with primary care staff [19, 22] to delineate key intervention targets. The intervention’s content was designed to address patients’ limited knowledge about naloxone, low overdose risk perception, and reluctance to have difficult conversations about overdose with clinicians. The intervention was delivered as a video directly to patients – without requiring an appointment – to minimize clinician effort, reduce bias in patient identification, and assuage clinician concerns that initiating conversations about overdose would stigmatize patients. The messages were conveyed as a fictional first-person narrative to elicit emotion and enhance recollection of the content, with foreshadowing and humor to help generate interest and sustain attention throughout the video [41, 44].
Study investigators wrote the draft content for the video, which was assessed for comprehension and acceptability in 11 cognitive interviews of patients and their caregivers, and further refined by the research team. Interviewees provided input on how the messages should be framed, whether they resonated, and whether they were stigmatizing. The research team then developed the animated video using Vyond Studio® software [45]. The narrator is a female patient prescribed LTOT for chronic pain. After foreshadowing the overdose emergency, she visits her primary care physician, who provides clinical information on the signs of an overdose, risk factors, and how to acquire and use naloxone. The narrator shares this information with her partner. She subsequently attends a party, uses both alcohol and prescribed opioid analgesics, and accidently experiences an overdose. Her partner revives her using naloxone he has with him, and she is transported to the emergency department. The couple later picks up a refill at the pharmacy under the standing order and engages in other preventive behaviors, including having a lock box for safer home opioid storage. Professional actors provided voiceovers for the main characters, and final editing was conducted using Adobe® Audition® software in a recording studio [46]. The video was embedded within a password-protected, single page website using WordPress® software and underwent usability testing with 3 patients.
Participants receiving LTOT who were randomized to the intervention arm received a link to the video and were required to play the video in its entirety at T0 to obtain renumeration for their research participation. One month after T0, participants in the intervention arm were emailed a weblink to view the video again with 3 additional weblinks that could be shared with family, friends, or caregivers.
Baseline data
Demographic survey questions (race, ethnicity, education, and income) were derived from the Behavioral Risk Factor Surveillance System and assessed at T0 [47]. Baseline (past year before T0) clinical characteristics were identified using the electronic health and pharmacy records.
Outcomes
We examined 2 primary outcomes: opioid risk behavior and naloxone uptake. Opioid risk behavior was assessed at T0, 4 months, and 8 months with the Opioid-Related Behaviors In Treatment (ORBIT) instrument, a validated, self-administered 10-item scale [48]. The ORBIT measures risk behaviors, such as using opioids for purposes other than pain or requesting early refills [19]. ORBIT items are presented on a 5-point Likert scale and can be used to assess behavior changes over time [48, 49]. We modified the ORBIT to measure behaviors over the previous 4 months. The ORBIT was administered to participants in the intervention prior to viewing the video at T0. Risk behavior was analyzed as a binary outcome, with a positive response defined as endorsing one or more risk behaviors on the ORBIT scale [50].
Naloxone dispensings were ascertained over a 12-month follow-up period using National Drug Codes (NDC) from electronic pharmacy data indicating that the products were sold by KPCO pharmacies and from claims demonstrating that a patient purchased naloxone from an external pharmacy. Participants were also asked on the survey if they or a family member had obtained naloxone. We conducted a post-hoc analysis in which the survey naloxone uptake data was combined with the pharmacy and claims data, and participants who had received naloxone prior to baseline were excluded. For another post-hoc analysis, naloxone uptake 12-months prior to T0 was compared to uptake 12-months after T0.
In addition to the primary outcomes, we assessed overdose and naloxone knowledge at T0, 4 months, and 8 months with the Prescription Opioid Overdose Knowledge Scale (Rx-OOKS). Rx-OOKS is a validated 25-item scale measuring knowledge of overdose risks, overdose warning signs, steps to address overdose, and appropriate use of naloxone. Rx-OOKS scores range from 0 to 25, with a higher Rx-OOKS score representing greater knowledge [51]. Missing Rx-OOKS item responses received a score of zero. Rx-OOKS was administered to participants after they viewed the video at T0. It was analyzed as a continuous variable.
We also used the Alcohol, Smoking, and Substance Involvement Screening Test (ASSIST) to examine the following secondary risk behavior outcomes: cannabis use, other drug use (heroin, cocaine, methamphetamine, hallucinogens, inhalants, other drugs) and non-medical sedative use [52, 53]. The ASSIST was assessed as a binary outcome, in which endorsement of one or more behavior was considered a positive response. We assessed hazardous drinking with the Alcohol Use Disorders Identification Test – Concise (AUDIT-C) [54]. The AUDIT-C is scored on a scale of 0 to 12, where a score of 0 indicates no alcohol use. We assessed the AUDIT-C as a binary outcome, in which scores of ≥4 for men and ≥3 for women represented positive responses.
At 12 months, we compared the incidence of opioid overdose and all-cause mortality between the study arms. Opioid overdoses were identified using International Classification of Diseases (ICD)-10 CM codes (online supplemental eTable 1) from emergency department and hospital records, and from Denver County paramedic records. To identify deaths and causes of deaths, identifiers for all patients were linked to the Colorado Department of Public Health and the Environment vital records. When available, participants’ medical records were reviewed to confirm opioid overdoses and the cause of death.
Statistical Methods
A priori statistical power and sample size calculations were based on a continuous ORBIT score. There were approximately 8,300 patients receiving LTOT who were eligible for the study, and prior work suggested that between 10% and 30% of these patients would participate in the trial. Based on a 10% participation rate and a 2-sided α = 0.05, we could detect a 0.31 difference in ORBIT scores between the intervention and usual care arms with 80% power. However, the ORBIT scores were skewed with little variability: mean at T0=1.6 (SD=2.3) on a scale of 0 to 40. For the analysis, we elected to dichotomize the ORBIT score as endorsing ≥1 opioid risk behavior vs. endorsing 0 behaviors.
Generalized linear mixed-effects models for repeated measures were used to assess the change in risk behavior and knowledge scores between the study arms over time. The use of mixed models accounted for the correlation between observations made by the same participant across time. Primary and secondary risk behaviors were analyzed as binary outcomes, using a binary distribution and log link function. The Rx-OOKS knowledge score was modeled as a continuous outcome, using a normal distribution and identity link function. Each model included the following variables: study arm (intervention or usual care), survey time point (T0, 4 months, 8 months), and interaction between the study arm and survey time point. Risk ratios or risk differences and 95% CIs comparing outcomes between study arms at each time point are reported. To account for missing survey data, we imputed 20 complete datasets by the method of fully conditional specification using all variables that could be potentially associated with the missing data. Each of the 20 complete datasets was analyzed using a generalized linear mixed model with repeated measures, and the parameter estimates obtained from each analyzed dataset were combined. We imputed missing data using PROC MI and combined parameter estimates using PROC MIANALZE in SAS. We also conducted sensitivity analyses for the ORBIT and Rx-OOKS that excluded missing survey data and only included individuals who completed the respective survey measures at each time point.
Receipt of at least one naloxone dispensation (in primary and post-hoc analysis), opioid overdose, and death were analyzed as dichotomous outcomes using a log-binomial model with the study arm as the exposure variable. Risk ratios and 95% confidence intervals were calculated. For the post-hoc analysis comparing naloxone uptake 12-months prior to T0 to uptake 12-months after T0, we used generalized linear mixed-effects model. We analyzed naloxone uptake as a binary variable and included the following variables in the model: study arm (intervention or usual care), study time point (pre-intervention, post-intervention), and interaction between the study arm and study time point.
All outcomes were analyzed by the intention-to-treat principle. A 5% significance level using 2-sided tests was applied in all analyses, and data were analyzed using SAS® Studio Software version 3.8 (SAS Institute Inc., Cary, North Carolina).
RESULTS
Participants (N=1,004; Figure 1) had a mean age of 60.2 years, 63.8% were female, and 41.6% completed college or more. Intervention (n=519) and usual care (n=485) participants were similar in terms of baseline demographic characteristics, substance use disorder diagnoses, medical comorbidities, and mean opioid dose (Table 1). All participants in the intervention arm watched the video at baseline; 35 participants or caregivers logged into the website to watch the video after baseline.
Figure 1.
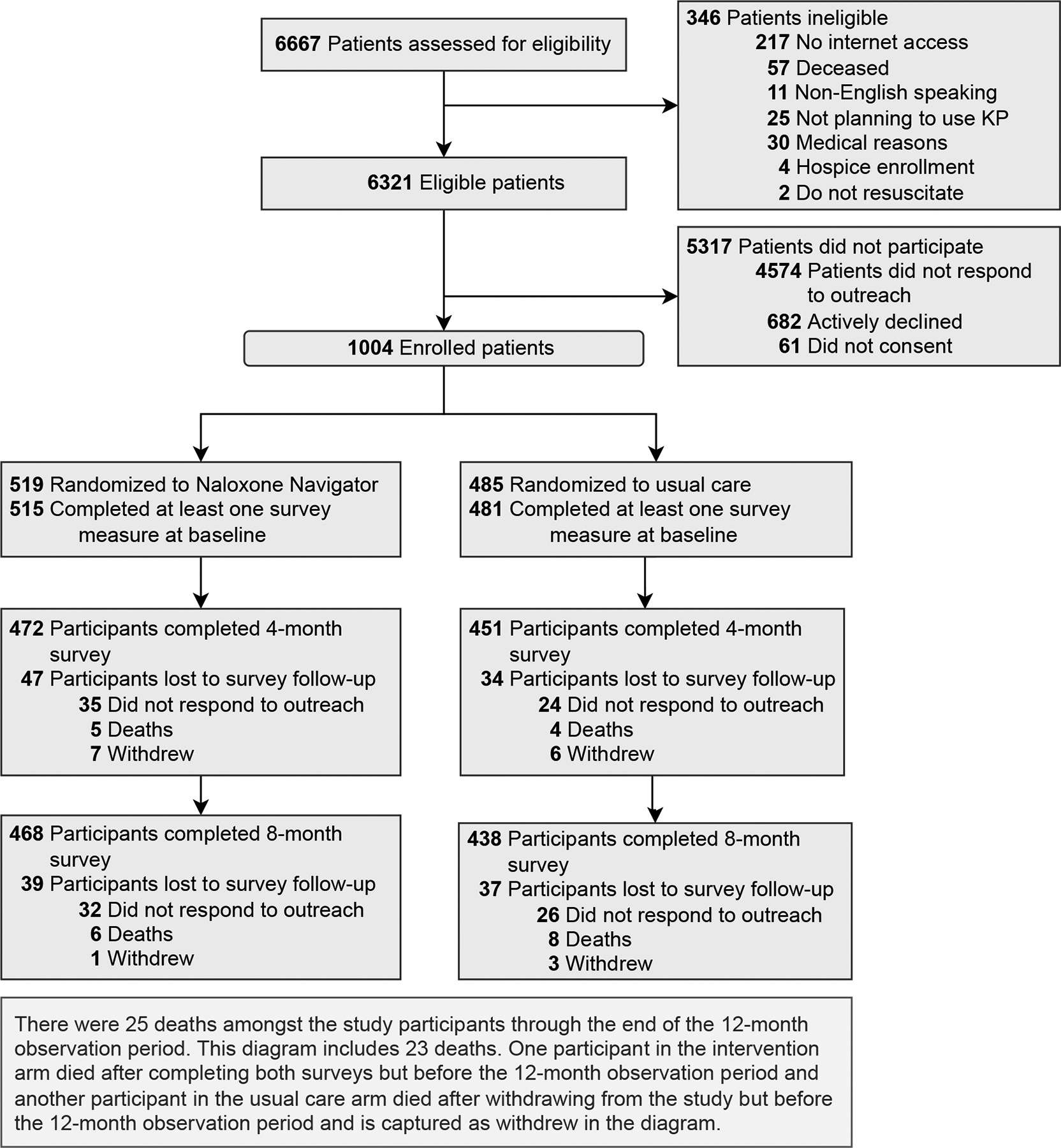
Consolidated Standards of Reporting Trials (CONSORT) Diagram of Screening, Enrollment, and Follow-up of Patients
Table 1.
Baseline Demographic and Clinical Characteristics of Study Participants, Overall and by Trial Arm
| Characteristic | Overall study participants (N=1004) |
Intervention arm (N=519) |
Usual care arm (N=485) |
|---|---|---|---|
| Age, mean (SD), y | 60.2 (12.5) | 60.2 (12.8) | 60.2 (12.2) |
| Female, n(%) | 641 (63.8) | 335 (64.6) | 306 (63.1) |
| Race/Ethnicity, n(%)* | |||
| Hispanic | 102 (10.2) | 48 (9.3) | 54 (11.1) |
| White | 779 (77.6) | 401 (77.3) | 378 (77.9) |
| Black | 40 (4.0) | 21 (4.1) | 19 (3.9) |
| All other racial and ethnic groups | 60 (6.0) | 33 (6.4) | 27 (5.6) |
| Missing | 23 (2.3) | 16 (3.1) | 7 (1.4) |
| Education, n(%)* | |||
| Less than high school | 23 (2.3) | 11 (2.1) | 12 (2.5) |
| Completed high school | 158 (15.7) | 82 (15.8) | 76 (15.7) |
| Attended some college | 375 (37.4) | 179 (34.5) | 196 (40.4) |
| Completed college or a higher degree | 418 (41.6) | 227 (43.7) | 191 (39.4) |
| Missing | 30 (3.0) | 20 (3.9) | 10 (2.1) |
| Annual household income, US dollars, n(%)* | |||
| Less than 20,000 | 159 (15.8) | 74 (14.3) | 85 (17.5) |
| 20,000 – <40,000 | 167 (16.6) | 85 (16.4) | 82 (16.9) |
| 40,000 – <75,000 | 282 (28.1) | 150 (28.9) | 132 (27.2) |
| 75,000 or more | 251 (25.0) | 135 (26.0) | 116 (23.9) |
| Missing | 145 (14.4) | 75 (14.5) | 70 (14.4) |
| Insurance, n(%)† | |||
| Commercial | 283 (28.2) | 143 (27.6) | 140 (28.9) |
| Medicaid | 139 (13.8) | 74 (14.3) | 65 (13.4) |
| Medicare | 529 (52.7) | 271 (52.2) | 258 (53.2) |
| Other | 53 (5.3) | 31 (6.0) | 22 (4.5) |
| Modified Charlson Comorbidity Index, median (IQR) ‡ | 1.0 (0–2.0) | 1.0 (0–2.0) | 1.0 (0–2.0) |
| Opioid use disorder, n(%)‡ | 46 (4.6) | 22 (4.2) | 24 (5.0) |
| Alcohol use disorder, n(%)‡ | 25 (2.5) | 14 (2.7) | 11 (2.3) |
| Tobacco use or nicotine use disorder, n(%)‡ | 217 (21.6) | 107 (20.6) | 110 (22.7) |
| Prior naloxone receipt, n(%)‡ | 87 (8.7) | 41 (7.9) | 46 (9.5) |
| Average daily opioid dose, median morphine milligram equivalents, median (IQR) § | 30.2 (15.7–60.0) | 30.2 (16.4–60.0) | 30.2 (15.1–60.9) |
Abbreviations: ORBIT - Opioid-Related Behaviors in Treatment modified to refer to past 4 months; AUDIT-C - Alcohol Use Disorders Identification Test - Concise; NIDA modified ASSIST – National Institute on Drug Abuse modified Alcohol, Smoking and Substance Involvement Screening Test; Rx-OOKS – Prescription Opioid Overdose Knowledge Scale
Assessed using survey measures; when denominators are indicated, the denominator is those who completed that Time 0 measure
Assessed at the time of study enrollment
Assessed using naloxone dispensations in the year prior to study enrollment and self-report at Time 0 survey
Assessed in the 6 months prior to study enrollment
The proportion of participants who reported one or more opioid risk behaviors (ORBIT) decreased over time in both arms (Figure 2). The most common reported risk behavior was “I have saved up my opioid medication, just in case I needed it later” (online supplemental eTable 2). From T0 to 8 months, reported risk behavior decreased from 59.9% to 43.4% in the intervention arm and from 52.0% to 38.8% in the usual care arm. However, risk behavior did not differ between the study arms over time (study arm by time interaction P-value = 0.93; Table 2). Across the three surveys, missingness ranged from 1.0% to 10.0% in the intervention arm and from 1.0% to 10.1% in the usual care arm. The sensitivity analysis that excluded missing survey data produced similar results (study arm by time interaction P-value = 0.93; online supplemental eTable 3).
Figure 2.
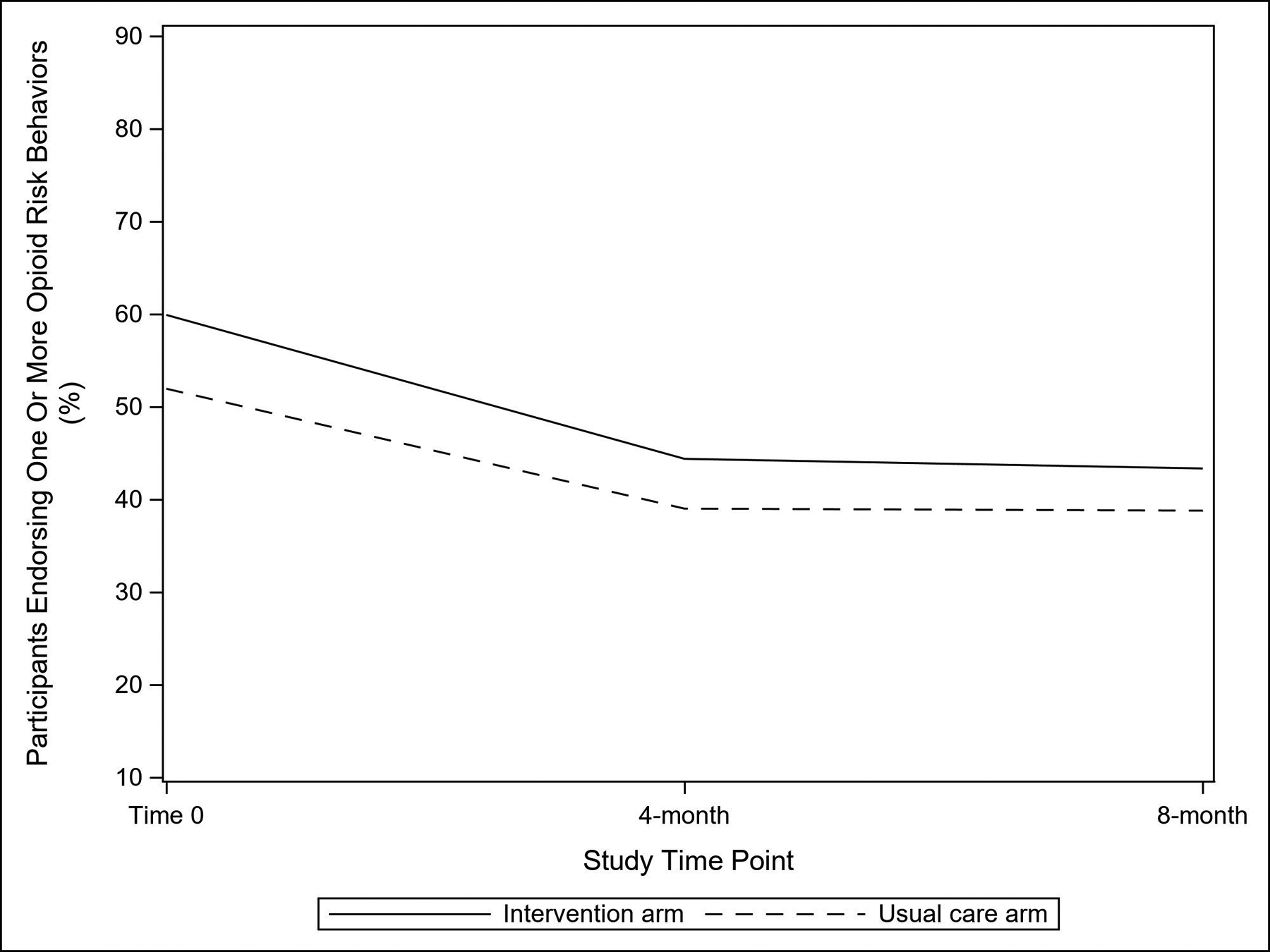
Proportion of Study Participants Endorsing One or More Opioid Risk Behaviors on the Opioid-Related Behaviors in Treatment Scale, by Trial Arm
Table 2.
Opioid Risk Behavior* Among Study Participants over Time, by Trial Arm
| Risk behavior | Proportion endorsing risk behavior (95% CI) |
Risk ratio (95% CI) |
Time × intervention P value |
|
|---|---|---|---|---|
| Intervention arm (n=519) |
Usual care arm (n=485) |
|||
| Opioid risk behavior | 0.93 | |||
| Time 0 | 59.9 (55.7, 64.1) | 52.0 (47.5, 56.4) | 1.15 (1.03, 1.29) | |
| 4 months | 44.4 (40.0, 48.8) | 39.0 (34.6, 43.5) | 1.14 (0.98, 1.32) | |
| 8 months | 43.4 (38.9, 47.8) | 38.8 (34.3, 43.3) | 1.12 (0.96, 1.30) | |
Opioid risk behavior was analyzed as a binary outcome, with a positive response defined as endorsing one or more risk behaviors on the ORBIT (Opioid-Related Behaviors in Treatment) scale
By the end of follow-up, 52 (10.0%) and 43 (8.9%) of the participants had been dispensed naloxone in the intervention and usual care arms, respectively. In the intervention arm, 3 (5.8%) of the 52 of the participants received naloxone outside of a KPCO pharmacy, and 6 (14.0%) of the 43 participants in the usual care arm received naloxone externally, as indicated by a claim. The difference in naloxone dispensations between the study arms was not statistically significant (risk ratio = 1.13, 95% confidence interval, 0.77 to 1.66). In both study arms, 87 participants had received naloxone prior to T0 and 35 participants reported that they or caregiver had acquired naloxone on the survey. In the post-hoc analysis that excluded the former and included the latter, 13.6% of the intervention and 11.4% of the usual care participants received naloxone during the follow-up period, and the difference was not statistically significant (risk ratio = 1.19, 95% confidence interval, 0.85 to 1.69). In the post-hoc analysis comparing 12-months prior to and 12-months after T0, naloxone uptake increased from 3.9% to 10.0% in the invention arm (P-value < 0.001) and from 5.4% to 8.9% in the usual care arm (P-value = 0.03); however, the difference between the study arms over time was not statistically significant (P-value = 0.17) (online supplemental eFigure 1).
After viewing the intervention video at T0, the mean knowledge (Rx-OOKS) score was 19.3 and decreased to 15.5 at 8 months. In the usual care arm, the mean knowledge score at T0 was 11.4 and increased to 14.0 at 8 months (Table 3, online supplemental eFigure 2). Knowledge was significantly greater in the intervention arm compared to usual care, but the difference between the arms attenuated over time (study arm by time interaction P < 0.001). Across the three surveys, missingness ranged from 2.7% to 9.8% in the intervention arm and from 1.4% to 9.9% in the usual care arm. The sensitivity analysis that excluded missing survey data produced similar results (study arm by time interaction P < 0.001; online supplemental eTable 4).
Table 3.
Opioid Overdose Prevention and Naloxone Knowledge Among Study Participants over Time, by Trial Arm
| Rx-OOKS | Rx-OOKS score* Mean (95% CI) |
Difference (95% CI) |
Time × intervention P value |
|
|---|---|---|---|---|
| Intervention arm (n=519) |
Usual care arm (n=485) |
|||
| Time 0 | 19.3 (18.9, 19.8) | 11.4 (11.0, 11.8) | 7.97 (7.41, 8.54) | <0.001 |
| 4 months | 14.8 (14.4, 15.1) | 13.2 (12.8, 13.6) | 1.57 (1.04, 2.10) | |
| 8 months | 15.5 (15.1, 15.8) | 14.0 (13.6, 14.3) | 1.50 (0.95, 2.05) | |
Abbreviations: Rx-OOKS – Prescription Opioid Overdose Knowledge Scale
Range of the Rx-OOKS scale is 0–25. Higher Rx-OOKS score represents greater knowledge
The secondary risk behavior outcomes – cannabis use, other drug use, non-medical sedative use and hazardous drinking – did not differ over time between the study arms (online supplemental eTable 5).
Across the follow-up, there was 1 (0.2%) opioid overdose in the intervention arm and 3 (0.6%) opioid overdoses in the usual care arm; one of the overdoses in the usual care arm was fatal. The difference between the arms was not statistically significant (risk ratio = 0.31, 95% confidence interval, 0.03 to 2.98). There were 12 (2.3%) and 13 (2.7%) all-cause deaths in intervention and usual care arms, respectively; the difference was not statistically significant (risk ratio = 0.86, 95% confidence interval, 0.40 to 1.87).
DISCUSSION
This pragmatic RCT demonstrated that exposure to a video-based overdose prevention and naloxone narrative increased opioid overdose and naloxone knowledge, without increasing opioid risk behaviors among patients prescribed LTOT. However, while naloxone uptake increased in both arms over time, the difference between the arms was not statistically significant.
Qualitative and survey data show that some providers have concerns about risk compensation. While data on naloxone-related risk compensation do not support this concern, the data has largely been derived from observational cohort and ecologic studies involving people who use illicit opioids [55, 56]. In this patient-level RCT, we did not find evidence that providing overdose education and facilitating access to naloxone increases the likelihood of risk behavior among patients receiving LTOT.
While the intervention effectively increased knowledge, it was insufficient to increase naloxone uptake relative to the control group. It is possible that cost considerations and stigma associated with requesting naloxone at the pharmacy counter remained barriers to obtaining naloxone for some intervention participants. It is also possible that exposure to the intervention – a single required viewing of a 6-minute video – was not intense enough to prompt participants to purchase naloxone. The intervention may therefore have to be repeated and augmented with a more resource-intensive approach such as naloxone co-dispensing, which has been shown to effectively increase naloxone uptake compared to usual care [28].
In the primary analysis, approximately 10% of participants who acquired naloxone obtained it outside of a KPCO pharmacy. It is possible that more participants would have acquired naloxone externally if we had conducted the trial after the Food and Drug Administration approved naloxone nasal spray for over-the-counter, nonprescription use in March 2023. However, it also possible that cost, stigma, and low risk perception among patients receiving LTOT remain barriers to uptake. Additional research is needed to assess the impact of over-the-counter status on access to naloxone.
The incidence rate of overdose was 0.4% (n=4) in both study arms, aligning with other published estimates among patients prescribed LTOT [57]. This suggests that, while patients receiving LTOT are at increased risk of overdose, the absolute risk of overdose in this population is low. However, since overdose is a very serious event and the population receiving LTOT in the US is large (approximately 5% of adults), expanding access to naloxone is a public health priority because it could potentially prevent thousands of overdoses in this population [14, 58].
The Centers for Disease Control and Prevention Clinical Practice Guideline for Prescribing Opioids for Pain encourages clinicians and practices to offer naloxone to patients prescribed opioids, as well as to provide education on overdose prevention and naloxone use to patients and their household members [59]. The Guideline further emphasizes that such efforts should focus on patients at high risk for overdose, including patients with a history of overdose, patients with a prior substance use disorder diagnosis, and patients being tapered to a lower opioid dose. Similarly, the World Health Organization recommends training on overdose management and making naloxone available to people who regularly use opioids and their families [60]. Given that the Naloxone Navigator intervention positively impacted overdose knowledge, clinicians and health care systems could use the video intervention to effectively counsel patients as part of a comprehensive effort to reduce the risks associated with opioid therapy.
This study had limitations. Participants were not blinded to their study arm assignment or the research topic (overdose and naloxone) and were repeatedly tested about naloxone knowledge with the Rx-OOKS instrument, factors which may have attenuated differences in both knowledge and naloxone uptake between the arms. Knowledge in the intervention arm decreased over time, and differences in knowledge between the study arms attenuated over time, suggesting that exposure to the intervention would need to be reinforced or repeated if implemented into practice. Although a priori statistical power was based on continuous ORBIT scores, we analyzed risk behavior as a dichotomous variable, which may have reduced the statistical power. Deaths that might have occurred outside of Colorado were not captured in the vital records. Lastly, the video was only tested in English; thus, the trial’s findings may not be generalizable to other languages and populations.
The Naloxone Navigator is a scalable intervention that effectively increased overdose prevention and naloxone knowledge among patients prescribed LTOT. While the intervention did not increase naloxone uptake relative to usual care, we did not find evidence that it increases opioid risk behaviors among patients receiving LTOT. We believe the intervention could be implemented across large health systems, complement other approaches to increase naloxone uptake, and be adapted to account for regulatory changes in naloxone, such as over-the-counter status and new formulations designed for higher potency synthetic opioids.
Supplementary Material
SUMMARY BOXES.
What is already known on this topic:
Uptake of take-home naloxone is low among patients prescribed long-term opioid therapy despite high rates of overdose.
While naloxone standing orders can help ensure access to naloxone, effective interventions to encourage patients to obtain naloxone and provide education on overdose risk and naloxone are needed.
What this study adds:
In this randomized clinical trial, patients prescribed long-term opioid therapy who watched a web-based, animated 6-minute video about overdose prevention and naloxone had increased knowledge about opioid overdose and naloxone and were not more likely to engage in risk behaviors than a similar group of patients who did not watch the video.
The educational intervention did not increase the likelihood of opioid risk behavior.
There was no difference in naloxone uptake across the groups but the intervention effectively increased knowledge.
How might these results change the focus of research or clinical practice?
Additional interventions to encourage naloxone uptake among patients prescribed long-term opioid therapy are needed. Overdose and naloxone knowledge content may need reinforcement over time.
Acknowledgments:
We appreciate the assistance and expertise of Kerstin Floyd, MD, Kathy Gleason, PhD, Ruth Bedoy, Emily Chenoweth, and members of the Data Safety and Monitoring Board (William Henderson, PhD, MPH, Marta Brooks, PharmD, and Adam Abraham, MD).
Funders:
Funding for this study was provided by the National Institute on Drug Abuse of the National Institutes of Health under award number R01DA042059. REDCap was supported by NIH/NCATS Colorado CTSA Grant Number UL1 TR002535. The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. All authors had full access to the complete data in the study and accept responsibility for publication.
Competing interests
All authors have completed the Unified Competing Interest form (available on request from the corresponding author) and declare support from the National Institutes of Health - National Institute on Drug Abuse for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years, no other relationships or activities that could appear to have influenced the submitted work. Outside of the listed affiliations, I.B. reports royalties from UpToDate, and all remaining authors declare that they do not have a conflict of interest.
Footnotes
Trial Registration: NCT03337009, first posted November 7, 2017
Prior Presentations
Baseline and preliminary results were presented at the Association for Multidisciplinary Education and Research in Substance use and Addiction, 46th Annual Meeting, Boston MA, November 10, 2022.
Transparency Declaration
The lead and senior authors (JMG and IAB, the manuscript’s guarantors) affirm that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered), have been explained.
Data Availability Statement
Investigators interested in using the data from this study must submit a written request. Requests must address regulatory and compliance requirements for data usage. Investigators must outline the necessary resources required to support their data request, including personnel and infrastructure. The original study team will evaluate each request based on scientific merit, ethical considerations, and alignment with the original study’s objectives.
Deidentified survey and electronic health record (EHR) data will be shared with investigators upon approval of written request. Investigators are responsible for obtaining necessary approvals and adhering to relevant laws and regulations and will be required to sign a Data Use Agreement (DUA) outlining the terms and conditions of data usage. The data sharing period is limited to three years following the publication of the primary study. The Naloxone Navigator video is available upon request; please send an email to: jason.m.glanz@kp.org. This Data Availability Statement is reflected in the Individual Participant Data (IPD) Sharing Statement of the clinicalTrials.gov registration (ClinicalTrials.gov number NCT03337009).
References
- 1.Centers for Disease Control and Prevention. Trends & statistics: Drug overdose death rates. 2023. Updated February 9, 2023. Available from: https://nida.nih.gov/research-topics/trends-statistics/overdose-death-rates Accessed May 25, 2023.
- 2.Breen P, Butt A. Deaths related to drug poisoning in England and Wales: 2021 registrations. In: Office for National Statistics, ed., 2022. [Google Scholar]
- 3.Mojtabai R National trends in long‐term use of prescription opioids. Pharmacoepidemiol Drug Saf 2018;27(5):526–34. [DOI] [PubMed] [Google Scholar]
- 4.Dunn KM, Saunders KW, Rutter CM, et al. Opioid prescriptions for chronic pain and overdose: A cohort study. Ann Intern Med 2010;152(2):85–92. doi: 152/2/85 [pii] 10.1059/0003-4819-152-2-201001190-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agnoli A, Xing G, Tancredi DJ, et al. Association of dose tapering with overdose or mental health crisis among patients prescribed long-term opioids. JAMA 2021;326(5):411–19. doi: 10.1001/jama.2021.11013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glanz JM, Xu S, Narwaney KJ, et al. Association between opioid dose reduction rates and overdose among patients prescribed long-term opioid therapy. Substance Abuse 2023;44(3):209–19. doi: 10.1177/08897077231186216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Community-based opioid overdose prevention programs providing naloxone - United States, 2010. MMWR Morb Mortal Wkly Rep 2012;61(6):101–5. [PMC free article] [PubMed] [Google Scholar]
- 8.Maxwell S, Bigg D, Stanczykiewicz K, Carlberg-Racich S. Prescribing naloxone to actively injecting heroin users: A program to reduce heroin overdose deaths. J Addict Dis 2006;25(3):89–96. doi: 10.1300/J069v25n03_11 [DOI] [PubMed] [Google Scholar]
- 9.Clark AK, Wilder CM, Winstanley EL. A systematic review of community opioid overdose prevention and naloxone distribution programs. J Addict Med 2014;8(3):153–63. doi: 10.1097/adm.0000000000000034 [DOI] [PubMed] [Google Scholar]
- 10.Policy Surveillance Program Staff. Naloxone overdose prevention laws. Updated January 1, 2022. Available from: https://pdaps.org/datasets/laws-regulating-administration-of-naloxone-1501695139 Accessed March 12, 2024.
- 11.Davis C, Carr D. State legal innovations to encourage naloxone dispensing. J Am Pharm Assoc (2003) 2017;57(2):S180–S84. doi: 10.1016/j.japh.2016.11.007 [DOI] [PubMed] [Google Scholar]
- 12.Weiner J, Murphy S, Behrends C. Expanding access to naloxone : A review of distribution strategies. Philadelphia, PA: Leonard Davis Institute of Health Economics; 2019. [Google Scholar]
- 13.Bohler RM, Freeman PR, Villani J, et al. The policy landscape for naloxone distribution in four states highly impacted by fatal opioid overdoses. Drug Alcohol Depend Rep 2023;6 doi: 10.1016/j.dadr.2022.100126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Office of the Surgeon General. U.S. Surgeon General’s advisory on naloxone and opioid overdose. Updated April 8, 2022. Available from: https://www.hhs.gov/surgeongeneral/reports-and-publications/addiction-and-substance-misuse/advisory-on-naloxone/index.html Accessed March 12, 2024.
- 15.Jones CM, Compton W, Vythilingam M, Giroir B. Naloxone co-prescribing to patients receiving prescription opioids in the Medicare Part D program, United States, 2016–2017. JAMA 2019;322(5):462–64. doi: 10.1001/jama.2019.7988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roberts AW. Naloxone prescribing among frequent opioid prescribers in Medicare Part D from 2013 to 2017: A retrospective study. J Gen Intern Med 2021;36(2):543–45. doi: 10.1007/s11606-020-05872-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Slocum S, Ozga JE, Joyce R, et al. If we build it, will they come? Perspectives on pharmacy-based naloxone among family and friends of people who use opioids: A mixed methods study. BMC Public Health 2022;22(1):735. doi: 10.1186/s12889-022-13078-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carre Z Zoe carre: Take home naloxone is not reaching those who most need it. In: BMJ Opinion, ed., 2019. [Google Scholar]
- 19.Binswanger IA, Koester S, Mueller SR, et al. Overdose education and naloxone for patients prescribed opioids in primary care: A qualitative study of primary care staff. J Gen Intern Med 2015;30(12):1837–44. doi: 10.1007/s11606-015-3394-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Winograd RP, Werner KB, Green L, et al. Concerns that an opioid antidote could “make things worse”: Profiles of risk compensation beliefs using the naloxone-related risk compensation beliefs (narrc-b) scale. Substance Abuse 2020;41(2):245–51. doi: 10.1080/08897077.2019.1616348 [DOI] [PubMed] [Google Scholar]
- 21.Winograd RP, Davis CS, Niculete M, et al. Medical providers’ knowledge and concerns about opioid overdose education and take-home naloxone rescue kits within veterans affairs health care medical treatment settings. Substance Abuse 2017;38(2):135–40. doi: 10.1080/08897077.2017.1303424 [DOI] [PubMed] [Google Scholar]
- 22.Mueller SR, Koester S, Glanz JM, et al. Attitudes toward naloxone prescribing in clinical settings: A qualitative study of patients prescribed high dose opioids for chronic non-cancer pain. J Gen Intern Med 2017;32(3):277–83. doi: 10.1007/s11606-016-3895-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wheeler E, Jones TS, Gilbert MK, Davidson PJ. Opioid overdose prevention programs providing naloxone to laypersons - United States, 2014. MMWR Morb Mortal Wkly Rep 2015;64(23):631–5. [PMC free article] [PubMed] [Google Scholar]
- 24.Davis CS, Walley AY, Bridger CM. Lessons learned from the expansion of naloxone access in Massachusetts and North Carolina. The Journal of Law, Medicine & Ethics 2015;43(1_suppl):19–22. doi: 10.1111/jlme.12208 [DOI] [PubMed] [Google Scholar]
- 25.The Network for Public Health Law. Opioid misuse and overdose prevention: 50-state survey. 2021. Updated February 2021. Available from: https://www.networkforphl.org/wp-content/uploads/2021/02/50-State-Survey-Legal-Interventions-to-Reduce-Overdose-Mortality-Naloxone-Access-Laws.pdf Accessed April 4, 2022.
- 26.Mercer F, Parkes T, Foster R, et al. Patient, family members and community pharmacists’ views of a proposed overdose prevention intervention delivered in community pharmacies for patients prescribed high-strength opioids for chronic non-cancer pain: An explorative intervention development study. Drug Alcohol Rev 2023;42(3):517–26. doi: 10.1111/dar.13554 [DOI] [PubMed] [Google Scholar]
- 27.Evoy KE, Hill LG, Groff L, et al. Naloxone accessibility without a prescriber encounter under standing orders at community pharmacy chains in Texas. JAMA 2018;320(18):1934–37. doi: 10.1001/jama.2018.15892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thompson EL, Rao PSS, Hayes C, Purtill C. Dispensing naloxone without a prescription: Survey evaluation of Ohio pharmacists. J Pharm Pract 2019;32(4):412–21. doi: 10.1177/0897190018759225 [DOI] [PubMed] [Google Scholar]
- 29.Bessen S, Metcalf SA, Saunders EC, et al. Barriers to naloxone use and acceptance among opioid users, first responders, and emergency department providers in New Hampshire, USA. Int J Drug Policy 2019;74:144–51. doi: 10.1016/j.drugpo.2019.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Green TC, Case P, Fiske H, et al. Perpetuating stigma or reducing risk? Perspectives from naloxone consumers and pharmacists on pharmacy-based naloxone in 2 states. J Am Pharm Assoc (2003) 2017;57(2s):S19–S27.e4. doi: 10.1016/j.japh.2017.01.013 [DOI] [PubMed] [Google Scholar]
- 31.Binswanger IA, Rinehart D, Mueller SR, et al. Naloxone co-dispensing with opioids: A cluster randomized pragmatic trial. J Gen Intern Med 2022. doi: 10.1007/s11606-021-07356-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Evoy KE, Groff L, Hill LG, et al. Impact of student pharmacist-led naloxone academic detailing at community pharmacies in Texas. J Am Pharm Assoc (2003) 2020;60(1):81–86. doi: 10.1016/j.japh.2019.09.007 [DOI] [PubMed] [Google Scholar]
- 33.Evoy KE, Hill LG, Davis CS. Considering the potential benefits of over-the-counter naloxone. Integr Pharm Res Pract 2021;10:13–21. doi: 10.2147/IPRP.S244709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peet ED, Powell D, Pacula RL. Trends in out-of-pocket costs for naloxone by drug brand and payer in the US, 2010–2018. JAMA Health Forum 2022;3(8):e222663. doi: 10.1001/jamahealthforum.2022.2663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu DT, Tamang S, Humphreys K. Promises and perils of the FDA’s over-the-counter naloxone reclassification. Lancet Reg Health Am 2023;23:100518. doi: 10.1016/j.lana.2023.100518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davis AC, Voelkel JL, Remmers CL, et al. Comparing Kaiser Permanente members to the general population: Implications for generalizability of research. Perm J 2023;27(2):87–98. doi: 10.7812/tpp/22.172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dispense supply of emer drugs for overdose victims, SB15–053 (Colo 2015) April 6, 2016. Accessed August 26, 2023. Https://statebillinfo.Com/bills/fiscal/15/sb053_f1.Pdf.
- 38.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42(2):377–81. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform 2019;95:103208. doi: 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mummah SA, Robinson TN, King AC, et al. Ideas (integrate, design, assess, and share): A framework and toolkit of strategies for the development of more effective digital interventions to change health behavior. J Med Internet Res 2016;18(12):e317. doi: 10.2196/jmir.5927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Montaño DE, D K. Theory of reasoned action, theory of planned behavior, and the integrated behavioral model, chapter 4. In: Glanz K, Rimer BK, Lewis FM, eds. Health behavior and health education: Theory, research, and practice. 4th ed. San Francisco: Jossey-Bass; 2008. [Google Scholar]
- 42.Champion VL, CS S. The health belief model, chapter 3. In: Glanz K, Rimer BK, Lewis FM, eds. Health behavior and health education: Theory, research, and practice. 4th ed. San Francisco: Jossey-Bass; 2008. [Google Scholar]
- 43.Bagri G, Jones GV. The role of first person perspective and vivid imagery in memory for written narratives. Educational Psychology in Practice 2018;34(3):229–44. doi: 10.1080/02667363.2018.1431522 [DOI] [Google Scholar]
- 44.Hoeken H, Van Vliet M. Suspense, curiosity, and surprise: How discourse structure influences the affective and cognitive processing of a story. Poetics 2000;27(4):277–86. [Google Scholar]
- 45.Vyond Studio. Goanimate, inc. [Computer Software]. San Mateo, California, USA; Available from https://vyond.com, 2017. [Google Scholar]
- 46.Adobe. Adobe Audition CC 2017 (Version 10) [Computer Software]. San Jose, California, USA; Available from https://adobe.com/products/audition.html, 2016. [Google Scholar]
- 47.Centers for Disease Control and Prevention (CDC). Behavioral risk factor surveillance system survey questionnaire. Atlanta, georgia: U.S. Department of Health and Human Services, centers for disease control and prevention,. 2014 [Google Scholar]
- 48.Larance B, Bruno R, Lintzeris N, et al. Development of a brief tool for monitoring aberrant behaviours among patients receiving long-term opioid therapy: The opioid-related behaviours in treatment (orbit) scale. Drug Alcohol Depend 2016;159:42–52. doi: 10.1016/j.drugalcdep.2015.11.026 [DOI] [PubMed] [Google Scholar]
- 49.Smith SM, Paillard F, McKeown A, et al. Instruments to identify prescription medication misuse, abuse, and related events in clinical trials: An acttion systematic review. The Journal of Pain 2015;16(5):389–411. doi: 10.1016/j.jpain.2015.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Campbell G, Noghrehchi F, Nielsen S, et al. Risk factors for indicators of opioid-related harms amongst people living with chronic non-cancer pain: Findings from a 5-year prospective cohort study. EClinicalMedicine 2020;28:100592. doi: 10.1016/j.eclinm.2020.100592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shoup JA, Mueller SR, Binswanger IA, et al. Modifying and evaluating the opioid overdose knowledge scale for prescription opioids: A pilot study of the rx-ooks. Pain Med 2020;21(10):2244–52. doi: 10.1093/pm/pnaa190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McNeely J, Strauss SM, Wright S, et al. Test-retest reliability of a self-administered alcohol, smoking and substance involvement screening test (ASSIST) in primary care patients. J Subst Abuse Treat 2014;47(1):93–101. doi: 10.1016/j.jsat.2014.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Humeniuk R, Ali R, Babor TF, et al. Validation of the alcohol, smoking and substance involvement screening test (ASSIST). Addiction 2008;103(6):1039–47. doi: 10.1111/j.1360-0443.2007.02114.x [DOI] [PubMed] [Google Scholar]
- 54.Harris AH, Bradley KA, Bowe T, et al. Associations between audit-c and mortality vary by age and sex. Popul Health Manag 2010;13(5):263–8. doi: 10.1089/pop.2009.0060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bruzelius E, Cerdá M, Davis CS, et al. Naloxone expansion is not associated with increases in adolescent heroin use and injection drug use: Evidence from 44 US states. International Journal of Drug Policy 2023;114:103980. doi: 10.1016/j.drugpo.2023.103980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tse WC, Djordjevic F, Borja V, et al. Does naloxone provision lead to increased substance use? A systematic review to assess if there is evidence of a ‘moral hazard’ associated with naloxone supply. International Journal of Drug Policy 2022;100:103513. doi: 10.1016/j.drugpo.2021.103513 [DOI] [PubMed] [Google Scholar]
- 57.Bohnert AS, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA 2011;305(13):1315–21. doi: 305/13/1315 [pii] 10.1001/jama.2011.370 [DOI] [PubMed] [Google Scholar]
- 58.Nielsen S, Scott N, Tidhar T, et al. The cost and impact of distributing naloxone to people who are prescribed opioids to prevent opioid-related deaths: Findings from a modelling study. Addiction 2022;117(4):1009–19. doi: 10.1111/add.15727 [DOI] [PubMed] [Google Scholar]
- 59.Dowell D, Ragan KR, Jones CM, et al. CDC clinical practice guideline for prescribing opioids for pain - United States, 2022. MMWR Recomm Rep 2022;71(3):1–95. doi: 10.15585/mmwr.rr7103a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.World Health Organization. Opioid overdose. 2023. Available from: https://www.who.int/news-room/fact-sheets/detail/opioid-overdose Accessed September 21, 2023.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Investigators interested in using the data from this study must submit a written request. Requests must address regulatory and compliance requirements for data usage. Investigators must outline the necessary resources required to support their data request, including personnel and infrastructure. The original study team will evaluate each request based on scientific merit, ethical considerations, and alignment with the original study’s objectives.
Deidentified survey and electronic health record (EHR) data will be shared with investigators upon approval of written request. Investigators are responsible for obtaining necessary approvals and adhering to relevant laws and regulations and will be required to sign a Data Use Agreement (DUA) outlining the terms and conditions of data usage. The data sharing period is limited to three years following the publication of the primary study. The Naloxone Navigator video is available upon request; please send an email to: jason.m.glanz@kp.org. This Data Availability Statement is reflected in the Individual Participant Data (IPD) Sharing Statement of the clinicalTrials.gov registration (ClinicalTrials.gov number NCT03337009).


