Abstract
The G and P genotypes of 3,601 rotavirus strains collected in the United Kingdom between 1995 and 1999 were determined (M. Iturriza-Gómara et al., J. Clin. Microbiol. 38:4394–4401, 2000). In 95.4% of the strains the most common G and P combinations, G1P[8], G2P[4], G3P[8], and G4P[8], were found. A small but significant number (2%) of isolates from the remaining strains were reassortants of the most common cocirculating strains, e.g., G1P[4] and G2P[8]. Rotavirus G9P[6] and G9P[8] strains, which constituted 2.7% of all viruses, were genetically closely related in their G components, but the P components of the G9P[8] strains were very closely related to those of cocirculating strains of the more common G types (G1, G3, and G4). In conclusion, genetic interaction by reassortment among cocirculating rotaviruses is not a rare event and contributes significantly to their overall diversity.
Rotaviruses are the major cause of viral gastroenteritis in infants and young children, producing a significant pediatric disease burden worldwide (3) and high childhood mortality in developing countries (33). Therefore, the epidemiology of rotaviruses has been studied by many groups (reviewed in reference 16), and intense efforts are devoted to developing a vaccine (32, 43, 46). One vaccine candidate was licensed for widespread use in the United States (6), but the recommendation for its usage was retracted after severe and seemingly vaccine-associated complications (gut intussusception) which emerged at the implementation stage (7). Although the correlates of protection, virus-specific secreted coproantibodies of immunoglobulin A and possibly immunoglobulin G subclasses, are reasonably well identified, the degree of cross-protection conveyed by a single or several natural infections (50) or vaccinations (47) is still poorly understood. In order to evaluate the effectiveness of vaccines, their protection against rotavirus strains cocirculating in the population at the time of vaccination has to be known.
Rotaviruses are a genus of the Reoviridae family and possess a genome of 11 segments of double-stranded RNA. There are at least five groups (A to E), and within group A several subgroups have been defined. Group- and subgroup-specific antigens are located on VP6, the middle layer protein of the trilayered rotavirus particle (35). The two outer layer proteins, VP4 (occurring as 60 spike-shaped dimers) and VP7 (occurring as 260 trimers), both elicit neutralizing antibodies which are type specific. Protease cleavage of VP4 results in two fragments, VP8*, the N-terminal fragment where most type-specific epitopes are located, and VP5*, which contains mainly cross-reactive epitopes. A dual typing classification system has been established defining at least 14 G (glycoprotein, VP7-specific) types and 20 P (protease-sensitive protein, VP4-specific) types. Of these, at least 10 different G types and 11 different P types have been found in human isolates (18).
At present it is not clear how the enormous antigenic and genomic diversity of rotavirus strains observed at any one geographical location and any one time is generated, maintained, or changing over time. For human rotaviruses, four mechanisms have been identified to account for the observed diversity: point mutations, occurring as singular events or accumulating sequentially over time; genomic reassortment in the progeny of two viruses after coinfection of a single cell; genome rearrangements (intramolecular recombination); and the introduction of animal rotaviruses into the human population. These mechanisms contribute to the diversity of rotavirus strains individually and in combination.
Point mutations occur frequently in rotaviruses (∼5 × 10−5/site/replication, amounting to approximately one mutation per single genome replication [4]). Some of them are conserved and passed on to progeny viruses in which they can accumulate. Such mutations can be used to define genetic lineages and sublineages (28, 31, 36), which are useful for classification and have epidemiological meaning, e.g., by defining outbreak strains (28). They may be very important in the analysis of other aspects of the transmission dynamics of rotaviruses (15, 37). Antibody escape mutations may contribute to the antigenic diversity and to the generation of monotypes within the different rotavirus serotypes (9, 44).
Genome reassortment of rotaviruses readily occurs in vitro under appropriate conditions (for review, see reference 45) but has also been identified as a mechanism for determining the progenitors of rotavirus strains isolated in nature, both in humans and animals (5, 11, 48, 49, 51). Interestingly, the VP4 and VP7 molecules, situated near each other on the outer layer of the rotavirus particle, may alter each other's antigenicity (8) or receptor specificity (38) when brought together from different parents in reassortment progeny. The relative contribution of reassortment to genomic and antigenic diversity of cocirculating viruses is under discussion. As rotaviruses of the same group reassort readily in doubly infected cells and VP4 and VP7 are encoded by different RNA segments (RNA 4 and RNA 7, 8, or 9 depending on strain, respectively), various G/P combinations have been detected in natural human and animal isolates (14). G1 to G4 and P[4] and P[8] are the most commonly found G and P types in human rotavirus strains. Rotaviruses of the G1P[8], G3P[8], and G4P[8] combinations are mainly of subgroup II, and viruses of the G2P[4] combination are mainly of subgroup I.
Genome rearrangements have been observed to occur both in vitro and in vivo (for review, see references 13 and 45). Their relative contribution to genomic and antigenic diversity in nature is probably small (compared to that of point mutations and reassortment) but is essentially unknown.
There is now increasing evidence that animal rotaviruses can infect humans, either by direct transmission of the virus or by contributing one or several genes to reassortants with essentially a human strain genetic background (12, 20, 39). The full significance of rotaviruses arising from an “animal reservoir” for human infections is not yet defined.
When the relative significance of these evolutionary pathways for rotavirus diversity is considered, reassortment stands out, either occurring between viruses of the same species or between viruses of different species. In this study, the genetic analysis of 147 strains from a large collection of fully G- and P-typed human rotaviruses (29) has shown that genetic interaction in vivo among cocirculating human rotavirus strains resulting in reassortant formation is not a rare event and that some of these events are of epidemiological significance.
(Beverley Isherwood was an undergraduate student of the University of Cambridge, and part of this work constituted a Part II project in partial fulfillment of the requirements for a B.A. in Natural Sciences.)
MATERIALS AND METHODS
Viruses.
The G and P types were determined for a total of 3,601 rotavirus strains. These viruses were genotyped as part of a large epidemiological survey from samples collected in 16 different locations throughout the United Kingdom between 1995 and 1999 (29).
Serology.
Subgrouping of rotavirus strains was carried out on a subset of 568 samples by enzyme-linked immunosorbent assay using monoclonal antibodies specific for subgroup I (255/60) and subgroup II (631/9) as described previously by Greenberg et al. (26).
G and P typing of rotavirus strains.
RNA extraction and nested reverse transcription (RT)-PCRs using VP4 and VP7 type-specific primers were carried out as previously described (21, 24, 30, 31).
Nucleotide sequencing of rotavirus cDNA.
cDNA sequencing of the VP7 genes of 39 G1P[8], 6 G1P[4], 14 G9P[6], and 19 G9P[8] rotavirus strains was performed. Consensus fragments of 616 nucleotides (nt) or 367 nt in length (nt positions 166 to 782 or 417 to 784 for the G9 or G1 strains, respectively) were used for multiple alignments and phylogenetic analyses. Partial VP4 nucleotide sequencing (of the VP8* subunit, nt positions 11 to 887) of strains of common G and P combinations (29 G1P[8], 4 G3P[8], 5 G4P[8], and 5 G2P[4] strains), of strains with unusual G and P combinations (2 G8P[8], 9 G9P[8], 1 G2P[8], 11 G1P[4], and 1 G4P[4] strains), and of two strains with an unusual P type (G1P[9]) was performed. All sequencing was performed as previously described, using primers Beg9 and End9 for VP7 genes and Con2 and Con3 for VP4 partial sequencing (21, 24, 28, 31). The sequence fragments used for phylogenetic analyses included the type-specific regions and were chosen as the minimum lineage-defining sequence lengths. Lineages within types were defined as clusters of sequences with >90% homology and were confirmed by two different tree-building methods and bootstrapping.
Phylogenetic analysis.
Phylogenetic analysis using the Clustal, maximum-likelihood, maximum-parsimony, and neighbor-joining methods was performed using the Megalign (DNAstar; Lasergene, Madison, Wis.) and Bionumerics (Applied Mathematics, Kortrijk, Belgium) software programs.
Sequences used.
The VP7 partial sequences of the G9 strains found in the United Kingdom have the following EMBL accession numbers: AJ401238 to AJ401267 inclusive. VP7 and VP4 sequences obtained from GenBank/EMBL were used for comparisons. The VP7 sequences were as follows: L14079 (116E); K02033 (Wa); D16343 (KU). The VP4 sequences were as follows: M96825 (Wa); M21014 (KU); U07753 (P[4]); M32559 (P[4]); AF076925 (P[6]); AB008289 (P[9]); D10971 (FRV1); D13403 (Cat2); D90260 (K8); D10970 (AU1).
RESULTS
G and P type combinations of rotavirus strains collected from 16 catchment areas in the United Kingdom from 1995 to 1996 and 1998 to 1999.
Both the G and P types were determined by RT-PCR in 3,601 of 4,021 (89.6%) rotavirus-positive stool specimens collected in the United Kingdom over a period of 4 rotavirus seasons (1995 through 1999). The data are summarized in Table 1 (29; unpublished results for 1998 to 1999). Although G1P[8], G2P[4], G3P[8], and G4P[8] were the four most frequently found cocirculating G and P combinations, representing 95.4% of all typed strains, several subsets of less commonly observed combinations were found in the United Kingdom over this period: (i) unusual combinations of common G and P types, G1P[4], G2P[8], and G4P[4], which represented 1.4% of all typed isolates (Table 1); (ii) an uncommon G/P type combination, G9P[6], which appeared in the United Kingdom first in 1995 to 1996 and represented 0.7% of all strains typed (Table 1); (iii) combinations of common G types with uncommon P types, G1P[6], G1P[9], G3P[6], G3P[9], and G4P[6], representing 0.6% of all strains, and vice versa, of uncommon G types with common P types, G8P[8] and G9P[8], which constituted 2% of all strains (Table 1).
TABLE 1.
G and P genotype combinations of the rotavirus strains typed by RT-PCRa
| G/P combination | No. (%) of combinations in different seasons
|
||||
|---|---|---|---|---|---|
| 1995–1996 | 1996–1997 | 1997–1998 | 1998–1999 | Total | |
| G1P[8] | 407 (56.9) | 919 (86.5) | 830 (73.1) | 632 (92.0) | 2,789 (77.5) |
| G2P[4] | 128 (17.9) | 20 (1.9) | 180 (15.9) | 27 (3.9) | 355 (9.9) |
| G3P[8] | 45 (6.3) | 45 (4.2) | 5 (0.4) | 2 (0.3) | 97 (2.7) |
| G4P[8] | 83 (11.6) | 45 (4.2) | 53 (4.7) | 13 (1.9) | 194 (5.4) |
| Subtotal | 663 (92.7) | 1,029 (96.8) | 1,068 (94.0) | 674 (98.1) | 3,434 (95.4) |
| G1P[4] | 11 (1.5) | 13 (1.2) | 9 (0.8) | 1 (0.1) | 34 (0.9) |
| G1P[6] | 2 (0.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (0.1) |
| G1P[9] | 0 (0.0) | 1 (0.1) | 2 (0.2) | 2 (0.3) | 5 (0.1) |
| G2P[8] | 6 (0.8) | 0 (0.0) | 3 (0.3) | 0 (0.0) | 9 (0.2) |
| G3P[6] | 11 (1.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 11 (0.3) |
| G3P[9] | 1 (0.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.0) |
| G4P[4] | 1 (0.1) | 2 (0.2) | 2 (0.2) | 1 (0.1) | 6 (0.2) |
| G4P[6] | 1 (0.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.0) |
| G8P[8] | 0 (0.0) | 0 (0.0) | 2 (0.2) | 0 (0.0) | 2 (0.1) |
| G9P[6] | 18 (2.5) | 0 (0.0) | 8 (0.7) | 0 (0.0) | 26 (0.7) |
| G9P[8] | 1 (0.1) | 18 (1.7) | 42 (3.7) | 9 (1.3) | 69 (1.9) |
| Subtotal | 52 (7.3) | 34 (3.2) | 68 (6.0) | 13 (1.9) | 167 (4.6) |
| Total typed | 715 | 1,063 | 1,136 | 687 | 3,601 |
Samples were collected during the seasons 1995 through 1999 from up to 16 different catchment areas throughout the United Kingdom. The data collected between 1995 and 1998 have been published previously (29).
Partial VP7 sequences and phylogenetic analysis of G1 strains.
The analyzed strains clustered into three different genetic lineages (I, II, III) and two sublineages, each within lineages I and III (Fig. 1). The lineages were confirmed by both Clustal and maximum-parsimony phylogenetic methods. Some of the lineage-defining amino acid substitutions have been described elsewhere (17, 36), but sublineages within lineage III had not been previously described. G1 viruses of lineage IV were not found among the United Kingdom strains investigated. The sequences of the six G1P[4] strains clustered exclusively within lineages I and II (Fig. 1). The VP7 sequences of the G1P[4] strains were more closely related to the VP7 sequences of G1P[8] strains (>96% homology at nucleotide level) within their corresponding lineages than to those G1P[4] strains of a different lineage (≤95% homology at nucleotide level) (Fig. 1 and Table 2).
FIG. 1.
Phylogenetic tree constructed from nucleotide sequences of the VP7 gene (nt 417 to 784) of the rotavirus strains G1P[8] and possible reassortants G1P[4] using Clustal and neighbor-joining methods. Laboratory number, rotavirus season, G/P combination, and geographical origin of the strains are indicated. Prototype strains KU and Wa were included (GenBank accession numbers D16343 and K02033, respectively). VP7 sequences derived from G1P[4] strains are underlined. Bootstrap values are indicated in the dendrogram. The calibration bar indicates percent homology.
TABLE 2.
Nucleotide sequence homology matrix of VP7 sequences of G1 rotavirus strains of lineages I-B and IIab
| Lineage and strain | % Nucleotide sequence homology
|
|||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 575-95/96 G1P[4] | 5 45-95/96 G1P[4] | 6 | 7 | 8 | 9 608-95/96 G1P[4] | 10 | 11 | 12 | 13 | 14 197-96/97 G1P[4] | 15 | 16 | 17 | 18 | 19 585-95/96 G1P[4] | 20 | 21 | 22 | |
| Lineage II | ||||||||||||||||||||||
| 1 497-95/96 | 100.00 | |||||||||||||||||||||
| 2 343-96/97 | 98.91 | 100.00 | ||||||||||||||||||||
| 3 432-95/96 | 99.39 | 98.57 | 100.00 | |||||||||||||||||||
| 4 575-95/96 G1P[4] | 96.49* | 98.77* | 99.43* | 100.00* | ||||||||||||||||||
| 5 45-95/96 G1P[4] | 96.49* | 97.81* | 99.18* | 99.14* | 100.00* | |||||||||||||||||
| 6 510-95/96 | 99.35 | 98.23 | 99.79 | 99.57* | 99.36* | 100.00 | ||||||||||||||||
| 7 379-96/97 | 96.61 | 97.87 | 99.21 | 97.91* | 97.99* | 99.21 | 100.00 | |||||||||||||||
| 8 4-96/97 | 96.49 | 98.11 | 99.49 | 98.36* | 98.32* | 99.66 | 99.19 | 100.00 | ||||||||||||||
| 9 608-95/96 G1P[4] | 96.49* | 98.11* | 99.71* | 98.81* | 98.43* | 99.77* | 99.04* | 99.68* | 100.00* | |||||||||||||
| Lineage I-B | ||||||||||||||||||||||
| 10 440-95/96 | 93.35 | 94.46 | 96.27 | 95.25 | 95.33 | 96.37 | 96.01 | 96.33 | 96.23 | 100.00 | ||||||||||||
| 11 323-95/96 | 95.42 | 94.28 | 95.98 | 95.84 | 95.81 | 96.11 | 95.53 | 95.99 | 95.93 | 99.83 | 100.00 | |||||||||||
| 12 261-96/97 | 95.55 | 95.85 | 96.17 | 95.85 | 96.02 | 96.18 | 95.69 | 96.02 | 96.01 | 99.24 | 99.24 | 100.00 | ||||||||||
| 13 263-96/97 | 92.98 | 94.34 | 96.11 | 94.44 | 94.79 | 96.13 | 95.46 | 95.77 | 95.81 | 98.60 | 98.65 | 99.88 | 100.00 | |||||||||
| 14 197-96/97 G1P[4] | 92.98 | 94.66 | 96.21 | 94.81 | 95.00 | 96.15 | 95.71 | 95.99 | 94.82 | 98.63* | 98.69* | 99.88* | 99.95* | 100.00* | ||||||||
| 15 493-95/96 | 95.15 | 95.45 | 95.73 | 95.59 | 95.73 | 95.73 | 95.87 | 95.59 | 95.59 | 98.05 | 97.99 | 98.75 | 99.01 | 99.01* | 100.00 | |||||||
| 16 437-95/96 | 92.72 | 94.95 | 96.12 | 94.05 | 94.57 | 95.81 | 95.52 | 95.52 | 95.35 | 98.01 | 98.15 | 99.30 | 98.87 | 98.90* | 99.40 | 100.00 | ||||||
| 17 123-96/97 | 94.75 | 94.79 | 95.59 | 94.94 | 95.09 | 95.09 | 95.25 | 94.94 | 94.93 | 97.70 | 97.70 | 98.88 | 98.77 | 98.77* | 99.08 | 98.93 | 100.00 | |||||
| 18 121-96/97 | 94.55 | 94.55 | 94.87 | 94.70 | 94.86 | 94.86 | 95.02 | 94.70 | 94.69 | 97.07 | 97.07 | 97.66 | 98.19 | 98.19* | 98.19 | 98.03 | 98.47 | 100.00 | ||||
| 19 585-95/96 G1P[4] | 94.25 | 93.56 | 95.27 | 94.41 | 94.46 | 94.59 | 94.33 | 94.46 | 94.45 | 97.29* | 97.29* | 98.57* | 98.19* | 98.19* | 98.05* | 98.62* | 98.39* | 97.95* | 100.00* | |||
| 20 124-96/97 | 95.16 | 95.45 | 96.25 | 95.60 | 95.75 | 95.75 | 95.89 | 95.60 | 95.59 | 98.39 | 98.39 | 99.51 | 99.41 | 99.41* | 98.94 | 99.25 | 99.22 | 98.03 | 98.39* | 100.00 | ||
| 21 271-96/97 | 92.86 | 95.05 | 96.12 | 94.73 | 95.03 | 96.01 | 95.91 | 95.91 | 95.78 | 98.47 | 98.38 | 99.69 | 99.59 | 99.59* | 99.01 | 98.98 | 99.08 | 97.87 | 98.15* | 99.71 | 100.00 | |
| Lineage IV | ||||||||||||||||||||||
| 22 Wa | 92.95 | 92.25 | 93.53 | 90.25 | 91.23 | 94.17 | 89.76 | 89.87 | 91.60 | 91.45 | 93.43 | 92.61 | 89.81 | 91.88 | 92.29 | 89.15 | 92.01 | 91.53 | 91.55 | 92.64 | 90.71 | 100.00 |
Reassortant G1P[4] strains are identified, and homology values between these and all strain of their corresponding lineage are marked with an asterisk. Homology values between G1P[4] reassortant strains of different lineages are indicated in bold.
Strain 608-95/96 G1P[4] in lineage II shared greater sequence homology (98.5% ± 1.1%; range, 96.5 to 99.7%) with strains 379-96/97 G1P[8] (99%), 4-96/97 G1P[8] (99.7%), 497-95/96 G1P[8] (96.5%), 343-95/96 G1P[8] (98.1%), 432-95/96 G1P[8] (98.7%), and 510-95/96 G1P[8] (99.8%), all within lineage II, than with the G1P[4] strains in lineage I-B, 585-95/96 and 197-97 (94.6% ± 0.23%; range, 94.4 to 95.0%, respectively). Similarly, G1P[4] strains 575-95/96 and 45-95/96 exhibit comparable properties. The same is true of strains of lineage I-B, in which strain 585-95/96 G1P[4] shared 98.2% ± 0.4% homology (range, 97.3% to 98.6%) with G1P[8] strains of lineage I-B, i.e., 440-95/96 (97.3%), 323-95/96 (97.3%), 271-96/97 (98.2%), 124-96/97 (98.4%), 493-95/96 (98.1%), 437-95/96 (98.6%), and 123-96/97 (98.4%), compared to 94.6% ± 0.23% homology (range, 94.4 to 95.0%) with G1P[4] strains of lineage II.
Partial VP7 sequences and phylogenetic analysis of G9 strains.
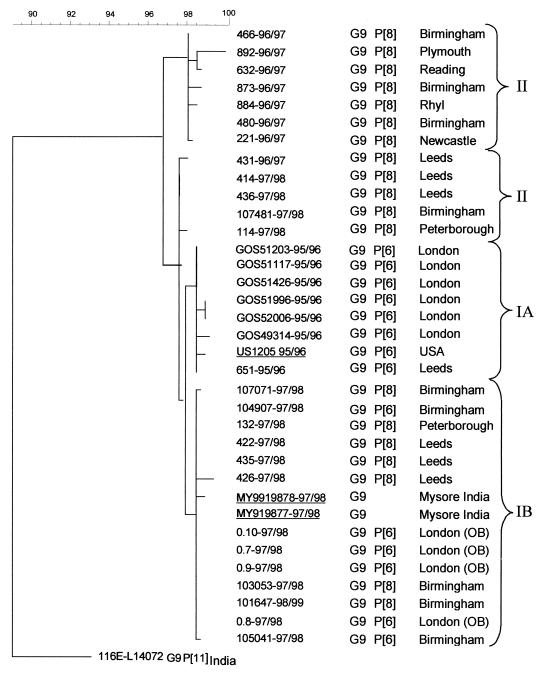
The VP7 sequences (nt 166 to 782) of the G9 strains were all very closely related (identities of ≥97%), but several lineages could be distinguished (Fig. 2). Within lineage I-B, the VP7 genes of G9P[6] and G9P[8] strains shared nearly identical sequences (e.g., 107071–97/98 G9P[8] Birmingham with 104907–97/98 G9P[6] Birmingham, 132–97/98 G9P[8] Peterborough, 422–97/98 G9P[8] Leeds, etc.).
FIG. 2.
Phylogenetic tree constructed from partial nucleotide sequences of the VP7 gene (nt 166 to 782) of the G9 rotavirus strains using the maximum-likelihood method. Nomenclature of the sequences indicates the laboratory number, season of isolation, the G and P types of the strains, and the geographical origin, OB indicates outbreak strains. The VP7 sequence of strain US1205 was provided by C. Kirkwood and that of strain 116E was obtained from GenBank (accession number L14072). Brackets indicate lineages I-A, I-B, II, and III. The calibration bar indicates percent homology. Strains from the United States and India are underlined. Sequences of Mysore strains were obtained from G. Kang, Vellore, India.
Partial VP4 sequences and phylogenetic analysis of P[8] strains.
All P[8] genes analyzed clustered in three previously defined lineages (24, 31, 36) (Fig. 3), but the following observations are noteworthy.
FIG. 3.
Phylogenetic tree constructed from partial nucleotide sequences of the VP8* fragment of VP4 genes (nt 11 to 887) of the rotavirus strains G1P[8], G3P[8], and G4P[8] and of possible reassortants G2P[8], G8P[8], and G9P[8] using the Clustal method and Megalign. Laboratory number, rotavirus season, G/P combination, and geographical origin of the strains are indicated. Representative strains of the different VP7 G1 lineages (Fig. 2) are indicated (∗). Strains Wa and KU (GenBank/EMBL accession no. M96825 and M2114, respectively) and a P[6] strain (GenBank/EMBL accession no. AF076925) were included for comparisons. Strains whose sequences were obtained from GenBank/EMBL are shaded. The calibration bar indicates percent divergence.
(i) No significant genetic differences were observed in the VP8* region of the VP4 gene between the putative reassortant viruses (G8P[8], G9P[8] and G2P[8]) and the strains of common G and P combinations (G1P[8], G3P[8], and G4P[8]). Reassortants and common types fell within the same lineages and sublineages (Fig. 3).
(ii) P[8] lineage I was found to be exclusively made up of G1 strains (Fig. 3).
(iii) P[8] lineage III was found less frequently than the other two lineages and in combination with VP7 genes of G4 and G9 but not of G1.
Partial VP4 sequences and phylogenetic analysis of P[4] strains.
The VP4 genes of G1P[4] (uncommon) were very closely related to those of representative G2P[4] (common) strains (Fig. 4; e.g., 621–95/96 G2P[4] Cambridge with 111–95/96 G1P[4] Belfast and 487–97/98 G1P[4] Plymouth; 30–96/97 G2P[4] Reading with 85–96/97 G1P[4] Reading; 525–95/96 G2P[4] Cambridge with 575–95/96 G1P[4] Cambridge; 1142–96/97 G2P[4] Plymouth and 468–96/97 G2P[4] Birmingham with 1070–96/97 G1P[4] Plymouth and 1131–96/97 G1P[4] Plymouth).
FIG. 4.
Phylogenetic tree constructed from nucleotide sequences of VP8* regions of the VP4 genes (nt 11 to 887) of rotavirus strain G2P[4] and possible reassortants G1P[4] and G4P[4], using the Clustal method and Megalign. Laboratory number, rotavirus season, G/P combination, and geographical origin of the strains are indicated. VP4 sequences of two P[4] strains (GenBank/EMBL accession no. U07753 and M32559) and a P[8] strain (Wa, GenBank/EMBL accession no. M96825) were included for comparison (shaded). Possible reassortant strains are underlined. The calibration bar indicates percent divergence.
Partial VP4 sequences and phylogenetic analysis of P[9] strains.
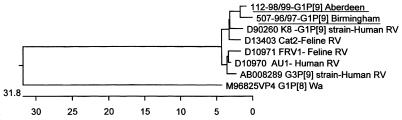
Partial VP4 gene sequences of two G1P[9] strains (nt 232 to 822) were compared with P[9] sequences obtained from the GenBank database (Fig. 5). Their partial sequences, like those of other human P[9] rotavirus strains found elsewhere (AU, K8), were closely related to the corresponding sequences of rotaviruses of feline origin (Cat2, FRV1).
FIG. 5.
Phylogenetic tree constructed from partial nucleotide sequences (nt 232 to 822) of the VP4 gene (corresponding to the hypervariable region of VP8*) of the P[9] rotavirus strains found in the United Kingdom (underlined) and reference human and feline rotavirus P[9] strains, using the Clustal method and Megalign. Nomenclature of the strains indicates the laboratoy number followed by the G/P type and the geographical origin of the isolates for the United Kingdom strains. Sequences of P[9] strains and of a P[8] strain (Wa) were used for comparison. Accession numbers, strains, and hosts from which the P[9] strains were isolated are indicated. The calibration bar indicates percent divergence.
VP6 serology and G/P combinations.
The subgroups were characterized for 568 fully G- and P-genotyped strains; 116 (20.4%) were of subgroup I, 365 (64.3%) were of subgroup II, 24 (4.2%) were of subgroups I and II both, and 63 (11.1%) were non-I, non-II. While most viruses of subgroups I and II were G2P[4] and G1P[8], G3P[8], or G4P[8], there were 10 exceptions (Table 3): one specimen each of the G1P[8], G3P[8], and G4P[8] viruses was of subgroup I, and seven of the G2P[4] viruses were of subgroup II. Thus, the number of strains with common G and P combinations but from uncommon subgroups amounts to 10 of 468 (2.2%) strains investigated and appears with a frequency similar to that for the unusual combinations of common G and P types (1.4%; Tables 1 and 3).
TABLE 3.
VP6-specific subgroup of 481 rotavirus strains with full G/P type identificationa
| G/P combination | No. of subgroup I strains for each GP combination | % Total no. of subgroup I strains | No. of subgroup II strains for each GP combination | % Total no. of subgroup II strains | Total no. of strains for G/P GP combinationb |
|---|---|---|---|---|---|
| G1P[8] | 1 | 0.9 | 265 | 72.6 | 266 |
| G2P[4] | 108 | 93.9 | 7 | 1.9 | 115 |
| G3P[8] | 1 | 0.9 | 36 | 9.9 | 37 |
| G4P[8] | 1 | 0.9 | 48 | 13.2 | 49 |
| G1P[4] | 3 | 2.6 | 4 | 1.1 | 7 |
| G2P[8] | 0 | 0.0 | 4 | 1.1 | 4 |
| G3P[9] | 1 | 0.0 | 0 | 0.0 | 1 |
| G4P[4] | 1 | 0.9 | 0 | 0.0 | 1 |
| G9P[6] | 0 | 0.0 | 1 | 0.3 | 1 |
| Total | 116 | 365 | 481 |
The numbers of strains with common G and P genotype combinations but with an unusual subgroup association are indicated in boldface.
Samples reacting as I + II or non-I, non-II were excluded.
DISCUSSION
Epidemiological surveys of rotavirus infections have been carried out since the late 1970s (reviewed in reference 16). Initially, G serotyping by means of monoclonal antibodies established that serotypes G1 to G4 predominated in Europe, North America, and Australia but also established that many rotaviruses were not typeable at the time (termed “non-G1 to G4” rotaviruses) and were very prevalent in South America, Africa, and Asia. Since the early 1990s, when genotyping became available for both the G and P components of the virus (21, 24), a more complete assessment has been possible and a more complex picture has emerged. Viruses with various G and P combinations were found, suggesting a diverse gene pool in the overall population of human rotaviruses and leading to speculations about their origin (16).
There is now strong evidence to suggest that cocirculating rotavirus strains continuously interact genetically by reassortment. Sequencing of the VP7 and VP4 genes of rotavirus strains with common and uncommon G/P combinations, selected from a large collection of strains found in the population of the United Kingdom between 1995 and 1999 (29), provided the evidence of reassortment between cocirculating rotavirus strains.
The comparison of sequence data obtained from G1P[8] strains and of the possible reassortant strains G1P[4] showed that the VP7 sequences of all the G1 strains segregated into lineages independent of their P type. The G1 sequences of G1P[4] strains are firmly embedded in the G1 lineages I and II of common G1P[8] strains and are not a component of a separate lineage. Similarly, VP4 sequences of G2P[4], G4P[4], and G1P[4] clustered together and were highly homologous. These findings strongly suggest that G2P[8], G1P[4], and G4P[4] strains originated through reassortment between the cocirculating G1P[8], G2P[4], and G4P[8] rotavirus strains. Also, the putative reassortant strains were found more commonly in seasons in which G1P[8], G2P[4], G3P[8], and G4P[8] were cocirculating at relatively high frequencies and were found rarely in seasons in which G1P[8] strains were overwhelmingly predominant (Table 1).
Further evidence for reassortment was obtained by comparing partial VP4 sequences obtained from P[8] strains in combination with the G1, G2, G3, G4, G8, or G9 type. Strains of P[8] lineages I and II were found more frequently than strains of lineage III. Particular associations were found between the P[8] lineages and the G types of the different strains. P[8] lineage II was found to contain rotavirus strains of G types G1, G2, G3, G4, G8, and G9, and the VP4 sequences of all these strains were highly homologous. The clustering within this lineage was not associated with the G type of the strains. This may suggest that these strains have all originated through reassortment during dual infection. By contrast, all the strains in P[8] lineage I were G1, which may indicate some constraint to reassortment involving these strains. However, a small number of G4P[8] strains of P[8] lineage I have been identified in a study in Finland (36). P[8] lineage III was found at much lower frequency, and only G4 or G9 strains were identified in this cluster; the lack of G1 strains associated with this lineage may explain its lower incidence. Also, this may suggest that there are constraints against reassortment between P[8] lineage III strains and G1 strains and perhaps also between P[8] and other G types; however, larger data sets are necessary to strengthen this hypothesis.
Reassortment opportunities depend on double infections of cells and hosts. Those have been found with a frequency of 2% in the United Kingdom collection (29). Mixed infections were identified by the presence of more than one amplicon corresponding to different G and/or P types in the same sample and included G1 + G2/P[4] + P[8], G1 + G2/P[4], G1 + G2/P[8], G2 + G4/P[4], G1 + G9/P[6], and G1 + G9/P[8], which could have given rise to the reassortant strains described in this study (G1P[4], G2P[8], G4P[4], and G9P[8]). In controlled animal experiments, isolates containing two parental strains and a reassortant strain or containing one parental strain and a reassortant strain were found (22). It is possible that the four common G/P combinations represent the genetically fittest rotavirus strains and that other combinations may be evolutionary dead ends. However, mixed infections and putative reassortant strains have been found at much higher frequency (up to 30%) in other countries, particularly in the developing world (1, 49), and there is evidence that putative reassortants can spread and become epidemiologically significant strains, e.g., G1P[4] in Argentina (2) or G2P[8] in Bangladesh (49). Also, the increase in the incidence and spread of infection with G9P[8] strains during 3 consecutive years in the United Kingdom (29) suggests that even if G1P[8], G2P[4], G3P[8], and G4P[8] strains are seemingly better adapted in genetic terms, there is still room for the successful introduction and cocirculation year after year for other rotavirus strains.
Strains G1 to G4 in combination with P types other than P[8] and P[4] and non-G1-to-G4 strains were relatively rare in the collection of samples analyzed here, with the exception of G9 strains. G9 strains were first found in the United Kingdom as G9P[6] in 1995 to 1996 (10, 28) and were displaced by G9P[8] strains in subsequent years. There was evidence that G9P[6] strains are a relatively recent introduction into the human population (28, 29) and that reassortment was the underlying mechanism for the origin of the G9P[8] strains, based on phylogenetic analysis of VP7 and VP4 gene sequences. The relative lack of diversity between the VP7 nucleotide sequences of the United Kingdom G9 strains and between the VP4 nucleotide sequences of the P[6] strains, the chronological clustering of the VP7 sequences of G9 strains into lineages, and the coexistence of more than one VP7 lineage coinciding with the time in which the incidence and spread of G9P[8] strains reached the highest levels all provide evidence for a relatively recent introduction of G9P[6] strains, their reassortment with common P[8] strains, and the spread of G9P[8] rotaviruses into the human population. In contrast, the VP4 nucleotide sequence of the G9P[8] strains revealed greater genetic variability, clustering in the same global lineages identified from sequences of P[8] rotavirus strains occurring in combination with other G types (Fig. 4) and not correlating with geographical or temporal clustering (25, 31, 36). Dual infections in 1995 to 1996, characterized by samples containing G1 and G9 in combination with P[6] and containing G1 and G9 in combination with P[8], which are a prerequisite for reassortment, were also found (28).
P[9] sequences in two G1 strains found in the United Kingdom were closely related to those of rotaviruses of feline origin, similar to recent findings in the United States (27). There is now increasing evidence that animal strains may infect humans and possibly become more prevalent in humans, e.g., G5P[8] in Brazil, G9P[11] in India, and G9P[6] in India and more recently in the United States and Europe (reviewed in reference 16).
Uncommon associations between G/P type and subgroup occurred at approximately 2%; such viruses have previously been shown to be in vivo reassortants (19, 34). As observed in other studies, the majority of the subgroup I strains were G2P[4] strains. However, a small percentage of G2P[4] strains were of subgroup II, which provided further evidence for reassortment. If it is assumed that a subgroup normally segregates with P type (P[4] with subgroup I and P[8] with subgroup II), then four G1P[4] strains which should be of subgroup I can be regarded as violating this rule; they may have arisen through the reassortment of the VP4 of G2P[4] subgroup I with the VP7 and VP6 of G1P[8] subgroup II or are possible double reassortants. The presence of VP6 reassortants suggests that this mechanism of creating diversity is not confined to the VP4 and VP7 genes of rotaviruses.
Reassortment appears to be the principal mechanism responsible for the enormous diversity within the rotavirus population. The ease with which rotaviruses can reassort in vitro and in vivo and the occasional isolation from humans of rotaviruses containing gene segments highly homologous to those of animal strains also support this evolutionary mechanism (5, 40, 41, 48).
The introduction of novel rotavirus strains into the human population in the developing world is thought to be associated with close contact among humans and between humans and domestic livestock, especially in areas prone to flooding or with a monsoon climate (1). Perhaps it is not surprising that in the developing countries, where crowded living conditions and closer contact with domestic animals are common, there is a greater diversity of rotaviruses in the human population than in developed countries. In time, G1P[8], G2P[4], G3P[8], and G4P[8] may no longer be considered the “common” human rotavirus types, at least in some parts of the world. The unusual strains found in the United Kingdom may have been introduced by importation from other countries where the above conditions are met and where “unusual” genotypes are more prevalent, or they may have been introduced into the human population either by zoonotic transmission or by reassortment between animal and human rotaviruses (29).
From the present study it can be estimated that 1 in 20 rotavirus infections in the United Kingdom, amounting to approximately 25,000 cases in England and Wales annually (29), is caused by rotavirus strains different from G1P[8], G2P[4], G3P[8], and G4P[8], which are considered to be the most common rotavirus strains cocirculating at present. Those strains that have unusual combinations of the above G and P types are unlikely to have a major impact in the levels of protection of the population or for vaccination purposes. However, approximately 60% of the possible reassortants observed have G and/or P types different from those given above and may have an animal origin. Furthermore, G9P[8] strains were found in the United Kingdom to be the third most common strains between 1997 and 1999, with an incidence higher than that of G3P[8] strains (29; unpublished data).
In summary, there is now compelling evidence to put forth reassortment among cocirculating human rotavirus strains as a continuously operative mechanism, as suggested by Gouvea and Brantly (23), and as analogous to that observed for influenza viruses, where the significance of reassortment for pathogenesis has been worked out in detail (42). Cosurveillance of animal and human rotavirus strains will be vital to gain a better understanding of the relationships between cocirculating rotaviruses, before and during the implementation of any vaccination program.
ACKNOWLEDGMENTS
This work was supported by a grant of the Public Health Laboratory Service, London, United Kingdom.
We are grateful to Lynne Bastow for typing and processing of the manuscript.
REFERENCES
- 1.Ahmed U M, Urasawa S, Taniguchi K, Urasawa T, Kobayashi N, Wakasugi F, Islam A I, Sahikh H A. Analysis of human rotavirus strains prevailing in Bangladesh in relation to nationwide floods brought by the 1988 monsoon. J Clin Microbiol. 1991;29:2273–2279. doi: 10.1128/jcm.29.10.2273-2279.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Argüelles H M, Villegas G A, Castello A, Abrami A, Ghiringhelli P D, Semorile L, Glikmann G. VP7 and VP4 genotyping of human group A rotavirus in Buenos Aires, Argentina. J Clin Microbiol. 2000;38:252–259. doi: 10.1128/jcm.38.1.252-259.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bern C, Martines J, Zoysa I, Glass R I. The magnitude of the global problem of diarrhoeal disease: a ten-year update. Bull W H O. 1992;70:705–714. [PMC free article] [PubMed] [Google Scholar]
- 4.Blackhall J, Fuentes A, Magnusson G. Genetic stability of a porcine rotavirus RNA segment during repeated plaque isolation. Virology. 1996;225:181–190. doi: 10.1006/viro.1996.0586. [DOI] [PubMed] [Google Scholar]
- 5.Browning G F, Snodgrass D R, Nakagomi O, Kaga E, Sarasini A, Gerna G. Human and bovine serotype G8 rotaviruses may be derived by reassortment. Arch Virol. 1992;125:121–128. doi: 10.1007/BF01309632. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Rotavirus vaccine for the prevention of rotavirus gastroenteritis among children. Recommendations of the Advisory Committee on Immunization Practices (ACIP) Morb Mortal Wkly Rep. 1999;48:1–20. [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Intussusception among recipients of rotavirus vaccine—United States. 1998–1999. Morb Mortal Wkly Rep. 1999;48:577–581. [PubMed] [Google Scholar]
- 8.Chen D Y, Estes M K, Ramig R F. Specific interactions between rotavirus outer capsid proteins VP4 and VP7 determine expression of a cross-reactive, neutralizing VP4-specific epitope. J Virol. 1992;66:432–439. doi: 10.1128/jvi.66.1.432-439.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coulson B S, Kirkwood C D, Masendycz P J, Bishop R F, Gerna G. Amino acids involved in distinguishing between monotypes of rotavirus G serotypes 2 and 4. J Gen Virol. 1996;77:239–245. doi: 10.1099/0022-1317-77-2-239. [DOI] [PubMed] [Google Scholar]
- 10.Cubitt W D, Steele A D, Iturriza M. Characterisation of rotaviruses from children treated at a London hospital during 1996: emergence of strains G9P2A[6] and G3P2A[6] J Med Virol. 2000;61:150–154. doi: 10.1002/(sici)1096-9071(200005)61:1<150::aid-jmv24>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 11.Cunliffe N A, Gondwe J S, Broadhead R L, Molyneux M E, Woods P A, Bresee J S, Glass R I, Gentsch J R, Hart C A. Rotavirus G and P types in children with acute diarrhea in Blantyre, Malawi, from 1997 to 1998: predominance of novel P[6]G8 strains. J Med Virol. 1999;57:308–312. [PubMed] [Google Scholar]
- 12.Das M, Dunn S J, Woode G N, Greenberg H B, Rao C D. Both surface proteins (VP4 and VP7) of an asymptomatic neonatal rotavirus strain (I321) have high levels of sequence identity with the homologous proteins of a serotype 10 bovine rotavirus. Virology. 1993;194:374–379. doi: 10.1006/viro.1993.1271. [DOI] [PubMed] [Google Scholar]
- 13.Desselberger U. Genome rearrangements of rotaviruses. Adv Virus Res. 1996;46:69–95. doi: 10.1016/s0065-3527(08)60070-6. [DOI] [PubMed] [Google Scholar]
- 14.Desselberger U. Reoviruses. In: Mahy B W J, Collier L, editors. Topley & Wilson's microbiology and microbial infections. 9th ed. 1. Virology. London, United Kingdom: E. Arnold; 1998. pp. 537–550. [Google Scholar]
- 15.Desselberger U, Estes M. Future rotavirus research. In: Gray J, Desselberger U, editors. Rotaviruses: methods and protocols. Totowa, N.J: Humana Press; 2000. pp. 239–258. [DOI] [PubMed] [Google Scholar]
- 16.Desselberger, U., M. Iturriza-Gómara, and J. Gray. Rotavirus epidemiology and surveillance. In D. Chadwick and J. Goode (ed.), Viral gastroenteritis. Novartis Foundation Symposium, vol. 238. John Wiley & Sons, Chichester, United Kingdom, in press. [DOI] [PubMed]
- 17.Diwarkarla C, Palombo E. Genetic and antigenic variation of capsid protein VP7 of serotype G1 human rotavirus isolates. J Gen Virol. 1999;80:341–344. doi: 10.1099/0022-1317-80-2-341. [DOI] [PubMed] [Google Scholar]
- 18.Estes M. Rotaviruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 1625–1655. [Google Scholar]
- 19.Garbarg-Chenon A, Bricout F, Nicolas J C. Study of genetic reassortment between two human rotaviruses. Virology. 1984;139:358–365. doi: 10.1016/0042-6822(84)90381-7. [DOI] [PubMed] [Google Scholar]
- 20.Gentsch J R, Das B K, Jiang B, Bhan M K, Glass R I. Similarity of the VP4 protein of human rotavirus strain 116E to that of the bovine B223 strain. Virology. 1993;194:424–430. doi: 10.1006/viro.1993.1280. [DOI] [PubMed] [Google Scholar]
- 21.Gentsch J R, Glass R I, Woods P, Gouvea V, Gorziglia M, Flores J, Das B K, Bhan M K. Identification of group A rotavirus gene 4 types by polymerase chain reaction. J Clin Microbiol. 1992;30:1365–1373. doi: 10.1128/jcm.30.6.1365-1373.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gombold J L, Ramig R F. Analysis of reassortment of genome segments in mice mixedly infected with rotaviruses SA11 and RRV. J Virol. 1986;57:110–116. doi: 10.1128/jvi.57.1.110-116.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gouvea V, Brantly M. Is rotavirus a population of reassortants? Trends Microbiol. 1995;3:159–162. doi: 10.1016/s0966-842x(00)88908-8. [DOI] [PubMed] [Google Scholar]
- 24.Gouvea V, Glass R I, Woods P, Taniguchi K, Clark H F, Forrester B, Fang Z Y. Polymerase chain reaction amplification and typing of rotavirus nucleic acid from stool specimens. J Clin Microbiol. 1990;28:276–282. doi: 10.1128/jcm.28.2.276-282.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gouvea V, Lima R C, Linhares R E, Clark H F, Nosawa C M, Santos N. Identification of two lineages (WA-like and F45-like) within the major rotavirus genotype P[8] Virus Res. 1999;59:141–147. doi: 10.1016/s0168-1702(98)00124-5. [DOI] [PubMed] [Google Scholar]
- 26.Greenberg H, McAuliffe V, Valdesuso J, Wyatt R, Flores J, Kalica A, Hoshino Y, Singh N. Serological analysis of the subgroup protein of rotavirus, using monoclonal antibodies. Infect Immun. 1983;39:91–99. doi: 10.1128/iai.39.1.91-99.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Griffin D D, Kirkwood C D, Parashar U D, Woods P A, Bresee J S, Glass R I, Gentsch J R The National Rotavirus Strain Surveillance System Collaborating Laboratories. Surveillance of rotavirus strains in the United States: identification of unusual strains. J Clin Microbiol. 2000;38:2784–2787. doi: 10.1128/jcm.38.7.2784-2787.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iturriza-Gómara M, Cubitt D, Steele D, Green J, Brown D, Kang G, Desselberger U, Gray J. Characterisation of rotavirus G9 strains isolated in the UK between 1995 and 1998. J Med Virol. 2000;61:510–517. doi: 10.1002/1096-9071(200008)61:4<510::aid-jmv15>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 29.Iturriza-Gómara M, Green J, Brown D W G, Ramsay M, Desselberger U, Gray J J. Molecular epidemiology of human group A rotavirus infections in the United Kingdom between 1995 and 1998. J Clin Microbiol. 2000;38:4394–4401. doi: 10.1128/jcm.38.12.4394-4401.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iturriza-Gómara M, Green J, Brown D W, Desselberger U, Gray J J. Comparison of specific and random priming in the reverse transcriptase polymerase chain reaction for genotyping group A rotaviruses. J Virol Methods. 1999;78:93–103. doi: 10.1016/s0166-0934(98)00168-2. [DOI] [PubMed] [Google Scholar]
- 31.Iturriza-Gómara M, Green J, Brown D W, Desselberger U, Gray J J. Diversity within the VP4 gene of rotavirus P[8] strains: implications for reverse transcription-PCR genotyping. J Clin Microbiol. 2000;38:898–901. doi: 10.1128/jcm.38.2.898-901.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Joensuu J, Koskenniemi E, Pang X L, Vesikari T. Randomised placebo-controlled trial of rhesus-human reassortant rotavirus vaccine for prevention of severe rotavirus gastroenteritis. Lancet. 1997;350:1205–1209. doi: 10.1016/S0140-6736(97)05118-0. [DOI] [PubMed] [Google Scholar]
- 33.Kapikian A Z. Overview of viral gastroenteritis. Arch Virol Suppl. 1996;12:7–19. doi: 10.1007/978-3-7091-6553-9_2. [DOI] [PubMed] [Google Scholar]
- 34.Krishnan T, Naik T N, Desselberger U. Molecular epidemiology of human rotaviruses: reassortment in vivo as a mechanism for strain diversity? J Infect. 1996;32:169–170. doi: 10.1016/s0163-4453(96)91653-9. [DOI] [PubMed] [Google Scholar]
- 35.López S, Espinosa R, Greenberg H B, Arias C F. Mapping the subgroup epitopes of rotavirus protein VP6. Virology. 1994;204:153–162. doi: 10.1006/viro.1994.1519. [DOI] [PubMed] [Google Scholar]
- 36.Maunula L, von Bonsdorff C H. Short sequences define genetic lineages: phylogenetic analysis of group A rotaviruses based on partial sequences of genome segments 4 and 9. J Gen Virol. 1998;79:321–332. doi: 10.1099/0022-1317-79-2-321. [DOI] [PubMed] [Google Scholar]
- 37.McLean A. Vaccination, evolution and changes in the efficacy of vaccines: a theoretical framework. Proc R Soc Lond B. 1995;261:389–393. doi: 10.1098/rspb.1995.0164. [DOI] [PubMed] [Google Scholar]
- 38.Mendez E, Arias C F, López S. Interactions between the two surface proteins of rotavirus may alter the receptor-binding specificity of the virus. J Virol. 1996;70:1218–1222. doi: 10.1128/jvi.70.2.1218-1222.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakagomi O, Isegawa Y, Ward R L, Knowlton D R, Kaga E, Nakagomi T, Ueda S. Naturally occurring dual infection with human and bovine rotaviruses as suggested by the recovery of G1P8 and G1P5 rotaviruses from a single patient. Arch Virol. 1994;137:381–388. doi: 10.1007/BF01309483. [DOI] [PubMed] [Google Scholar]
- 40.Nakagomi O, Nakagomi T. Genetic diversity and similarity among mammalian rotaviruses in relation to interspecies transmission of rotavirus. Arch Virol. 1991;120:43–55. doi: 10.1007/BF01310948. [DOI] [PubMed] [Google Scholar]
- 41.Nakagomi O, Nakagomi T. Molecular evidence for naturally occurring single VP7 gene substitution reassortant between human rotaviruses belonging to two different genogroups. Arch Virol. 1991;119:67–81. doi: 10.1007/BF01314324. [DOI] [PubMed] [Google Scholar]
- 42.Palese P. Reassortment continuum. In: Notkins A L, Oldstone M B A, editors. Concepts in viral pathogenesis. New York, N.Y: Springer-Verlag; 1984. pp. 144–151. [Google Scholar]
- 43.Pérez-Schael I, Guntiñas M J, Pérez M, Pagone V, Rojas A M, Gonzalez R, Cunto W, Hoshino Y, Kapikian A Z. Efficacy of the rhesus rotavirus-based quadrivalent vaccine in infants and young children in Venezuela. N Engl J Med. 1997;337:1181–1187. doi: 10.1056/NEJM199710233371701. [DOI] [PubMed] [Google Scholar]
- 44.Raj P, Matson D O, Coulson B S, Bishop R F, Taniguchi K, Urasawa S, Greenberg H B, Estes M K. Comparisons of rotavirus VP7-typing monoclonal antibodies by competition binding assay. J Clin Microbiol. 1992;30:704–711. doi: 10.1128/jcm.30.3.704-711.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramig R F. Genetics of the rotaviruses. Annu Rev Microbiol. 1997;51:225–255. doi: 10.1146/annurev.micro.51.1.225. [DOI] [PubMed] [Google Scholar]
- 46.Rennels M B, Glass R I, Dennehy P H, Bernstein D I, Pichichero M E, Zito E T, Mack M E, Davidson B L, Kapikian A Z. Safety and efficacy of high-dose rhesus-human reassortant rotavirus vaccines—report of the National Multicenter Trial. United States Rotavirus Vaccine Efficacy Group. Pediatrics. 1996;97:7–13. [PubMed] [Google Scholar]
- 47.Rennels M B, Ward R L, Mack M E, Zito E T. Concurrent oral poliovirus and rhesus-human reassortant rotavirus vaccination: effects on immune responses to both vaccines and on efficacy of rotavirus vaccines. The US Rotavirus Vaccine Efficacy Group. J Infect Dis. 1996;173:306–313. doi: 10.1093/infdis/173.2.306. [DOI] [PubMed] [Google Scholar]
- 48.Santos N, Lima R, Nozawa C, Linhares R, Gouvea V. Detection of porcine rotavirus type G9 and of a mixture of types G1 and G5 associated with Wa-like VP4 specificity: evidence for natural human-porcine genetic reassortment. J Clin Microbiol. 1999;37:2734–2736. doi: 10.1128/jcm.37.8.2734-2736.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Unicomb L E, Podder G, Gentsch J R, Woods P A, Hasan K Z, Faruque A S, Albert M J, Glass R I. Evidence of high-frequency genomic reassortment of group A rotavirus strains in Bangladesh: emergence of type G9 in 1995. J Clin Microbiol. 1999;37:1885–1891. doi: 10.1128/jcm.37.6.1885-1891.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Velazquez F R, Matson D O, Calva J J, Guerrero L, Morrow A L, Carter Campbell S, Glass R I, Estes M K, Pickering L K, Ruiz Palacios G M. Rotavirus infections in infants as protection against subsequent infections. N Engl J Med. 1996;335:1022–1028. doi: 10.1056/NEJM199610033351404. [DOI] [PubMed] [Google Scholar]
- 51.Zao C L, Yu W N, Kao C L, Taniguchi K, Lee C Y, Lee C N. Sequence analysis of VP1 and VP7 genes suggests occurrence of a reassortant of G2 rotavirus responsible for an epidemic of gastroenteritis. J Gen Virol. 1999;80:1407–1415. doi: 10.1099/0022-1317-80-6-1407. [DOI] [PubMed] [Google Scholar]