Abstract
Egress of four important alphaherpesviruses, equine herpesvirus 1 (EHV-1), herpes simplex virus type 1 (HSV-1), infectious laryngotracheitis virus (ILTV), and pseudorabies virus (PrV), was investigated by electron microscopy of infected cell lines of different origins. In all virus-cell systems analyzed, similar observations were made concerning the different stages of virion morphogenesis. After intranuclear assembly, nucleocapsids bud at the inner leaflet of the nuclear membrane, resulting in enveloped particles in the perinuclear space that contain a sharply bordered rim of tegument and a smooth envelope surface. Egress from the perinuclear cisterna primarily occurs by fusion of the primary envelope with the outer leaflet of the nuclear membrane, which has been visualized for HSV-1 and EHV-1 for the first time. The resulting intracytoplasmic naked nucleocapsids are enveloped at membranes of the trans-Golgi network (TGN), as shown by immunogold labeling with a TGN-specific antiserum. Virions containing their final envelope differ in morphology from particles within the perinuclear cisterna by visible surface projections and a diffuse tegument. Particularly striking was the addition of a large amount of tegument material to ILTV capsids in the cytoplasm. Extracellular virions were morphologically identical to virions within Golgi-derived vesicles, but distinct from virions in the perinuclear space. Studies with gB- and gH-deleted PrV mutants indicated that these two glycoproteins, which are essential for virus entry and direct cell-to-cell spread, are dispensable for egress. Taken together, our studies indicate that the deenvelopment-reenvelopment process of herpesvirus maturation also occurs in EHV-1, HSV-1, and ILTV and that membrane fusion processes occurring during egress are substantially different from those during entry and direct viral cell-to-cell spread.
Herpesviruses are large DNA-containing enveloped viruses that replicate in the nuclei of infected cells. Based on biological parameters and sequence data, the family Herpesviridae is divided into the subfamilies Alpha-, Beta-, and Gammaherpesvirinae (58). Despite their biological diversity, many steps of herpesvirus morphogenesis seem to be conserved. Attached herpesvirus virions penetrate the cell membrane by direct fusion between the viral envelope and the plasma membrane, and the deenveloped nucleocapsids reach the nucleopores by movement along cellular microtubuli, where the genomic DNA is released into the nucleus (19, 50, 51, 52). Progeny nucleocapsids assemble in the nucleus and exit this compartment by budding at the inner nuclear membrane into the perinuclear space (35, 47). The subsequent events of envelopment and egress remain controversial. Whereas for herpes simplex virus type 1 (HSV-1), a model that entails vesicular transport of enveloped virions through the secretory pathway with concomitant in situ modification of virion glycoproteins was initially proposed, for varicella-zoster virus (VZV), pseudorabies virus (PrV), and human cytomegalovirus (HCMV), a pathway involving deenvelopment of perinuclear virions by fusion at the outer leaflet of the perinuclear cisterna and secondary envelopment of intracytoplasmic capsids in the trans-Golgi area has been described (2, 6, 10, 11, 15, 19, 24, 25, 43, 49, 59, 61). Recent biochemical studies of HSV-1 were also consistent with a deenvelopment-reenvelopment pathway (3, 60). The majority of reports on the ultrastructure of herpesvirus replication concentrate on the human alphaherpesviruses HSV-1, HSV-2, and VZV and the porcine pathogen PrV (5, 8, 12, 14, 15, 19, 21, 22, 30, 31, 47, 51, 57, 59). In addition, morphogenesis of the betaherpesvirus HCMV has been studied in some detail (16, 17, 42, 43, 55, 56). In contrast, morphogenesis of many important animal pathogens, such as gallid herpesvirus 1 (infectious laryngotracheitis virus [ILTV]) and equine herpesvirus 1 (EHV-1), has only incompletely been analyzed ultrastructurally. In particular, no comprehensive comparative analysis has been performed under conditions as identical as possible for the different viruses. Therefore, in our electron microscopy (EM) study, different steps of replication of EHV-1, HSV-1, ILTV, and PrV were analyzed in cell culture, focusing on the events during virus egress.
MATERIALS AND METHODS
Cells and viruses.
The pathogenic wild-type EHV-1 strain Rac L11 was propagated on RK13 or Edmin337 cells (38). Cells were infected at a multiplicity of infection (MOI) of 0.5 to 1 and incubated at 37°C. HSV-1 strain HFEM was generously provided by A. Minson, University of Cambridge, Cambridge, United Kingdom. African green monkey kidney (Vero), BHK21, and Hep-2 cells were infected with an MOI of 0.1 and incubated at 37°C. PrV strain Kaplan (26) was propagated as previously described (19, 37). The in vitro and in vivo growth properties of the pathogenic ILTV strain A489 (kindly provided by D. Lütticken, Intervet International, Boxmeer, The Netherlands) have been reported previously (13). A chicken hepatoma cell line, LMH (27) (obtained from D. N. Tripathy, University of Illinois, Urbana), was propagated in minimum essential medium supplemented with 10% fetal calf serum. Confluent cell monolayers were infected with ILTV at an MOI of 1. After 2 h at 37°C, the inoculum was replaced by fresh medium that contained only 5% fetal calf serum. gB and gH deletion mutants of PrV were propagated on complementing cells as described previously (1, 37). For EM, noncomplementing cells were infected with transcomplemented virions at an MOI of 1 and analyzed 16 h postinfection (p.i.).
EM.
For routine EM, noninfected and infected cell cultures in petri dishes or T75 culture flasks (Costar) were fixed at different times after infection (8, 12, 14, 16, or 18 h p.i.) for 60 min with 2.5% glutaraldehyde buffered in 0.1 M Na-cacodylate (300 mosmol) (pH 7.2) (Merck, Darmstadt, Germany). They were then scraped off the plate, pelleted by low-speed centrifugation, and embedded in LMP agarose (Biozym, Oldendorf, Germany). Small pieces were postfixed in 1.0% aqueous OsO4 (Polysciences Europe, Eppelheim, Germany) and stained with uranyl acetate. After stepwise dehydration in ethanol, cells were cleared in propylene oxide, embedded in glycid ether 100 (Serva, Heidelberg, Germany) and polymerized at 59°C for 4 days. For intracellular labeling of the trans-Golgi network (TGN) protein 38 (44), uninfected porcine kidney (PSEK) cells and wild-type PrV-infected PSEK cells were fixed with 0.5% glutaraldehyde in phosphate-buffered saline (PBS) (pH 7.2) for 30 min, embedded in LMP agarose (Biozym), and postfixed in the fixative described above for 30 min. Thereafter, samples were blocked with 0.5 M NH4Cl in PBS for 60 min, washed in PBS, stained in 0.5% aqueous uranyl acetate for 15 min, dehydrated in ethanol with a progressive decrease in temperature, embedded in the acrylic resin Lowicryl K4M (Lowi, Waldkraiburg, Germany) at −35°C, and polymerized by UV light (λ, 360 nm) (9).
Postembedding labeling of ultrathin sections was performed after blocking of surfaces with 1% cold water–fish gelatin, 0.02 M glycine, 1% fraction V bovine serum albumin (BSA; Sigma, Deisenhofen, Germany) in PBS, by either overnight incubation at 4°C or 2 h of incubation at room temperature with anti-rat TGN38 serum (44) diluted in PBS-BSA. The antiserum cross-reacts with TGN38 homologous proteins in numerous other species, including pigs (G. Banting, personal communication). Diluted 10-nm-diameter gold particle-tagged goat anti-rabbit antibodies or gold particle-tagged protein A (GAR10 or PAG10; British BioCell, Intl., Cambridge, United Kingdom)-labeled antibodies were added for 60 min at room temperature, and excess antibodies were removed by washing. The specificity of the reaction was controlled on uninfected and infected PSEK cells by using gold conjugate without primary antibody and by using non-TGN protein-specific antibodies (anti-Newcastle disease virus antibodies; data not shown).
Ultrathin sections of conventionally embedded material and labeled Lowicryl sections, counterstained with uranyl acetate and lead salts, were examined with an electron microscope (EM 400 T; Philips, Eindhoven, The Netherlands).
RESULTS
Egress of capsids from the nucleus.
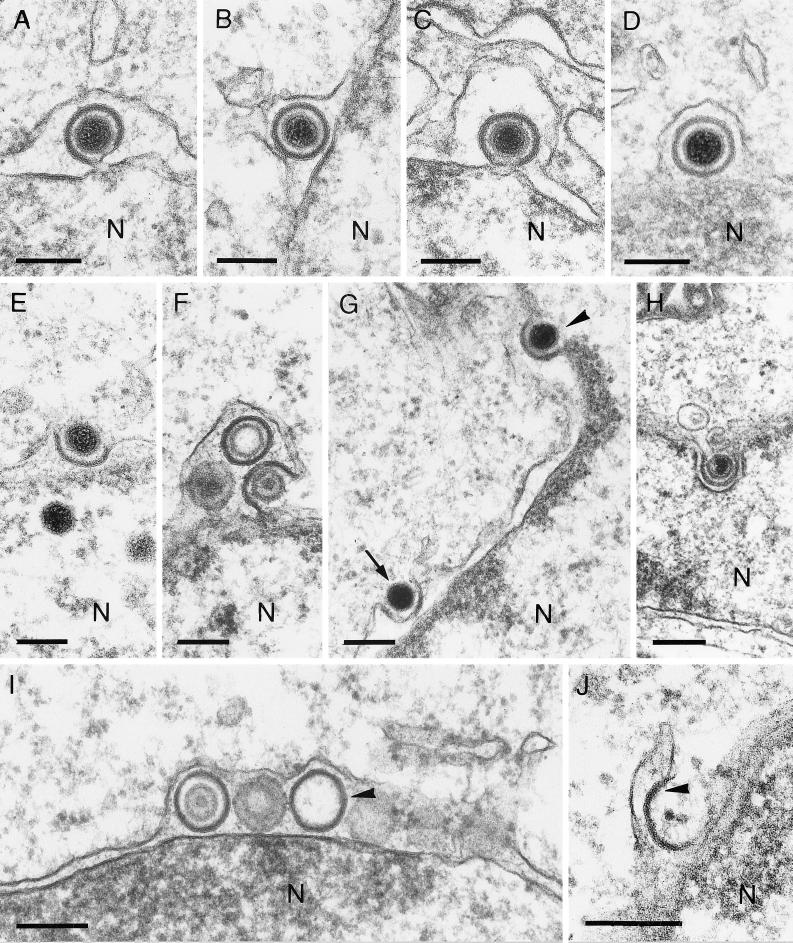
Intranuclear capsids were found to exit from the nucleus into the cytoplasm by budding through the inner leaflet of the nuclear membrane into the perinuclear cisterna. First, these nucleocapsids, as well as occasionally empty capsids, were observed in intimate contact with the inner nuclear membrane, accompanied by the appearance of a sharply bordered rim of electron-dense material between the nucleocapsid and the membrane at the budding site. No surface projections could be detected at the envelope of intracisternal virions. These particles with a smooth envelope were of nearly identical sizes in all viruses analyzed and were characterized by a clear halo between the sharply bordered rim of primary tegument and the nucleocapsid (Fig. 1A to D [see Fig. 5A to D]). Sporadically, vesicles that probably originated from budding of condensed tegument into the perinuclear cisterna without capsids were observed (Fig. 1I).
FIG. 1.
Exit of nucleocapsids from the nucleus. Enveloped EHV-1 (A), HSV-1 (B), ILTV (C), and PrV (D) particles in the perinuclear cisterna are shown. Release of EHV-1 (E), HSV-1 (F), and PrV (H) from the nuclear cisterna results from direct fusion. Simultaneous observation of primary envelopment (arrowhead) and deenvelopment (arrow) of HSV-1 particles is demonstrated in panel G. Capsidless HSV-1 particles (arrowhead) in the perinuclear cisterna (I) and fused with the outer lamella of the nuclear membrane (J [arrowhead]) were also observed. The bars represent 150 nm in panels A to F and H and 200 nm in panels G and I. N designates the nucleus.
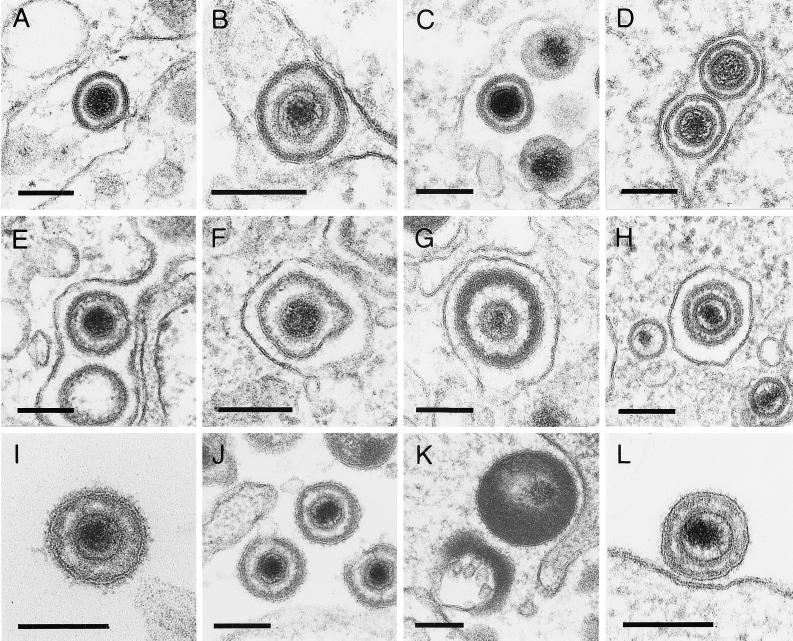
FIG. 5.
Morphology of virus particles. Virions in the perinuclear cisterna after primary envelopment: EHV-1 (A), HSV-1 (B), ILTV (C), and PrV (D); virions in Golgi vesicles after secondary envelopment: EHV-1 (E), HSV-1 (F), ILTV (G), and PrV (H); extracellular virions after virus egress: EHV-1 (I), HSV-1 (J), ILTV (K), and PrV (L). Bars, 150 nm.
Capsids were released from the perinuclear space by fusion of the primary envelope with the outer leaflet of the perinuclear cisterna as previously shown for PrV (Fig. 1H) (19) and demonstrated here for the first time for EHV-1 (Fig. 1E) and HSV-1 (Fig. 1F and G). In Fig. 1G, primary envelopment and deenvelopment of HSV-1 were observed adjacent in a single thin section. Occasionally, capsidless intracisternal L-particles were also found to fuse with the outer cytoplasmic leaflet of the perinuclear cisterna (Fig. 1J). In rare cases, intracisternal capsids were also found to be released after vesiculation at the outer leaflet followed by fusion of the particle envelope with the vesicle membrane in the vicinity of the Golgi apparatus (19; data not shown).
Secondary envelopment and virus egress.
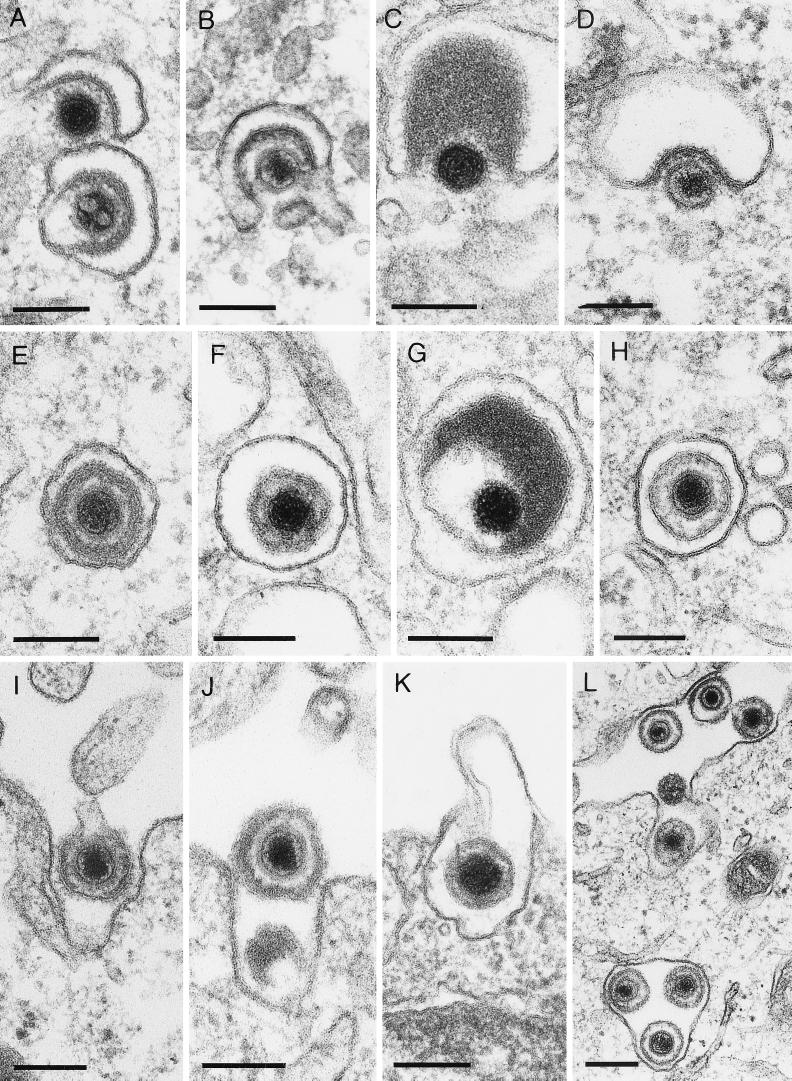
Deenveloped intracytoplasmic nucleocapsids were detected in the Golgi area adjacent to or partially wrapped by membranes, as shown for EHV-1 (Fig. 2A), HSV-1 (Fig. 2B and 3A), ILTV (Fig. 2C), and PrV (Fig. 2D) (19). This secondary envelopment process resulted in intravesicular enveloped virus particles (Fig. 2E to H). In ILTV, the addition of enormous amounts of tegument material (Fig. 2C and G and 3B) was particularly noteworthy. This excess tegument was also present in extracellular virions (Fig. 5K), but was never observed in perinuclear virus particles (Fig. 5C). Labeling of PrV-infected porcine kidney cells with a TGN-specific antiserum directed against the cytosolic domain of rat TGN38 (44), which cross-reacts with the porcine homolog, demonstrated that the subcellular compartment in which secondary envelopment occurred was the TGN (Fig. 4). Enveloped virus particles resulting from this budding process through TGN-derived membranes (Fig. 5E to G) were morphologically distinguishable from virions after primary envelopment at the inner nuclear membrane (Fig. 5A to D) in all viruses analyzed. The main difference was the presence of visible surface projections, which were only observed on the envelope of virions after secondary budding (Fig. 5E to H). Furthermore, mature particles frequently exhibited a spherical shape with various diameters. In EHV-1, HSV-1, and PrV particles, the tegument showed a lower electron density and not the sharply bordered arrangement observed in virions after primary envelopment. Thus, mature intravesicular EHV-1, HSV-1, and PrV particles were characterized by a slightly varied diameter, distinct surface projections, and a diffuse tegument (Fig. 5E to G). Because of the incorporation of highly variable amounts of tegument into ILTV virions, the shapes and diameters of the resulting virus particles were irregular, and the position of the nucleocapsid inside the virion frequently was strikingly eccentric (Fig. 2C and G and 5K), in contrast to the situation observed in the other viruses.
FIG. 2.
Secondary envelopment and virus egress. Envelopment of EHV-1 (A), HSV-1 (B), ILTV (C), and PrV (D) at membranes of the trans-Golgi area. Particles of EHV-1 (E), HSV-1 (F), ILTV (G), and PrV (H) within Golgi vesicles are shown, as is egress of EHV-1 (I), HSV-1 (J), ILTV (K), and PrV (L) by fusion of vesicles with the plasma membrane. The bars represent 150 nm in panels A to K and 250 nm in panel L.
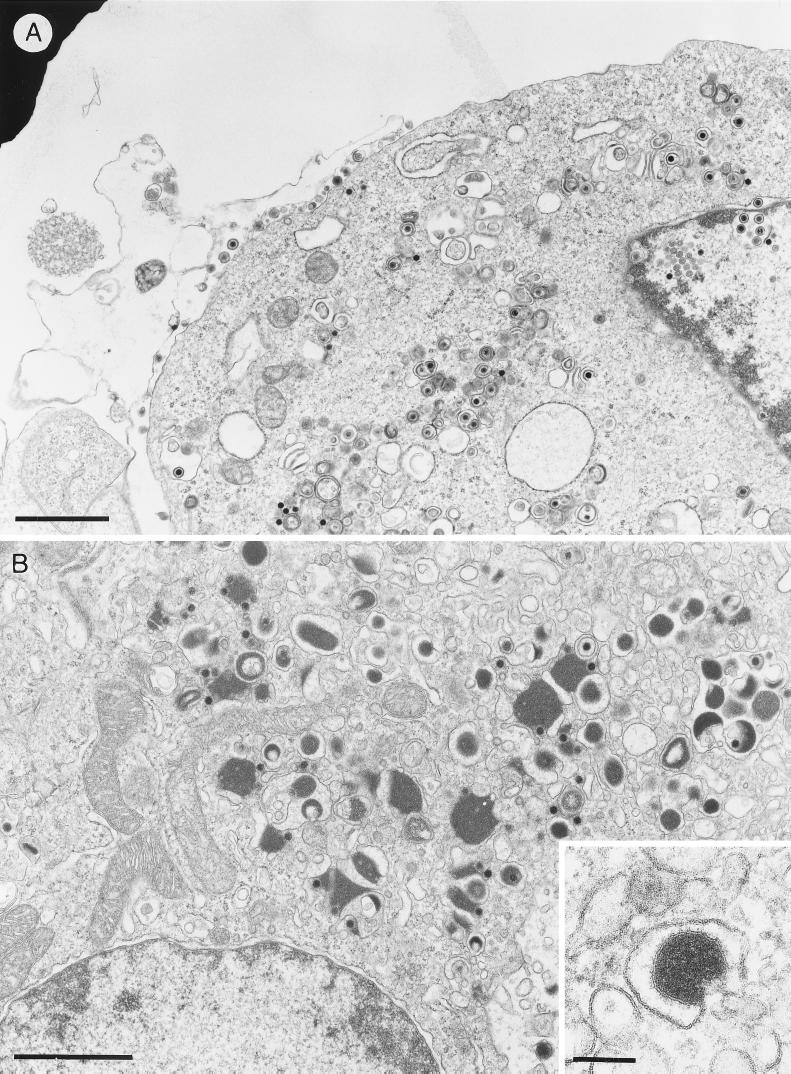
FIG. 3.
Overview of Vero cells infected with HSV-1 (A) and LMH cells infected with ILTV (B). The inset shows the formation of an ILTV L-particle. The bars represent 1.5 μm in panels A and B and 150 nm in the panel B inset.
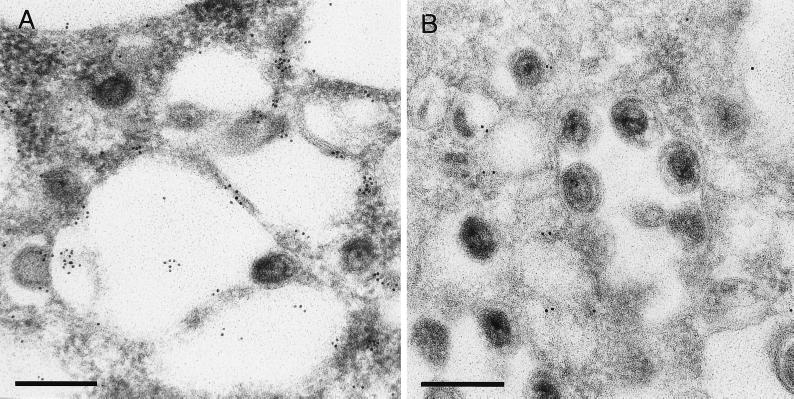
FIG. 4.
Immunolabeling of TGN38 protein. Ultrathin sections of Lowicryl-embedded PrV-infected porcine kidney cells were processed as described in Materials and Methods and incubated with two different dilutions (A, 1:100; B, 1:1,000) of anti-TGN38 serum (44) followed by 10-nm-diameter gold particle-tagged secondary antibodies. The bars represent 250 nm.
In all cells producing EHV-1, HSV-1, ILTV, or PrV, formation of virus particles lacking nucleocapsids (L-particles), covered by distinct surface projections, was regularly observed, but it appeared to be most striking in ILTV, due to the packaging of sometimes enormous amounts of tegument (Fig. 3B) compared to, e.g., HSV-1 infection (Fig. 3A). After secondary envelopment, either single mature virions or L-particles were located in Golgi vesicles (Fig. 2E to H), or virions accumulated in larger vacuoles (not shown). Egress of virus progeny occurred by exocytosis of virus-containing vesicles in all viruses analyzed (Fig. 2I to L).
In summary, for all viruses analyzed, enveloped particles inside the perinuclear cisterna clearly differed in ultrastructure from secondarily enveloped virions inside Golgi-derived vesicles and released extracellular virions. In contrast, virions inside Golgi vesicles and extracellular virions were morphologically indistinguishable (Fig. 5).
Glycoprotein requirements for virus egress.
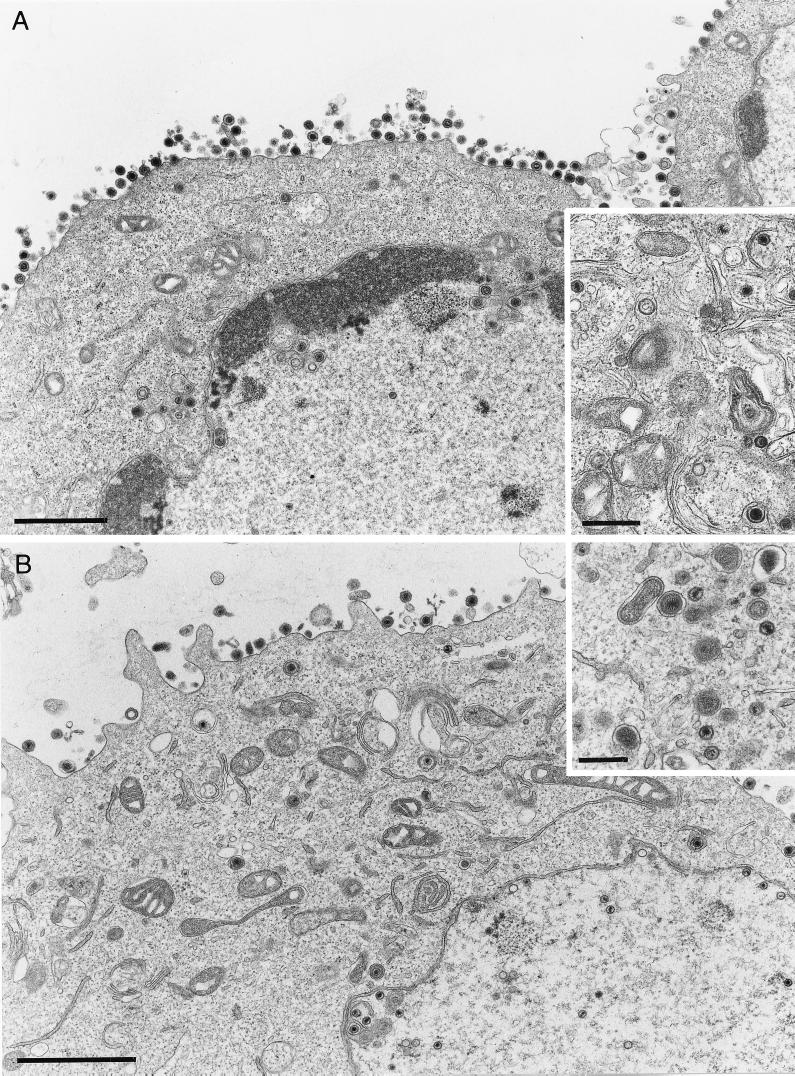
Fusion of the envelope of extracellular virus particles with the cell membrane is one of the initiating events of herpesvirus infection. For this process, the presence of gB and gH has invariably been shown to be necessary (51), whereas the absence of gD and gL can be tolerated in the presence of compensatory mutations (28, 48, 49). Moreover, gB and gH are strictly required for direct viral cell-to-cell spread and for membrane fusion after transient expression (29, 33). Since the egress of perinuclear virions into the cytoplasm requires fusion of the primary envelope with the outer leaflet of the nuclear membrane, we analyzed whether gB- or gH-deleted virus mutants are defective at this step in virion morphogenesis. To this end, noncomplementing RK13 cells were infected with transcomplemented gB (PrV-gB−) or gH deletion mutants (PrV-gH−) of PrV. As shown in Fig. 6, all stages of virion morphogenesis were observed irrespective of the absence of gB (Fig. 6A) or gH (Fig. 6B). These included budding into the perinuclear space, the presence of intracytoplasmic nucleocapsids, and observation of numerous apparently fully enveloped virus particles outside the cell. Thus, we conclude that fusion during egress is effected by mechanisms fundamentally different from fusion during entry or direct cell-to-cell spread.
FIG. 6.
Ultrastructure of cells infected with gB or gH deletion mutants of PrV. Noncomplementing RK13 cells were infected with PrV-gB− (A) or PrV-gH− (B) at an MOI of 1 and analyzed 16 h after infection. All stages of virus maturation, including numerous extracellular virus particles, can be observed. The bars represent 1.5 μm in panels A and B and 500 nm in the insets.
DISCUSSION
In this study, egress of EHV-1, HSV-1, and ILTV was analyzed by EM and compared with previous results with PrV (19) to obtain a comprehensive picture of similarities and differences in alphaherpesvirus replication. Although cell lines of different origins (porcine, equine, chicken, and primate) were analyzed, no cell-specific effects on the ultrastructure of virus morphogenesis were detected; rather, morphogenesis generally proved to be very similar. Exit of capsids from the nucleus started by budding at the inner nuclear membrane, resulting in acquisition of a primary envelope. This event was observed for each of the four virus species investigated. Since virions apparently did not accumulate in the perinuclear cisterna, we assume that the passage through the two leaflets of the nucleus is a rapid process. Comparison of micrographs of the four viruses analyzed showed that, under identical preparative conditions, all virus particles within the perinuclear cisterna exhibit the same morphology. This is an important observation, since different preparative conditions may affect virus morphology and therefore falsify the results (35, 41). The centrally located nucleocapsid is surrounded by an electron-lucent halo, a sharply bordered rim of uniform thickness of primary tegument and a smooth envelope without surface projections. In addition, primarily enveloped virions exhibit a uniform diameter. Surprisingly, budding of tegument material into the perinuclear cisterna without associated capsids was also detected, resulting in perinuclear L-particles. This indicates that the presence of capsids is not a prerequisite for budding.
The following steps of maturation of virions located in the perinuclear cisterna are still controversial. One model, developed for HSV-1, proposes a single budding event of nucleocapsids at the inner leaflet of the nuclear membrane, followed by virion transport inside vesicles through the secretory pathway to the Golgi complex and exocytosis at the cell surface (6, 7, 11, 24, 47). Another model proposes deenvelopment of virions during transit through the outer leaflet of the perinuclear membrane and a secondary envelopment step in the trans-Golgi region. The latter model is primarily supported by results with Epstein-Barr virus, PrV, VZV, and HCMV (8, 15, 18, 19, 25, 34, 36, 43, 59, 61). Recently, analyses using mutated HSV-1 glycoproteins also yielded results that were congruent with the deenvelopment-reenvelopment model (3, 60).
Micrographs from areas near the perinuclear cisterna indicated that all viruses studied exit the nucleus primarily by deenvelopment at the cisterna. We are aware of the fact that static electron micrographs do not prove the directionality of the observed processes. However, in this report, we show for the first time fusion stages of EHV-1 and HSV-1 capsids with the outer leaflet of the nuclear membrane, which parallels previous findings with PrV (19). These fusion processes are essential for the deenvelopment-reenvelopment model, and their observation lends further support for this pathway of alphaherpesvirus egress. Only occasionally does vesiculation seem to occur. Secondary envelopment of released nucleocapsids occurred at membranes of the trans-Golgi area, as shown by immunolabeling of the TGN-resident protein 38. Accumulations of enveloped virions in structures resembling endosomes or lysosomes, as described for HSV-1 (4), were not observed. Moreover, the ultrastructure of virions in Golgi-derived vesicles differed from that of particles in the perinuclear cisterna. The most prominent differences were the enlarged and less dense tegument and the presence of surface projections at the envelope, which resembled the projections seen on mature extracellular virions. Ultrastructural differences between particles in Golgi vesicles and the perinuclear cisterna were previously described for ILTV (20) and for the betaherpesvirus human herpesvirus 6 (46).
The most striking difference between the virus species studied was the large amount of tegument incorporated into ILT virions during secondary envelopment. This excess tegument material caused the virions to have varied diameters and irregular shapes. Since these heavily tegumented virions comprise the majority of extracellular capsid-containing ILTV particles, this finding demonstrates that final tegumentation and envelopment occur at the TGN. To summarize, our data on EHV-1, HSV-1, and ILTV are consistent with results published for PrV and VZV and support a primary envelopment-deenvelopment-secondary envelopment model for all alphaherpesviruses.
A notable but poorly understood phenomenon is the formation of tegument containing particles without capsids or nucleocapsids, designated as dense or L-particles, which had previously been described for EHV-1, HSV-1, PrV, and HCMV (23, 32, 45, 53–56). Our analysis demonstrated the existence of two forms of capsidless particles. One of them was localized within the perinuclear cisterna, and the other was present within Golgi-derived vesicles or vacuoles or was at the cell surface. Therefore, it appears likely that the interaction between viral tegument and membrane proteins without a need for the presence of capsids is sufficient for the intranuclear and intracytoplasmic budding processes.
In HSV-1, fusion of virion envelope and cell membrane during penetration requires the presence of four viral glycoproteins, namely, gB, gD, gH, and gL (51). For PrV, similar requirements for this fusion process were reported. However, compensatory mutations may render gD and gL dispensable for this process in PrV, which, as known today, leaves only gB and gH as strictly necessary for membrane fusion. This correlates with results from in vitro fusion assays after transient expression of PrV glycoproteins, which indicated that expression of gB and gH resulted in membrane fusion, in particular when a gB mutant with an increased fusogenic potential had been used (29). Therefore, it was of interest to analyze whether these glycoproteins are also required for membrane fusion during egress of virus particles from the perinuclear space. Our data show that PrV mutants lacking either of the two glycoproteins still mature in a fashion morphologically indistinguishable from wild-type PrV, which proves that egress from the perinuclear space proceeds without these fusogenic glycoproteins and thus is distinct from other virus-induced membrane fusions. Concerning the results obtained with PrV-gH−, our data parallel those described previously by others (40). However, our findings on unimpaired virion morphogenesis in PrV-gB− contrast with previous reports that seemed to indicate that PrV-gB− virions accumulated in the perinuclear space, leading to the hypothesis that gB is required for virion exit from the nucleus (39). Our data show that gB is not required for egress. So far, the reason for these differences in observations is unclear, but it should be noted that different mutations were present in the virus mutants. Whereas Peeters et al. inserted a premature stop codon into the gB gene, but otherwise retained all coding sequences for gB (39), we used a gB deletion mutant that lacks most of the gB open reading frame (37).
In summary, this report provides the first comprehensive comparative ultrastructural analysis of the egress of four different alphaherpesviruses. Extensive electron microscopical analysis of infected cells, prepared by identical methods and under identical conditions, yielded comparable results between the viruses in terms of morphogenetic steps in cultured cells. The main stages of the egress process of EHV-1, HSV-1, ILTV, and PrV, as visualized by electron microscopy, were similar between the different virus species. Further studies will be aimed at understanding in detail the molecular mechanisms involved in egress and the elucidation of common functions of the participating viral gene products.
ACKNOWLEDGMENTS
We are indebted to G. Banting for the generous gift of anti-TGN38 serum. We thank C. Möller and P. Meyer for excellent technical assistance in electron microscopical preparations and H. Stephan and E. Zorn for photographic assistance.
Parts of this study were supported by the Deutsche Forschungsgemeinschaft: grants Fu 395/1 (to W.F.), Me 854/4 (to T.C.M.), and Os 143/2 (to N.O.).
REFERENCES
- 1.Babic N, Klupp B G, Makoschey B, Karger A, Flamand A, Mettenleiter T C. Glycoprotein gH of pseudorabies virus is essential for penetration and propagation in cell culture and in the nervous system of mice. J Gen Virol. 1996;77:2277–2285. doi: 10.1099/0022-1317-77-9-2277. [DOI] [PubMed] [Google Scholar]
- 2.Brack A R, Dijkstra J M, Granzow H, Klupp B G, Mettenleiter T C. Inhibition of virion maturation by simultaneous deletion of glycoproteins E, I, and M of pseudorabies virus. J Virol. 1999;73:5364–5372. doi: 10.1128/jvi.73.7.5364-5372.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Browne H, Bell S, Minson T, Wilson D W. An endoplasmic reticulum-retained herpes simplex virus glycoprotein H is absent from secreted virions: evidence for reenvelopment during egress. J Virol. 1996;70:4311–4316. doi: 10.1128/jvi.70.7.4311-4316.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brunetti C R, Dingwell K S, Wale C, Graham F L, Johnson D C. Herpes simplex virus gD and virions accumulate in endosomes by mannose 6-phosphate-dependent and -independent mechanisms. J Virol. 1998;72:3330–3339. doi: 10.1128/jvi.72.4.3330-3339.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campadelli-Fiume G, Arsenakis M, Farabegoli F, Roizman B. Entry of herpes simplex virus in BJ cells that constitutively express viral glycoprotein D is by endocytosis and results in degradation of the virus. J Virol. 1988;62:159–167. doi: 10.1128/jvi.62.1.159-167.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campadelli-Fiume G, Farabegoli F, Di Gaeta S, Roizman B. Origin of unenveloped capsids in the cytoplasm of cells infected with herpes simplex virus 1. J Virol. 1991;65:1589–1595. doi: 10.1128/jvi.65.3.1589-1595.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campadelli-Fiume G, Brandimarti R, DiLazzaro C, Ward P L, Roizman B, Torrisi M R. Fragmentation and dispersal of Golgi proteins and redistribution of glycoproteins and glycolipids processed through the Golgi apparatus after infection with herpes simplex virus 1. Proc Natl Acad Sci USA. 1993;90:2798–2802. doi: 10.1073/pnas.90.7.2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Card J P, Rinaman L, Lynn R B, Lee B-H, Meade R P, Miselis R R, Enquist L W. Pseudorabies virus infection of the rat nervous system: ultrastructural characterization of viral replication, transport, and pathogenesis. J Neurosci. 1993;13:2515–2539. doi: 10.1523/JNEUROSCI.13-06-02515.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carlemalm E, Garavito R M, Villiger W. Resin development for electron microscopy and an analysis of embedding at low temperature. J Microsc. 1982;126:123–143. doi: 10.1111/j.1365-2818.1982.tb00358.x. [DOI] [PubMed] [Google Scholar]
- 10.Darlington R W, Moss L H., III Herpesvirus envelopment. J Virol. 1968;2:48–55. doi: 10.1128/jvi.2.1.48-55.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DiLazzaro C, Campadelli-Fiume G, Torrisi M R. Intermediate forms of glycoconjugates are present in the envelope of herpes simplex virions during their transport along the exocytic pathway. Virology. 1995;214:619–623. doi: 10.1006/viro.1995.0073. [DOI] [PubMed] [Google Scholar]
- 12.Felluga B. Electron microscopic observations on pseudorabies virus development in a line of pig kidney cells. Ann Sclavo. 1963;5:412–424. [Google Scholar]
- 13.Fuchs W, Ziemann K, Teifke J P, Werner O, Mettenleiter T C. The nonessential UL50 gene of avian infectious laryngotracheitis virus encodes a functional dUTPase which is not a virulence factor. J Gen Virol. 2000;81:627–638. doi: 10.1099/0022-1317-81-3-627. [DOI] [PubMed] [Google Scholar]
- 14.Fuller O A, Lee W-C. Herpes simplex virus type 1 entry through a cascade of virus-cell interactions requires different roles of gD and gH in penetration. J Virol. 1992;66:5002–5012. doi: 10.1128/jvi.66.8.5002-5012.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gershon A A, Sherman D L, Zhu Z, Gabel C A, Ambron R T, Gershon M D. Intracellular transport of newly synthesized varicella-zoster virus: final envelopment in the trans-Golgi network. J Virol. 1994;68:6372–6390. doi: 10.1128/jvi.68.10.6372-6390.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gibson W. Molecular biology of human cytomegalovirus. In: Becker Y, Darai G, editors. Frontiers of virology. 2. Molecular aspects of human cytomegalovirus diseases. Heidelberg, Germany: Springer-Verlag; 1993. pp. 303–329. [Google Scholar]
- 17.Gibson W. Structure and assembly of the virion. Intervirology. 1996;39:389–400. doi: 10.1159/000150509. [DOI] [PubMed] [Google Scholar]
- 18.Gong M, Kieff E. Intracellular trafficking of two major Epstein-Barr virus glycoproteins, gp350/220 and gp110. J Virol. 1990;64:1507–1516. doi: 10.1128/jvi.64.4.1507-1516.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Granzow H, Weiland F, Jöns A, Klupp B G, Karger A, Mettenleiter T C. Ultrastructural analysis of the replication cycle of pseudorabies virus in cell culture: a reassessment. J Virol. 1997;71:2072–2082. doi: 10.1128/jvi.71.3.2072-2082.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo P, Scholz E, Turek J, Nodgreen R, Maloney B. Assembly pathway of avian infectious laryngotracheitis virus. Am J Vet Res. 1993;54:2031–2039. [PubMed] [Google Scholar]
- 21.Harson R, Grose C. Egress of varicella-zoster virus from the melanoma cell: a tropism for the melanocyte. J Virol. 1995;69:4994–5010. doi: 10.1128/jvi.69.8.4994-5010.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang A S, Wagner R R. Penetration of herpes simplex virus into human epidermoid cells. Proc Soc Exp Biol Med. 1966;116:863–869. doi: 10.3181/00379727-116-29392. [DOI] [PubMed] [Google Scholar]
- 23.Irmiere A, Gibson W. Isolation and characterization of a noninfectious virion-like particle released from cells infected with human strains of cytomegalovirus. Virology. 1983;130:118–133. doi: 10.1016/0042-6822(83)90122-8. [DOI] [PubMed] [Google Scholar]
- 24.Johnson D C, Spear P G. Monensin inhibits the processing of herpes simplex virus glycoproteins, their transport to the cell surface, and the egress of virions from infected cells. J Virol. 1982;43:1102–1112. doi: 10.1128/jvi.43.3.1102-1112.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones F, Grose C. Role of cytoplasmic vacuoles in varicella-zoster virus glycoprotein trafficking and virion envelopment. J Virol. 1988;62:2701–2711. doi: 10.1128/jvi.62.8.2701-2711.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaplan A S, Vatter A. A comparison of herpes simplex and pseudorabies virus. Virology. 1959;7:394–407. doi: 10.1016/0042-6822(59)90068-6. [DOI] [PubMed] [Google Scholar]
- 27.Kawaguchi T, Nomura K, Hirayama Y, Kitagawa T. Establishment and characterization of a chicken hepatocellular carcinoma cell line, LMH. Cancer Res. 1987;47:4460–4464. [PubMed] [Google Scholar]
- 28.Klupp B G, Mettenleiter T C. Glycoprotein L-independent infectivity of pseudorabies virus is mediated by a gD-gH fusion protein. J Virol. 1999;73:3014–3022. doi: 10.1128/jvi.73.4.3014-3022.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klupp B G, Nixdorf R, Mettenleiter T C. Pseudorabies virus glycoprotein M inhibits membrane fusion. J Virol. 2000;74:6760–6768. doi: 10.1128/jvi.74.15.6760-6768.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCracken R M, Clarke J K. A thin-section study of the morphogenesis of Aujeszky's disease virus in synchronously infected cell cultures. Arch Gesamte Virusforsch. 1971;34:189–210. doi: 10.1007/BF01242992. [DOI] [PubMed] [Google Scholar]
- 31.McCracken R M, Dow C. An electron microscopic study of Aujeszky's disease. Acta Neuropathol. 1973;25:207–219. doi: 10.1007/BF00685200. [DOI] [PubMed] [Google Scholar]
- 32.McLauchlan J, Rixon F J. Characterization of enveloped tegument structures (L particles) produced by alphaherpesviruses: integrity of the tegument does not depend on the presence of capsid or envelope. J Gen Virol. 1992;73:269–276. doi: 10.1099/0022-1317-73-2-269. [DOI] [PubMed] [Google Scholar]
- 33.Mettenleiter T C. Aujeszky's disease (pseudorabies) virus: the virus and molecular pathogenesis—state of the art, June 1999. Vet Res. 2000;31:99–115. doi: 10.1051/vetres:2000110. [DOI] [PubMed] [Google Scholar]
- 34.Muraki Y, Yamada M, Yoshida M, Yamada S, Padilla J, Hatano Y, Hiramatsu Y, Uno F, Nii S. Electron microscopic observations of aberrant capsids of pseudorabies virus. J Electron Microsc. 1996;45:223–231. doi: 10.1093/oxfordjournals.jmicro.a023436. [DOI] [PubMed] [Google Scholar]
- 35.Nii S. Electron microscopic study on the development of herpesviruses. J Electron Microsc. 1992;41:414–423. [PubMed] [Google Scholar]
- 36.Nii S, Morgan C, Rose H M. Electron microscopy of herpes simplex virus. II. Sequence and development. J Virol. 1968;2:517–536. doi: 10.1128/jvi.2.5.517-536.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nixdorf R, Klupp B G, Karger A, Mettenleiter T C. Effects of truncation of the carboxy terminus of pseudorabies virus glycoprotein B on infectivity. J Virol. 2000;74:7137–7145. doi: 10.1128/jvi.74.15.7137-7145.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Osterrieder N, Neubauer A, Brandmüller C, Kaaden O-R, O'Callaghan D J. The equine herpesvirus 1 IR6 protein influences virus growth at elevated temperature and is a major determinant of virulence. Virology. 1996;226:243–251. doi: 10.1006/viro.1996.0652. [DOI] [PubMed] [Google Scholar]
- 39.Peeters B, de Wind N, Hooisma M, Wagenaar F, Gielkens A, Moormann R. Pseudorabies virus envelope glycoproteins gp50 and gII are essential for virus penetration, but only gII is involved in membrane fusion. J Virol. 1992;66:894–905. doi: 10.1128/jvi.66.2.894-905.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peeters B, de Wind N, Broer R, Gielkens A, Moormann R. Glycoprotein H of pseudorabies virus is essential for entry and cell-to-cell spread of the virus. J Virol. 1992;66:3888–3892. doi: 10.1128/jvi.66.6.3888-3892.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Puvion-Dutilleul F, Pichard E, Laithier M, Leduc E H. Effect of dehydrating agents on DNA organization in herpes viruses. J Histochem Cytochem. 1987;35:635–645. doi: 10.1177/35.6.3033063. [DOI] [PubMed] [Google Scholar]
- 42.Radsak K, Kern H, Reis B, Reschke M, Mockenhaupt T, Eickmann M. Human cytomegalovirus—aspects of viral morphogenesis and of processing and transport of viral glycoproteins. In: Barbanti-Brodano G, editor. DNA tumor viruses: oncogenic mechanisms. New York, N.Y: Plenum Press; 1995. pp. 295–312. [Google Scholar]
- 43.Radsak K, Eickmann M, Mockenhaupt T, Bogner E, Kern H, Eis-Hübinger A, Reschke M. Retrieval of human cytomegalovirus glycoprotein B from the infected cell surface for virus envelopment. Arch Virol. 1996;141:557–572. doi: 10.1007/BF01718317. [DOI] [PubMed] [Google Scholar]
- 44.Reaves B, Banting G. Perturbation of the morphology of the trans-Golgi network following brefeldin A treatment: redistribution of a TGN-specific integral membrane protein, TGN38. J Cell Biol. 1992;116:85–94. doi: 10.1083/jcb.116.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rixon F J, Addison C, McLauchlan J. Assembly of enveloped tegument structures (L particles) can occur independently of virion maturation in herpes simplex virus type 1-infected cells. J Gen Virol. 1992;73:277–284. doi: 10.1099/0022-1317-73-2-277. [DOI] [PubMed] [Google Scholar]
- 46.Roffman E, Albert J P, Goff J P, Frenkel N. Putative site for the aquisition of human herpesvirus 6 virion tegument. J Virol. 1990;64:6308–6313. doi: 10.1128/jvi.64.12.6308-6313.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roizman B, Sears A E. Herpes simplex viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Vol. 2. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2231–2295. [Google Scholar]
- 48.Schmidt J, Klupp B G, Karger A, Mettenleiter T C. Adaptability in herpesviruses: glycoprotein D-independent infectivity of pseudorabies virus. J Virol. 1997;71:17–24. doi: 10.1128/jvi.71.1.17-24.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schröder C, Keil G. Bovine herpesvirus 1 requires glycoprotein H for infectivity and direct spreading and glycoproteins gHW450 and gB for glycoprotein D-independent cell-to-cell spread. J Gen Virol. 1999;80:57–61. doi: 10.1099/0022-1317-80-1-57. [DOI] [PubMed] [Google Scholar]
- 50.Sodeik B, Ebersold M W, Helenius A. Microtubule-mediated transport of incoming herpes simplex virus 1 capsids to the nucleus. J Cell Biol. 1997;136:1007–1021. doi: 10.1083/jcb.136.5.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spear P G. Entry of alphaherpesviruses into cells. Semin Virol. 1993;4:167–180. [Google Scholar]
- 52.Spear P G, Wittels M, Fuller A O, WuDunn D, Johnson R. Herpes simplex virus: pathway of entry into cells. In: Compans R W, Helenius A, Oldstone M B A, editors. Cell biology of viral entry, replication and pathogenesis. New York, N.Y: Alan R. Liss; 1988. pp. 163–175. [Google Scholar]
- 53.Szilagyi J F, Cunningham C. Identification and characterization of a novel non-infectious herpes simplex virus-related particle. J Gen Virol. 1991;72:661–668. doi: 10.1099/0022-1317-72-3-661. [DOI] [PubMed] [Google Scholar]
- 54.Talbot P, Almeida J D. Human cytomegalovirus purification of enveloped virions and dense bodies. J Gen Virol. 1977;36:345–349. doi: 10.1099/0022-1317-36-2-345. [DOI] [PubMed] [Google Scholar]
- 55.Topilko A, Michelson S. Morphological and cytochemical analysis of human cytomegalovirus inoculum: correlation of free particles in inoculum with counterparts in infected cells. Res Virol. 1994;145:65–73. doi: 10.1016/s0923-2516(07)80008-2. [DOI] [PubMed] [Google Scholar]
- 56.Topilko A, Michelson S. Hyperimmediate entry of human cytomegalovirus virions and dense bodies into human fibroblasts. Res Virol. 1994;145:75–82. doi: 10.1016/s0923-2516(07)80009-4. [DOI] [PubMed] [Google Scholar]
- 57.Torrisi R M, DiLazzaro C, Pavan A, Pereira L, Campadelli-Fiume G. Herpes simplex virus envelopment and maturation studied by fracture label. J Virol. 1992;66:554–561. doi: 10.1128/jvi.66.1.554-561.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van Regenmortel H V M, Fauquet C M, Bishop D H L, Carstens E, Estes M K, Lemon S, Maniloff J, Mayo M A, McGeoch D, Pringle C R, Wickner R B, editors. Virus taxonomy. Seventh Report of the International Committee on Taxonomy of Viruses. New York, N.Y: Academic Press; 2000. [Google Scholar]
- 59.Whealy E M, Card J P, Meade R P, Robbins A K, Enquist L W. Effect of brefeldin A on alphaherpesvirus membrane protein glycosylation and virus egress. J Virol. 1991;65:1066–1081. doi: 10.1128/jvi.65.3.1066-1081.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Whiteley A, Bruun B, Minson T, Browne H. Effects of targeting herpes simplex virus type 1 gD to the endoplasmic reticulum and trans-Golgi network. J Virol. 1999;73:9515–9520. doi: 10.1128/jvi.73.11.9515-9520.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhu Z, Gershon M D, Hao Y, Ambron R T, Gabel C A, Gershon A A. Envelopment of varicella-zoster virus: targeting of viral glycoproteins to the trans-Golgi network. J Virol. 1995;69:7951–7959. doi: 10.1128/jvi.69.12.7951-7959.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]