Abstract
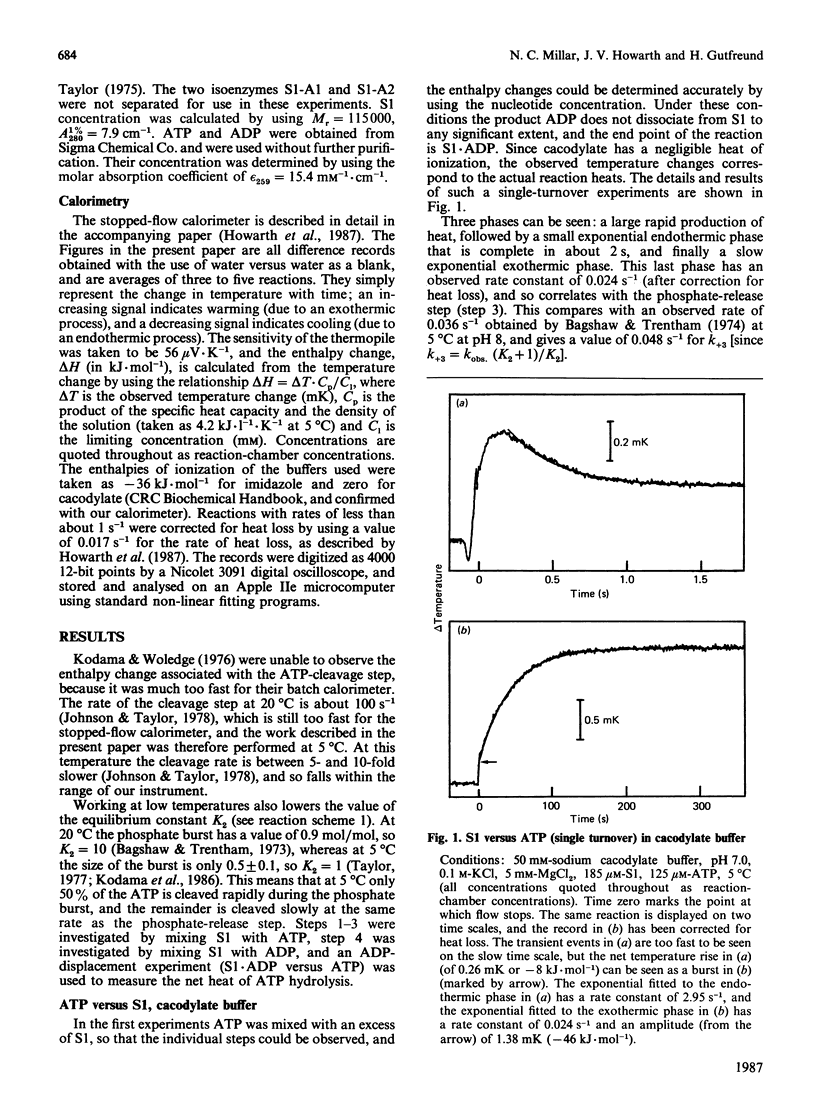
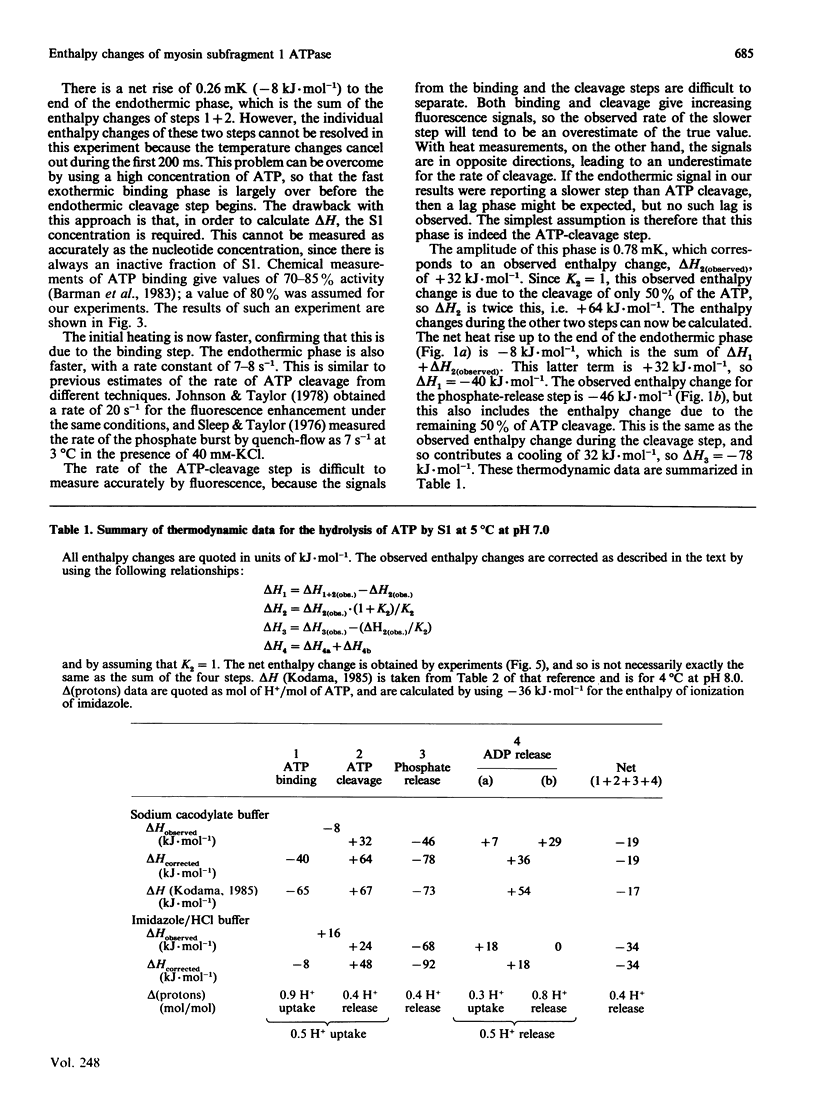
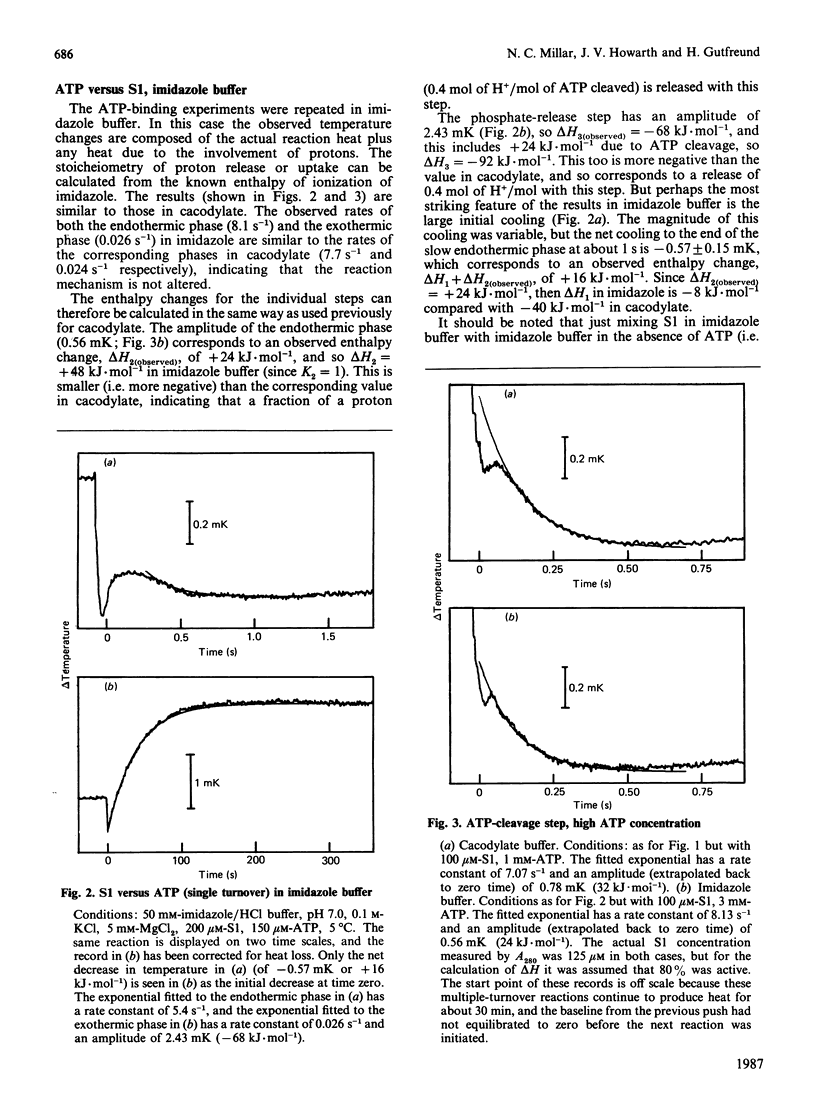
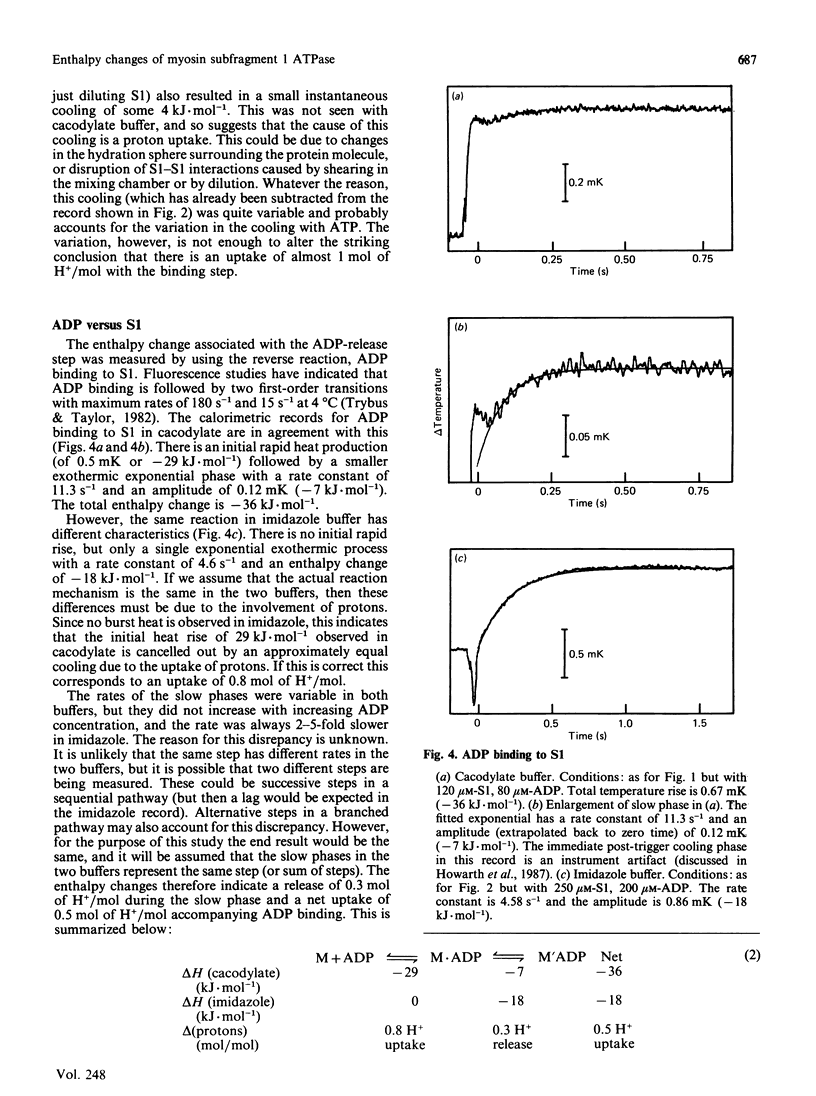
1. The enthalpy changes during individual reaction steps of the myosin subfragment 1 ATPase were studied with the use of a new stopped-flow calorimeter [Howarth, Millar & Gutfreund (1987) Biochem. J. 248, 677-682]. 2. At 5 degrees C and pH 7.0, the endothermic on-enzyme ATP-cleavage step was observed directly (delta H = +64 kJ.mol-1). 3. ADP binding is accompanied by a biphasic enthalpy change. 4. The release and uptake of protons was investigated by the use of two buffers with widely different heats of ionization. 5. Protons are involved in all four principal steps of the myosin subfragment 1 ATPase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberty R. A. Effect of pH and metal ion concentration on the equilibrium hydrolysis of adenosine triphosphate to adenosine diphosphate. J Biol Chem. 1968 Apr 10;243(7):1337–1343. [PubMed] [Google Scholar]
- Bagshaw C. R., Trentham D. R. The characterization of myosin-product complexes and of product-release steps during the magnesium ion-dependent adenosine triphosphatase reaction. Biochem J. 1974 Aug;141(2):331–349. doi: 10.1042/bj1410331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagshaw C. R., Trentham D. R. The reversibility of adenosine triphosphate cleavage by myosin. Biochem J. 1973 Jun;133(2):323–328. doi: 10.1042/bj1330323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barman T. E., Hillaire D., Travers F. Evidence for the two-step binding of ATP to myosin subfragment 1 by the rapid-flow-quench method. Biochem J. 1983 Mar 1;209(3):617–626. doi: 10.1042/bj2090617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chock S. P., Eisenberg E. Heavy meromyosin Mg-ATPase: presteady-state and steady-state Hplus release. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4915–4919. doi: 10.1073/pnas.71.12.4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chock S. P. The mechanism of the skeletal muscle myosin ATPase. III. Relationship of the H+ release and the protein absorbance change induced by ATP to the initial Pi burst. J Biol Chem. 1979 May 10;254(9):3244–3248. [PubMed] [Google Scholar]
- GREEN I., MOMMAERTS W. F. Adeno-sinetriphosphatase systems of muscle. I. An electrotitrimetric method of measurement. J Biol Chem. 1953 Jun;202(2):541–549. [PubMed] [Google Scholar]
- Gajewski E., Steckler D. K., Goldberg R. N. Thermodynamics of the hydrolysis of adenosine 5'-triphosphate to adenosine 5'-diphosphate. J Biol Chem. 1986 Sep 25;261(27):12733–12737. [PubMed] [Google Scholar]
- Hibberd M. G., Trentham D. R. Relationships between chemical and mechanical events during muscular contraction. Annu Rev Biophys Biophys Chem. 1986;15:119–161. doi: 10.1146/annurev.bb.15.060186.001003. [DOI] [PubMed] [Google Scholar]
- Howarth J. V., Millar N. C., Gutfreund H. A stopped-flow calorimeter for biochemical applications. Biochem J. 1987 Dec 15;248(3):677–682. doi: 10.1042/bj2480677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. A., Taylor E. W. Intermediate states of subfragment 1 and actosubfragment 1 ATPase: reevaluation of the mechanism. Biochemistry. 1978 Aug 22;17(17):3432–3442. doi: 10.1021/bi00610a002. [DOI] [PubMed] [Google Scholar]
- Kodama T., Fukui K., Kometani K. The initial phosphate burst in ATP hydrolysis by myosin and subfragment-1 as studied by a modified malachite green method for determination of inorganic phosphate. J Biochem. 1986 May;99(5):1465–1472. doi: 10.1093/oxfordjournals.jbchem.a135616. [DOI] [PubMed] [Google Scholar]
- Kodama T. Reaction heats and heat capacity changes for intermediate steps of the ATP hydrolysis catalyzed by myosin subfragment 1. J Biol Chem. 1981 Mar 25;256(6):2928–2933. [PubMed] [Google Scholar]
- Kodama T. Thermodynamic analysis of muscle ATPase mechanisms. Physiol Rev. 1985 Apr;65(2):467–551. doi: 10.1152/physrev.1985.65.2.467. [DOI] [PubMed] [Google Scholar]
- Kodama T., Woledge R. C. Calorimetric studies of the interaction of myosin with ADP. J Biol Chem. 1976 Dec 10;251(23):7499–7503. [PubMed] [Google Scholar]
- Koretz J. F., Taylor E. W. Transient state kinetic studies of proton liberation by myosin and subfragment 1. J Biol Chem. 1975 Aug 25;250(16):6344–6350. [PubMed] [Google Scholar]
- Marsh D. J., de Bruin S. H., Gratzer W. B. An investigation of heavy meromyosin-ADP binding equilibria by proton release measurements. Biochemistry. 1977 Apr 19;16(8):1738–1742. doi: 10.1021/bi00627a034. [DOI] [PubMed] [Google Scholar]
- PODOLSKY R. J., MORALES M. F. The enthalpy change of adenosine triphosphate hydrolysis. J Biol Chem. 1956 Feb;218(2):945–959. [PubMed] [Google Scholar]
- Shriver J. W. The structure of myosin and its role in energy transduction in muscle. Biochem Cell Biol. 1986 Apr;64(4):265–276. doi: 10.1139/o86-038. [DOI] [PubMed] [Google Scholar]
- Sleep J. A., Taylor E. W. Intermediate states of actomyosin adenosine triphosphatase. Biochemistry. 1976 Dec 28;15(26):5813–5817. doi: 10.1021/bi00671a019. [DOI] [PubMed] [Google Scholar]
- Taylor E. W. Mechanism of actomyosin ATPase and the problem of muscle contraction. CRC Crit Rev Biochem. 1979;6(2):103–164. doi: 10.3109/10409237909102562. [DOI] [PubMed] [Google Scholar]
- Taylor E. W. Transient phase of adenosine triphosphate hydrolysis by myosin, heavy meromyosin, and subfragment 1. Biochemistry. 1977 Feb 22;16(4):732–739. doi: 10.1021/bi00623a027. [DOI] [PubMed] [Google Scholar]
- Trentham D. R., Eccleston J. F., Bagshaw C. R. Kinetic analysis of ATPase mechanisms. Q Rev Biophys. 1976 May;9(2):217–281. doi: 10.1017/s0033583500002419. [DOI] [PubMed] [Google Scholar]
- Trybus K. M., Taylor E. W. Transient kinetics of adenosine 5'-diphosphate and adenosine 5'-(beta, gamma-imidotriphosphate) binding to subfragment 1 and actosubfragment 1. Biochemistry. 1982 Mar 16;21(6):1284–1294. doi: 10.1021/bi00535a028. [DOI] [PubMed] [Google Scholar]
- Weeds A. G., Taylor R. S. Separation of subfragment-1 isoenzymes from rabbit skeletal muscle myosin. Nature. 1975 Sep 4;257(5521):54–56. doi: 10.1038/257054a0. [DOI] [PubMed] [Google Scholar]


