ABSTRACT
In China, children aged 12 months receive only a single dose of the varicella vaccine and the incidence of varicella remains high. This study aims to evaluate the changes in immunity among children aged 1–3 years following a single dose of the varicella vaccine, providing a scientific basis for determining the optimal age for a second vaccination. This prospective cohort study employed glycoprotein enzyme-linked immunosorbent assay (gpELISA) for antibody detection. The changes in IgG antibody levels over time post-vaccination were analyzed using a restricted cubic spline (RCS) fitted binary logistic regression model. Varicella surveillance data were collected from the National Notifiable Disease Reporting System (NNDRS). Following a peak in varicella incidence in 2019, the incidence shifted toward older age groups. The cohort study results revealed a seropositivity rate of 100% in children during the 18 months post-vaccination, which subsequently declined to 71.6% by the 42 months. The geometric mean concentration (GMC) decreased from 307.6mIU/mL to 115.2mIU/mL. Additionally, 14 children contracted varicella during the follow-up period, resulting in a breakthrough rate of 2.85%. RCS analysis indicated that antibody levels fell below the protective threshold 18.69 months post-vaccination, with a non-linear decline in the odds ratio(OR) of maintaining antibody concentrations ≥ 50mIU/mL(p < .001, Pnonlinear ≤ 0.001). This study demonstrates that the long-term protective efficacy of a single dose of the varicella vaccine diminishes over time in children, underscoring the necessity of implementing a two-dose vaccination strategy. The findings provide scientific evidence for determining the optimal timing for administering the second dose of the vaccine.
KEYWORDS: Varicella vaccine, prospective study, restricted cubic spline, waning immunity, vaccination strategy
Introduction
Varicella, caused by the varicella-zoster virus (VZV), is a common childhood disease characterized by widespread papules and vesicles. It can lead to severe complications such as pneumonia and encephalitis.1 Several developed countries have successfully controlled this disease by incorporating a two-dose varicella vaccination schedule into their national immunization programs, typically administering the first dose to children aged 12 to 15 months and the second dose as a booster between 4 to 6 years of age.2–5
In China, the varicella vaccine manufactured by GlaxoSmithKline was introduced in 1997, recommending a single-dose regimen for children aged 12 months to 12 years. The introduction of domestically produced varicella vaccines in 2000 further facilitated vaccine dissemination.6 Currently, four domestic companies in China are involved in varicella vaccine production,7 these vaccines use the Oka strain, which is widely recognized for its safety and efficacy. The potency of these vaccines ranges from 1000 to 3000 PFU (plaque-forming units) per dose, adhering to the national standard set by the Chinese Pharmacopoeia. This standardization ensures consistency in vaccine quality across different manufacturers. However, the vaccine has not been included in the national expanded immunization program, resulting in an overall single-dose coverage rate of only 61.1%,8 with significant regional disparities. Under moderate vaccine coverage, the incidence of varicella increased from 3.17 cases per 100,000 in 2005 to 70.14 cases per 100,000 in 2019.9 In the United States, although the widespread use of the varicella vaccine has significantly reduced infection rates, mortality, and healthcare costs, outbreaks of varicella have been reported in schools with high vaccination coverage, suggesting a potential correlation between the duration post-vaccination and the risk of breakthrough infections.5,10 Consequently, adjusting the current varicella vaccination strategy, particularly the optimal timing for the second dose,11,12 has become a focal point in public health. Given the relatively limited real-world data on long-term protection post-vaccination, this study employs a prospective cohort design to track the immune response in children aged 1–3 years following a single dose of the varicella vaccine. The study aims to comprehensively describe the immune profile of this cohort over 42 months, analyze the trends in IgG antibody levels, and, using a RCS fitted binary logistic regression model,13 integrate surveillance data from 2018 to 2023. The goal is to provide scientific evidence for determining the optimal timing for the second dose of the varicella vaccine, thereby effectively preventing and controlling varicella outbreaks among adolescents in China.
Methods
Varicella surveillance
In Jiangsu Province, China, varicella is reported and managed as a Class C infectious disease, a process that began in July 2017. All cases diagnosed by hospital staff or county-level Centers for Disease Control and Prevention must be recorded in the National Notifiable Disease Reporting System (NNDRS), an internet-based reporting system. The incidence rate of varicella is calculated as the number of cases per 100,000 individuals, with population data provided by the National Bureau of Statistics of China.
Study design
In May 2019, a prospective observational cohort study was initiated in Rugao County, Nantong City, Jiangsu Province. Utilizing the “Jiangsu Provincial Immunization Integrated Service Management Information System,” we identified children who had received a single dose of the varicella vaccine within one month. A sample of these children, all residents of Nantong City, was selected for the study. The study involved approximately three years of follow-up, with semiannual assessments of the participants’ health status and incidence of varicella, concluding at the end of 2022. Additionally, venous blood samples were collected from each participant at intervals of 1 to 1.5 years to assess changes in varicella antibody levels. Children who contracted varicella or received additional varicella-containing vaccines during the sample collection period were excluded from the cohort.
Ethical considerations
This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Jiangsu Provincial Center for Disease Control and Prevention (approval number: JSJK2019-B004-02). Prior to enrollment, informed consent was obtained from the parents or legal guardians of all participating children. The consent process included a detailed explanation of the study’s purpose, procedures, potential risks and benefits, and measures to protect participant privacy and data confidentiality. Participants were informed of their right to withdraw from the study at any time without consequences. All data were anonymized and stored securely to ensure participant confidentiality.
Vaccines
The varicella attenuated live vaccines used in this study were produced by Changchun BCHT Biotechnology (also known as Baike), Changchun Keygen Biological Products, Changsheng Biotechnology, and Shanghai Institute of Biologic Products. These vaccines use the Oka strain, which is widely recognized for its safety and efficacy. The potency of these vaccines ranges from 1000 to 3000 PFU (plaque-forming units) per dose. The vaccine should be refrigerated at 2 − 8°C in a medical refrigerator before use. Each dose should be administered subcutaneously with a 0.5 mL injection at the attachment site of the lateral deltoid muscle of the upper arm.
Laboratory assay
Blood samples were frozen at −20°C until analysis. Varicella-specific IgG antibodies were detected using an enzyme-linked immunosorbent assay (ELISA), with all tests conducted at the central laboratory of the Affiliated Suqian First People’s Hospital of Nanjing Medical University. To avoid bias, all assays were performed by the same technician using glycoprotein ELISA (gpELISA) kits from Institut Virion/Serion GmbH. The ELISA kit determines specific IgG antibodies against the viral envelope gp of VZV bound to the surface of microtitration wells. IgG antibody titers were measured within the range of 15–2,000 mIU/mL. Samples with antibody concentrations > 2,000 mIU/mL were marked as 2,000 mIU/mL. Concentrations > 100 mIU/mL were considered positive, while concentrations < 50 mIU/mL were considered negative. Equivocal results (50–100 mIU/ml) were retested and categorized as a positive or negative finding using the same methods.
Statistical analysis
Data analysis was performed using Microsoft Excel, R software (version 4.2.2), and SPSS V.22.0 (IBM Corp, Armonk, NY). GMCs and seropositivity rates with 95% confidence intervals (95% CI) were calculated and stratified by gender and age. Analysis of variance (ANOVA) was used to compare GMCs, with a two-tailed p-value of .05 considered statistically significant. A two-segment logistic regression model was applied using RCS to examine the relationship between post-vaccination immunity duration and antibody levels in children. The number of knots in the RCS fit was selected based on the model’s maximum R2 to prevent overfitting. The x-axis in the analysis represented the duration of immunity after vaccination, while the y-axis depicted the odds ratio OR for predicted IgG antibody concentrations ≥ 50 mIU/ml. OR values were plotted, with color intervals indicating the corresponding 95% confidence intervals (CI) of the OR. The association trend was evaluated using both overall and non-linear Wald χ2 tests. A p-value less than 0.05 from the overall Wald χ2 test indicated statistical significance, suggesting a linear association trend if the non-linear Wald χ2 test yielded a p-value greater than 0.05. Conversely, if both tests resulted in p-values less than 0.05, a non-linear association trend between the variables was suggested.
Results
Changes in epidemiological characteristics of varicella in Jiangsu Province
Between 2018 and 2023, Jiangsu Province reported a cumulative total of 581,594 varicella cases, with an average incidence rate of 93.0 per 100,000 individuals. Age-specific distribution analysis revealed two distinct peaks in the 0–9 and 10–19 age groups. Notably, from 2021 onwards, there was a significant rise in the proportion of cases within the 10–19 age group (Figure 1).
Figure 1.
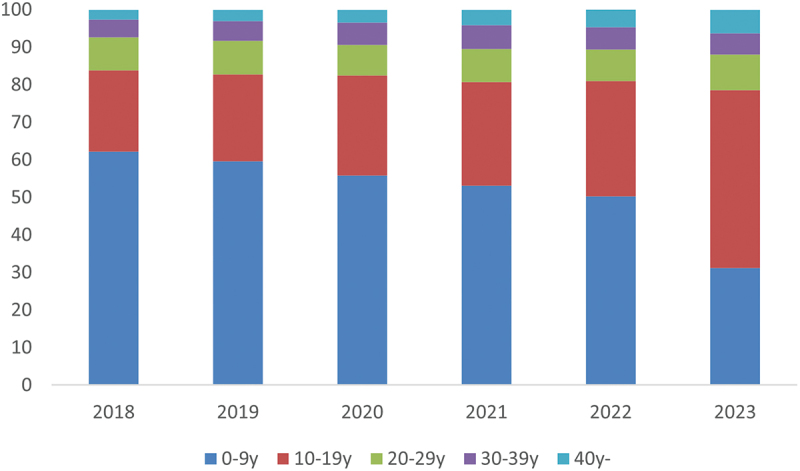
Proportion of varicella cases by age and year in Jiangsu Province, 2018-2023.
Analysis of immunity decline in participants after a single dose of varicella vaccine
In May 2019, the study enrolled 497 healthy children (248 males and 257 females) with an average age of 2.42 ± 0.65 years. Ultimately, 471 participants completed the first round of blood sampling in December 2020 (18 months), 450 completed the second round in December 2021 (30 months), and 418 completed the third round in December 2022 (42 months). During the follow-up period, 14 participants contracted varicella, all of which occurred during the first round of blood sampling.
As shown in Tables 1 and 2, there were significant differences in overall seropositivity rates and GMCs of varicella IgG antibodies in 2020, 2021, and 2022 after a single dose of the varicella vaccine (p < .01). Over time, the seropositivity rate decreased from 100.0% to 71.6%, and the GMC decreased from 307.6 mIU/mL to 115.2 mIU/mL. Further analysis indicated that among children aged 1, 2, and 3 years, seropositivity rates in 2022 compared to 2020 decreased by approximately 24.5% to 38.4%, and GMCs decreased by approximately 60.7% to 67.8%. Additionally, the study found no statistically significant impact of gender and age on seropositivity rates and GMCs (p > .05).
Table 1.
Overall immunity in terms of GMC to varicella in children aged 1-3 years in the follow up 42 months (mIU/mL).
| Characteristics | N | a18 months post vaccination | b30 months post vaccination | c42 months post vaccination |
|---|---|---|---|---|
| GMC 95%CI | GMC 95%CI | GMC 95%CI | ||
| Sex | ||||
| Boy | 228 | 310.7(291.8,329.7) | 169.9(158.9,180.9) | 117.1(106.3,127.9) |
| Girl | 243 | 304.7(284.8,324.6) | 165.9(154.9,176.8) | 114.1(103.2,124.9) |
| Age(years) | ||||
| 1- | 100 | 324.3(291.2,357.4) | 152.5(138.9,166.1) | 104.4(91.4,117.4) |
| 2- | 268 | 307.9(290.6,325.2) | 172.5(161.9,183.1) | 121.1(111.4,132.8) |
| 3- | 103 | 292.3(261.9,322.7) | 169.4(152.0,186.7) | 109.0(92.1,126.0) |
| Total | 471 | 307.6(293.9,321.2) | 167.5(160.2,175.6) | 115.2(107.6,122.9) |
aP18 months vs. 30 months <0.001; bP18 months vs. 42 months <0.001; cP30 months vs. 42 months <0.001.
Table 2.
Overall immunity in terms of seropositivity to varicella in children aged 1-3 years in the follow up 42 months (%).
| Characteristics | N | a18 months post vaccination | b30 months post vaccination | c42 months post vaccination |
|---|---|---|---|---|
| GMC 95%CI | GMC 95%CI | GMC 95%CI | ||
| Sex | ||||
| Boy | 228 | 100 | 92.8(87.9,95.3) | 74.6(67.5,80.1) |
| Girl | 243 | 100 | 95.6(914,97.4) | 68.6(61.3,75.1) |
| Age(years) | ||||
| 1- | 100 | 100 | 93.6(85.0,97.6) | 68.1(56.6,77.7) |
| 2- | 268 | 100 | 94.4(90.7,96.6) | 76.5(70.1,81.9) |
| 3- | 103 | 100 | 92.5(85.3,96.3) | 61.6(50.2,71.9) |
| Total | 471 | 100 | 94.2(91.7,96.0) | 76.8(72.6,80.6) |
aP18 months vs. 30 months <0.001; bP18 months vs. 42 months <0.001; cP30 months vs. 42 months <0.001.
The trend of varicella IgG antibody levels over time after immunization
Using RCS analysis, we observed a significant nonlinear relationship between the duration of immunity after a single dose of the varicella vaccine and the OR of maintaining varicella IgG antibody concentrations ≥ 50 mIU/mL (p < .001, Pnonlinear <0.001). The RCS curve analysis highlighted a critical time point at 18.69 months post-vaccination, where the OR for antibody levels reached an inflection point, indicating a subsequent nonlinear trend of initial increase followed by a decline (Figure 2).
Figure 2.
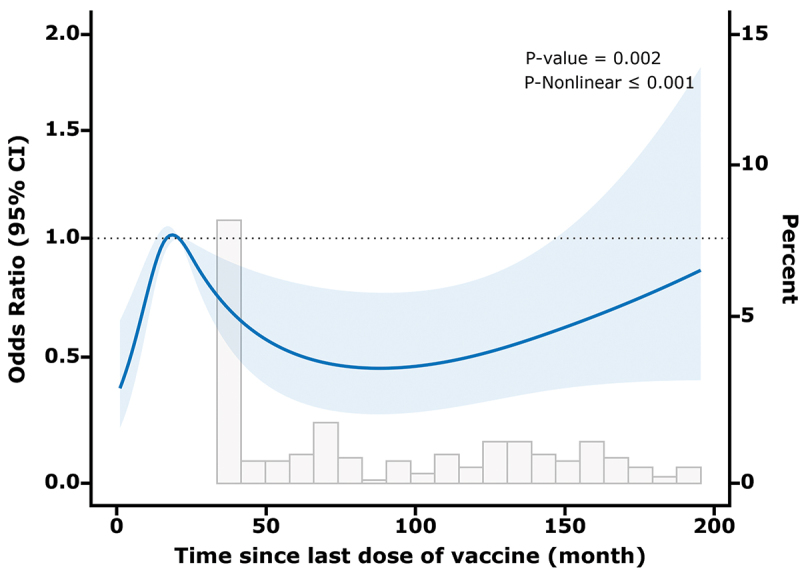
Association between duration after a single dose of the varicella vaccine and IgG antibody concentrations (≥50 mIU/mL) with the RCS function. A Model with 4 knots located at 5th, 35th, 65th, and 90th percentiles. Y-axis represents the or to present IgG antibody concentrations (≥50 mIU/mL) for any value of duration after immunization compared to individuals.
Discussion
Varicella has seen significant epidemiological changes in China. Since the introduction of the varicella vaccine in 1997, substantial progress has been made in disease prevention and control.14 However, following the classification of varicella as a Category C notifiable disease in Jiangsu Province in 2017, the incidence rate of 10–19 years old group increased significantly.2,15-17
This study provides empirical data on the waning immunity in Chinese children aged 1–3 years following a single dose of the varicella vaccine, which is crucial for informing national vaccination policies. As varicella vaccine coverage increases, the long-term efficacy of vaccination has become a key public health concern. Our findings indicate that the seropositivity rate reached 100% in the 18 months post-vaccination, but declined significantly to 71.6% by the 42 months. The GMC similarly decreased from 307.6 mIU/mL to 115.2 mIU/mL, underscoring the waning immunity over time. This aligns with international trends, where the vaccination strategy has shifted from a single dose to a two-dose regimen to enhance long-term protection. The Centers for Disease Control and Prevention (CDC) in the United States recommends a two-dose schedule based on evidence that a single dose may not provide sustained immunity.2,18,19 The epidemiological data from Rugao County during the study period reveal a moderate level of varicella circulation, with relatively stable, lower incidence rates from 2019 to 2023 suggest that the observed antibody dynamics in our cohort primarily reflect the vaccine-induced immunity rather than significant natural boosting effects. However, the occurrence of 14 breakthrough cases within the first 18 months of the study indicates that some participants did experience exposure to wild-type VZV, which could have influenced their antibody levels. These epidemiological findings underscore the complexity of interpreting vaccine effectiveness in real-world settings and highlight the importance of considering background disease incidence when evaluating long-term vaccine-induced immunity.
Given the low one-dose vaccination rate of 61.1% for the varicella vaccine, enhancing this initial coverage should be a public health priority. Our findings show that immunity declines over time with a single dose, suggesting the need for a two-dose regimen. However, without sufficient initial coverage, the benefits of a two-dose strategy may be limited. Increasing one-dose coverage would strengthen herd immunity, reduce virus circulation, and decrease breakthrough infections, even before introducing a second dose. Therefore, while a two-dose regimen is eventually necessary, improving one-dose coverage should be prioritized to ensure a stronger foundation for future vaccination efforts.
The study also revealed that 14 participants contracted varicella, resulting in a breakthrough rate of 2.85%, which is higher than the 0.70% reported in a U.S. study.20 This disparity highlights the differences in varicella vaccination strategies and efficacy across regions and underscores the need to optimize vaccination policies globally. Previous research indicates that the breakthrough rate for varicella increases from 1.2% at one year post-vaccination to 13.5% at five years,21 consistent with our observation of declining seropositivity over time. Given the effectiveness of a single dose in reducing severe varicella cases, the risk of breakthrough disease,5 and reduced external exposure due to decreased shedding of wild-type VZV,22 a two-dose regimen is essential for optimal protection and prevention of outbreaks.2
While most studies focus on changes in seropositivity or antibody concentrations post-vaccination,23–26 our research utilizes cross-sectional data (2018–2023) and employs RCS to model the independent effect of time since immunization on antibody levels. This approach provides a more precise and intuitive depiction of the OR for antibody levels ≥ 50mIU/mL over time. The RCS-fitted binary logistic regression model indicates a nonlinear relationship between post-vaccination duration and antibody concentration, with a critical turning point at approximately 18.69 months, where antibody levels fall below protective thresholds. This finding aligns with our prospective data, showing a 100% seropositivity rate and a GMCs of 307.6 mIU/mL at the first blood draw (18 months post-vaccination), followed by a significant decline at subsequent intervals. These insights are crucial for determining the optimal timing for administering the second vaccine dose to maintain long-term immunity.
However, the study has several limitations. First, it did not account for all factors that might influence vaccine efficacy, such as children’s nutritional and health status. Additionally, the follow-up period for vaccinated children was relatively short. Furthermore, this study focused solely on humoral immunity, as reflected by IgG titers, and did not assess cellular immunity. Both humoral and cellular immunity play crucial roles in protection against VZV infectious disease. Humoral immunity, mediated by antibodies, helps neutralize free virus particles, while cellular immunity, primarily involving T cells, is essential for recognizing and eliminating virus-infected cells. The absence of cellular immunity assessment in this study limits our understanding of the complete immune response to the varicella vaccine. Despite this limitation, the evaluation of humoral immunity provides valuable insights into the vaccine’s efficacy and the duration of antibody-mediated protection. Further prospective studies incorporating both humoral and cellular immunity assessments are needed to provide a more comprehensive understanding of the long-term protection against varicella following vaccination.
In summary, this study demonstrates that although a single dose of the varicella vaccine provides effective short-term protection, antibody levels in children decline significantly over time. RCS model analysis indicates that 18.69 months post-vaccination is a critical point where antibody levels begin to fall. These findings provide valuable insights for determining the optimal timing for the second dose of the varicella vaccine to ensure sustained immunity.
Acknowledgments
The authors acknowledge the staff of Rugao CDC for their invaluable support in the collection and analysis of the data.
Biography
Wang Wen a Deputy Chief Physician at the Department of Rheumatology and Immunology, the Affiliated Suqian First People’s Hospital of Nanjing Medical University in Suqian, Jiangsu Province, China. My primary research interest lies in the prevention and treatment of vaccine-related diseases for special populations, such as diabetics, pregnant women, individuals with immunodeficiency, and healthcare workers. My work involves the study of various diseases including chickenpox, whooping cough, and shingles. My goal is to improve the health and well-being of these vulnerable groups through innovative immunization strategies and effective disease management.
Funding Statement
This manuscript was funded by the Opening Foundation of Key Laboratory [grant number JSHD2022043] [grant number JSHD2022043], the Jiangsu Provincial Geriatric Health Research Project [grant number LKM2023005], Suqian Sci&Tech Program [grant number SY202312] and Jiangsu Provincial Health Commission Preventive Medicine and Hematology Prevention Research Project [grant number Ym2023099].
Disclosure statement
No potential conflict of interest was reported by the author(s).
Author contributions
X.S. and W.W. designed the study. X.S., L.Z. ZG.W. and W.W. collected and analyzed the data. All authors had full access to the data and had final responsibility for the decision to submit for publication. All authors have read and approved the article.
Data availability statement
The data that support the findings of this study are available on request from the corresponding author upon reasonable request. The data are not publicly available due to privacy or ethical restrictions.
References
- 1.Gershon AA. Is chickenpox so bad, what do we know about immunity to varicella zoster virus, and what does it tell us about the future? J Infect. 2017;74(Suppl 1):27–6. doi: 10.1016/S0163-4453(17)30188-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marin M, Güris D, Chaves SS, Schmid S, Seward JF. Prevention of varicella: recommendations of the advisory committee on immunization practices (ACIP). MMWR Recomm Rep. 2007;56:1–40. [PubMed] [Google Scholar]
- 3.Marin M, Marti M, Kambhampati A, Jeram SM, Seward JF. Global varicella vaccine effectiveness: a meta-analysis. Pediatrics. 2016;137(3):e20153741. doi: 10.1542/peds.2015-3741. [DOI] [PubMed] [Google Scholar]
- 4.Wiese-Posselt M, Siedler A, Mankertz A, Sauerbrei A, Hengel H, Wichmann O, Poethko-Müller C. Varicella-zoster virus seroprevalence in children and adolescents in the pre-varicella vaccine era, Germany. Bmc Infect Dis. 2017;17(1):356. doi: 10.1186/s12879-017-2461-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wutzler P, Bonanni P, Burgess M, Gershon A, Sáfadi MA, Casabona G. Varicella vaccination - the global experience. Expert Rev Vaccines. 2017;16(8):833–843. doi: 10.1080/14760584.2017.1343669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y, Zhang L, Sun X, Cao Y, Wang Z, Liu L, Xu Y, Zhou M, Liu Y. Effectiveness and failure rate of the varicella vaccine in an outbreak in Jiangsu, China: a 1: 2 matched case-control study. Hum Vaccin Immunother. 2020;16(3):506–512. doi: 10.1080/21645515.2019.1665959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu Q, Rivailler P, Xu S, Xu W, Longnecker RM. Comparison of the whole-genome sequence of an oka varicella vaccine from China with other oka vaccine strains reveals sites putatively critical for vaccine efficacy. J Virol. 2019;93(9). doi: 10.1128/JVI.02281-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu A, Sun T. Meta-analysis of varicella vaccine coverage among Chinese children. Chin J Vaccines Immun. 2017;23:698–704. doi: 10.1186/s12889-023-16304-4. [DOI] [Google Scholar]
- 9.Sui H, Li J, Wang M, Liu Y, Yin D. Varicella epidemiology in china, 2005-2015. Chin J Vaccines Immun. 2019;25:155–159. doi: 10.46234/ccdcw2023.218. [DOI] [Google Scholar]
- 10.Yin M, Xu X, Liang Y, Ni J. Effectiveness, immunogenicity and safety of one vs. two-dose varicella vaccination: a meta-analysis. Expert Rev Vaccines. 2018;17(4):351–362. doi: 10.1080/14760584.2018.1433999. [DOI] [PubMed] [Google Scholar]
- 11.Lopez AS, Guris D, Zimmerman L, Gladden L, Moore T, Haselow DT, Loparev VN, Schmid DS, Jumaan AO, Snow SL. One dose of varicella vaccine does not prevent school outbreaks: is it time for a second dose? Pediatrics. 2006;117(6):1070–1077. doi: 10.1542/peds.2005-2085. [DOI] [PubMed] [Google Scholar]
- 12.Sun X, Dai C, Wang K, Liu Y, Jin X, Wang C, Yin Y, Ding Z, Lu Z, Wang W, et al. A dynamic compartmental model to explore the optimal strategy of varicella vaccination: an epidemiological study in Jiangsu province, China. Trop Med Infect Dis. 2022;8(1):17. doi: 10.3390/tropicalmed8010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.R C. Restricted cubic spline regression . A brief introduction. Toronto: Institute for Clinical Evaluative Sciences; 2016. [Google Scholar]
- 14.Suo L, Lu L, Zhao D, Pang X. Impact of a 2-dose voluntary vaccination strategy on varicella epidemiology in Beijing, 2011–2017. Vaccine. 2020;38(20):3690–3696. doi: 10.1016/j.vaccine.2020.01.087. [DOI] [PubMed] [Google Scholar]
- 15.Sun X, Xu Y, Zhu Y, Tang F. Impact of non-pharmaceutical interventions on the incidences of vaccine-preventable diseases during the COVID-19 pandemic in the eastern of China. Hum Vaccin Immunother. 2021;17(11):4083–4089. doi: 10.1080/21645515.2021.1956227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bai Y, Shen L, Sun M, Yang Z, Chen Z, Zhai J, Xue M, Shao Z, Liu K, Zheng C. The short and long-term impact of nonpharmaceutical interventions on the prevalence of varicella in xi’an during the COVID-19 pandemic. J Med Virol. 2023;95:e29020. doi: 10.1002/jmv.29020. [DOI] [PubMed] [Google Scholar]
- 17.Sabale U, Jarmale L, Murtagh J, Pawaskar M, Bencina G, Santangelo OE. Impact assessment of immunization and the COVID-19 pandemic on varicella across Europe using digital epidemiology methods: a descriptive study. PLOS ONE. 2023;18(4):e283465. doi: 10.1371/journal.pone.0283465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lopez AS, Cardemil C, Pabst LJ, Cullen KA, Leung J, Bialek SR. Two-dose varicella vaccination coverage among children aged 7 years--six sentinel sites, United States, 2006-2012. MMWR Morb Mortal Wkly Rep. 2014;63(8):174–177. [PMC free article] [PubMed] [Google Scholar]
- 19.Lopez AS, Zhang J, Marin M. Epidemiology of varicella during the 2-dose varicella vaccination program — United States, 2005–2014. MMWR Morb Mortal Wkly Rep. 2016;65(34):902–905. doi: 10.15585/mmwr.mm6534a4. [DOI] [PubMed] [Google Scholar]
- 20.Knuf M, Zepp F, Helm K, Maurer H, Prieler A, Kieninger-Baum D, Douha M, Willems P. Antibody persistence for 3 years following two doses of tetravalent measles–mumps–rubella–varicella vaccine in healthy children. Eur J Pediatr. 2012;171(3):463–470. doi: 10.1007/s00431-011-1569-4. [DOI] [PubMed] [Google Scholar]
- 21.Zhu YF, Li YF, Du Y, Zeng M. Epidemiological characteristics of breakthrough varicella infection during varicella outbreaks in Shanghai, 2008–2014. Epidemiol Infect. 2017;145(10):2129–2136. doi: 10.1017/S0950268817000772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonanni P, Gershon A, Gershon M, Kulcsár A, Papaevangelou V, Rentier B, Sadzot-Delvaux C, Usonis V, Vesikari T, Weil-Olivier C, et al. Primary versus secondary failure after varicella vaccination: implications for interval between 2 doses. Pediatr Infect Dis J. 2013;32(7):305–313. doi: 10.1097/INF.0b013e31828b7def. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bakri FG, Abdelrahim ZM, Alkalbani AS, Khrais GM, Shamroukh DS, Hayajneh FA, Mahafza A. Seroprevalence of measles, mumps, rubella, and varicella among physicians and nurses in Jordan. Turk J Med Sci. 2016;46:614–619. doi: 10.3906/sag-1502-115. [DOI] [PubMed] [Google Scholar]
- 24.Yun JH, Lee E, Choi JH, Ki HK, Park J. Seroprevalence of varicella-zoster virus and measles among healthcare workers in a tertiary medical center in Korea. Vaccines (Basel). 2022;10(11):1956. doi: 10.3390/vaccines10111956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choi B, Kwon S. Seroprevalence comparison of different varicella vaccines among Turkish children. Hum Vaccin Immunother. 2022;18(5):2067443. doi: 10.1080/21645515.2022.2067443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bollaerts K, Riera-Montes M, Heininger U, Hens N, Souverain A, Verstraeten T, Hartwig S. A systematic review of varicella seroprevalence in European countries before universal childhood immunization: deriving incidence from seroprevalence data. Epidemiol Infect. 2017;145(13):2666–2677. doi: 10.1017/S0950268817001546. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author upon reasonable request. The data are not publicly available due to privacy or ethical restrictions.


