Abstract
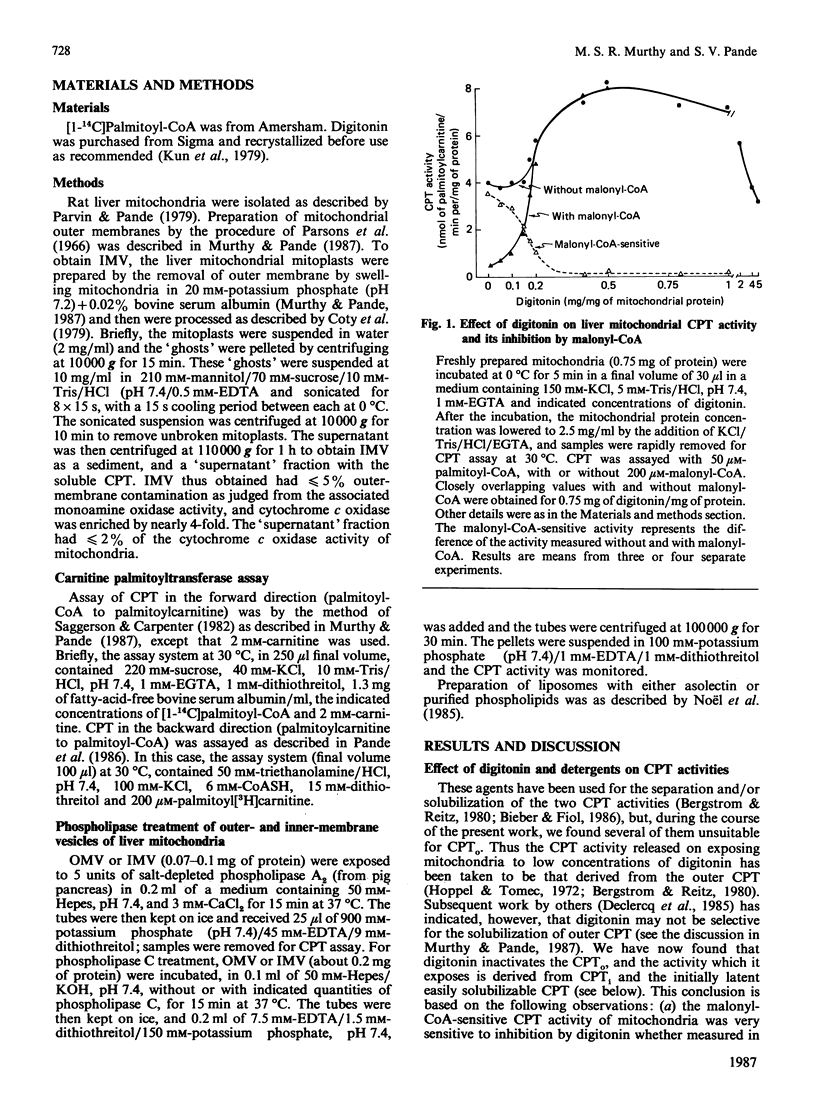
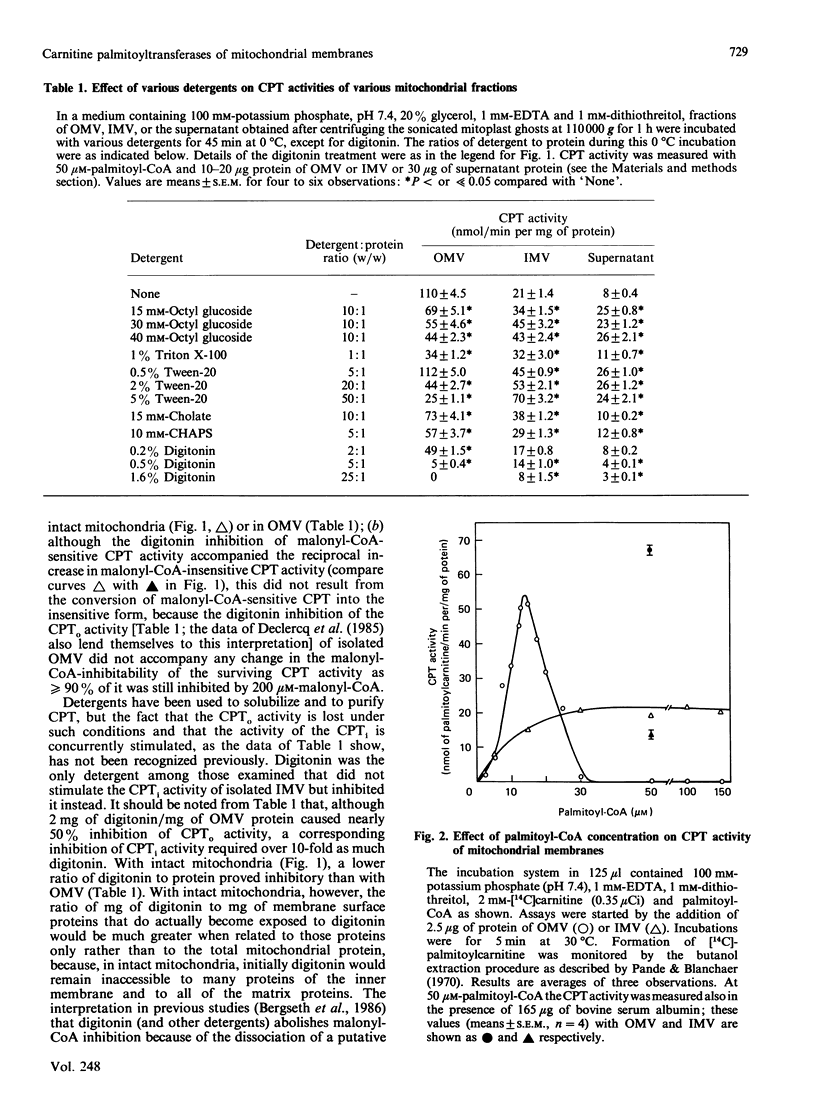
Recent evidence has shown that the outer, overt, malonyl-CoA-inhibitable carnitine palmitoyltransferase (CPTo) activity resides in the mitochondrial outer membrane [Murthy & Pande (1987) Proc. Natl. Acad. Sci. U.S.A. 84, 378-382]. A comparison of CPTo activity of rat liver mitochondria with the inner, initially latent, carnitine palmitoyltransferase (CPTi) of the mitochondrial inner membrane has revealed that the presence of digitonin and several other detergents inactivates CPTo activity. The CPTi activity, in contrast, was markedly stimulated by various detergents and phospholipid liposomes. These findings explain why in previous studies, which used digitonin or other detergents to expose, separate and purify the CPT activities, the inferences were drawn that (a) the ratio of latent to overt CPT was quite high, (b) both the CPT activities could be ascribed to one active protein recovered, and (c) the observed lack of malonyl-CoA inhibition indicated possible loss/separation of a putative malonyl-CoA-inhibition-conferring protein. Although both CPTo and CPTi were found to catalyse the forward and the backward reactions, CPTo showed greater capacity for the forward reaction and CPTi for the backward reaction. The easily solubilizable CPT, released on sonication of mitoplasts or of intact mitochondria under hypo-osmotic conditions, resembled CPTi in its properties. When octyl glucoside was used under appropriate conditions, 40-50% of the CPTo of outer membranes became solubilized, but it showed limited stability and decreased malonyl-CoA sensitivity. Malonyl-CoA-inhibitability of CPTo was decreased also on exposure of outer membranes to phospholipase C. When outer membranes that had been exposed to octyl glucoside or to phospholipase C were subjected to a reconstitution procedure using asolectin liposomes, the malonyl-CoA-inhibitability of CPTo was restored. A role of phospholipids in the malonyl-CoA sensitivity of CPTo is thus indicated.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergseth S., Lund H., Bremer J. Is carnitine palmitoyltransferase inhibited by a malonyl-CoA-binding unit in the mitochondria? Biochem Soc Trans. 1986 Aug;14(4):671–672. doi: 10.1042/bst0140671. [DOI] [PubMed] [Google Scholar]
- Bergstrom J. D., Reitz R. C. Studies on carnitine palmitoyl transferase: the similar nature of CPTi (inner form) and CPTo (outer form). Arch Biochem Biophys. 1980 Oct 1;204(1):71–79. doi: 10.1016/0003-9861(80)90008-9. [DOI] [PubMed] [Google Scholar]
- Bieber L. L., Fiol C. J. Characterization and properties of carnitine acyltransferases. Biochem Soc Trans. 1986 Aug;14(4):674–676. doi: 10.1042/bst0140674. [DOI] [PubMed] [Google Scholar]
- Brass E. P., Hoppel C. L. Effect of carnitine on mitochondrial oxidation of palmitoylearnitine. Biochem J. 1980 May 15;188(2):451–458. doi: 10.1042/bj1880451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremer J. Carnitine--metabolism and functions. Physiol Rev. 1983 Oct;63(4):1420–1480. doi: 10.1152/physrev.1983.63.4.1420. [DOI] [PubMed] [Google Scholar]
- Clarke P. R., Bieber L. L. Effect of micelles on the kinetics of purified beef heart mitochondrial carnitine palmitoyltransferase. J Biol Chem. 1981 Oct 10;256(19):9869–9873. [PubMed] [Google Scholar]
- Clarke P. R., Bieber L. L. Isolation and purification of mitochondrial carnitine octanoyltransferase activities from beef heart. J Biol Chem. 1981 Oct 10;256(19):9861–9868. [PubMed] [Google Scholar]
- Coty W. A., Wehrle J. P., Pedersen P. L. Measurement of phosphate transport in mitochondria and in inverted inner membrane vesicles of rat liver. Methods Enzymol. 1979;56:353–359. doi: 10.1016/0076-6879(79)56032-7. [DOI] [PubMed] [Google Scholar]
- Declercq P. E., Venincasa M. D., Mills S. E., Foster D. W., McGarry J. D. Interaction of malonyl-CoA and 2-tetradecylglycidyl-CoA with mitochondrial carnitine palmitoyltransferase I. J Biol Chem. 1985 Oct 15;260(23):12516–12522. [PubMed] [Google Scholar]
- Farrell S. O., Fiol C. J., Reddy J. K., Bieber L. L. Properties of purified carnitine acyltransferases of mouse liver peroxisomes. J Biol Chem. 1984 Nov 10;259(21):13089–13095. [PubMed] [Google Scholar]
- Fiol C. J., Bieber L. L. Sigmoid kinetics of purified beef heart mitochondrial carnitine palmitoyltransferase. Effect of pH and malonyl-CoA. J Biol Chem. 1984 Nov 10;259(21):13084–13088. [PubMed] [Google Scholar]
- Hoppel C. L., Tomec R. J. Carnitine palmityltransferase. Location of two enzymatic activities in rat liver mitochondria. J Biol Chem. 1972 Feb 10;247(3):832–841. [PubMed] [Google Scholar]
- Hostetler K. Y., Hoppel C. L., Romine J. S., Sipe J. C., Gross S. R., Higginbottom P. A. Partial deficiency of muscle carnitine palmitoyltransferase with normal ketone production. N Engl J Med. 1978 Mar 9;298(10):553–557. doi: 10.1056/NEJM197803092981007. [DOI] [PubMed] [Google Scholar]
- Kiorpes T. C., Hoerr D., Ho W., Weaner L. E., Inman M. G., Tutwiler G. F. Identification of 2-tetradecylglycidyl coenzyme A as the active form of methyl 2-tetradecylglycidate (methyl palmoxirate) and its characterization as an irreversible, active site-directed inhibitor of carnitine palmitoyltransferase A in isolated rat liver mitochondria. J Biol Chem. 1984 Aug 10;259(15):9750–9755. [PubMed] [Google Scholar]
- Kopec B., Fritz I. B. Comparison of properties of carnitine palmitoyltransferase I with those of carnitine palmitoyltransferase II, and preparation of antibodies to carnitine palmitoyltransferases. J Biol Chem. 1973 Jun 10;248(11):4069–4074. [PubMed] [Google Scholar]
- Kopec B., Fritz I. B. Properties of a purified carnitine palmitoyltransferase, and evidence for the existence of other carnitine acyltransferases. Can J Biochem. 1971 Aug;49(8):941–948. doi: 10.1139/o71-136. [DOI] [PubMed] [Google Scholar]
- Kun E., Kirsten E., Piper W. N. Stabilization of mitochondrial functions with digitonin. Methods Enzymol. 1979;55:115–118. doi: 10.1016/0076-6879(79)55016-2. [DOI] [PubMed] [Google Scholar]
- Lund H., Bremer J. Carnitine acetyltransferase. Effect of malonyl-CoA, fasting and clofibrate feeding in mitochondria from different tissues. Biochim Biophys Acta. 1983 Jan 7;750(1):164–170. doi: 10.1016/0005-2760(83)90216-3. [DOI] [PubMed] [Google Scholar]
- Lund H. Carnitine palmitoyltransferase: characterization of a labile detergent-extracted malonyl-CoA-sensitive enzyme from rat liver mitochondria. Biochim Biophys Acta. 1987 Mar 13;918(1):67–75. doi: 10.1016/0005-2760(87)90010-5. [DOI] [PubMed] [Google Scholar]
- Miyazawa S., Ozasa H., Osumi T., Hashimoto T. Purification and properties of carnitine octanoyltransferase and carnitine palmitoyltransferase from rat liver. J Biochem. 1983 Aug;94(2):529–542. doi: 10.1093/oxfordjournals.jbchem.a134384. [DOI] [PubMed] [Google Scholar]
- Moore K. H., Radloff J. F., Koen A. E., Hull F. E. Incomplete fatty acid oxidation by heart mitochondria: beta-hydroxy fatty acid production. J Mol Cell Cardiol. 1982 Aug;14(8):451–459. doi: 10.1016/0022-2828(82)90151-1. [DOI] [PubMed] [Google Scholar]
- Murthy M. S., Pande S. V. Malonyl-CoA binding site and the overt carnitine palmitoyltransferase activity reside on the opposite sides of the outer mitochondrial membrane. Proc Natl Acad Sci U S A. 1987 Jan;84(2):378–382. doi: 10.1073/pnas.84.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noël H., Goswami T., Pande S. V. Solubilization and reconstitution of rat liver mitochondrial carnitine acylcarnitine translocase. Biochemistry. 1985 Aug 13;24(17):4504–4509. doi: 10.1021/bi00338a003. [DOI] [PubMed] [Google Scholar]
- Pande S. V., Blanchaer M. C. Preferential loss of ATP-dependent long-chain fatty acid activating enzyme in mitochondria prepared using Nagarse. Biochim Biophys Acta. 1970 Feb 10;202(1):43–48. doi: 10.1016/0005-2760(70)90216-x. [DOI] [PubMed] [Google Scholar]
- Pande S. V., Murthy M. S., Noël H. Differential effects of phosphatidylcholine and cardiolipin on carnitine palmitoyltransferase activity. Biochim Biophys Acta. 1986 Jun 27;877(2):223–230. doi: 10.1016/0005-2760(86)90298-5. [DOI] [PubMed] [Google Scholar]
- Parsons D. F., Williams G. R., Chance B. Characteristics of isolated and purified preparations of the outer and inner membranes of mitochondria. Ann N Y Acad Sci. 1966 Jul 14;137(2):643–666. doi: 10.1111/j.1749-6632.1966.tb50188.x. [DOI] [PubMed] [Google Scholar]
- Parvin R., Pande S. V. Enhancement of mitochondrial carnitine and carnitine acylcarnitine translocase-mediated transport of fatty acids into liver mitochondria under ketogenic conditions. J Biol Chem. 1979 Jun 25;254(12):5423–5429. [PubMed] [Google Scholar]
- Saggerson D. Carnitine palmitoyltransferase in extrahepatic tissues. Biochem Soc Trans. 1986 Aug;14(4):679–681. doi: 10.1042/bst0140679. [DOI] [PubMed] [Google Scholar]
- Saggerson E. D., Carpenter C. A. Malonyl CoA inhibition of carnitine acyltransferase activities: effects of thiol-group reagents. FEBS Lett. 1982 Jan 11;137(1):124–128. doi: 10.1016/0014-5793(82)80329-3. [DOI] [PubMed] [Google Scholar]
- West D. W., Chase J. F., Tubbs P. K. The separation and properties of two forms of carnitine palmitoyltransferase from ox liver mitochondria. Biochem Biophys Res Commun. 1971 Mar 5;42(5):912–918. doi: 10.1016/0006-291x(71)90517-1. [DOI] [PubMed] [Google Scholar]


