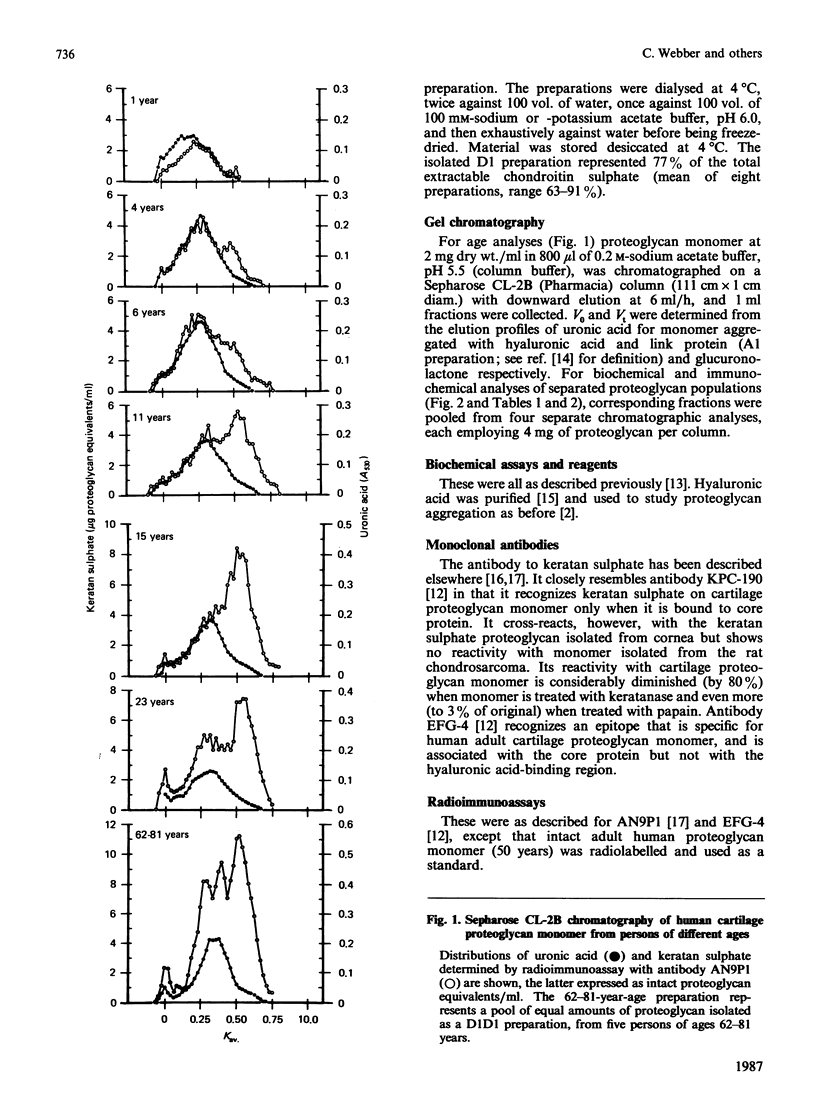
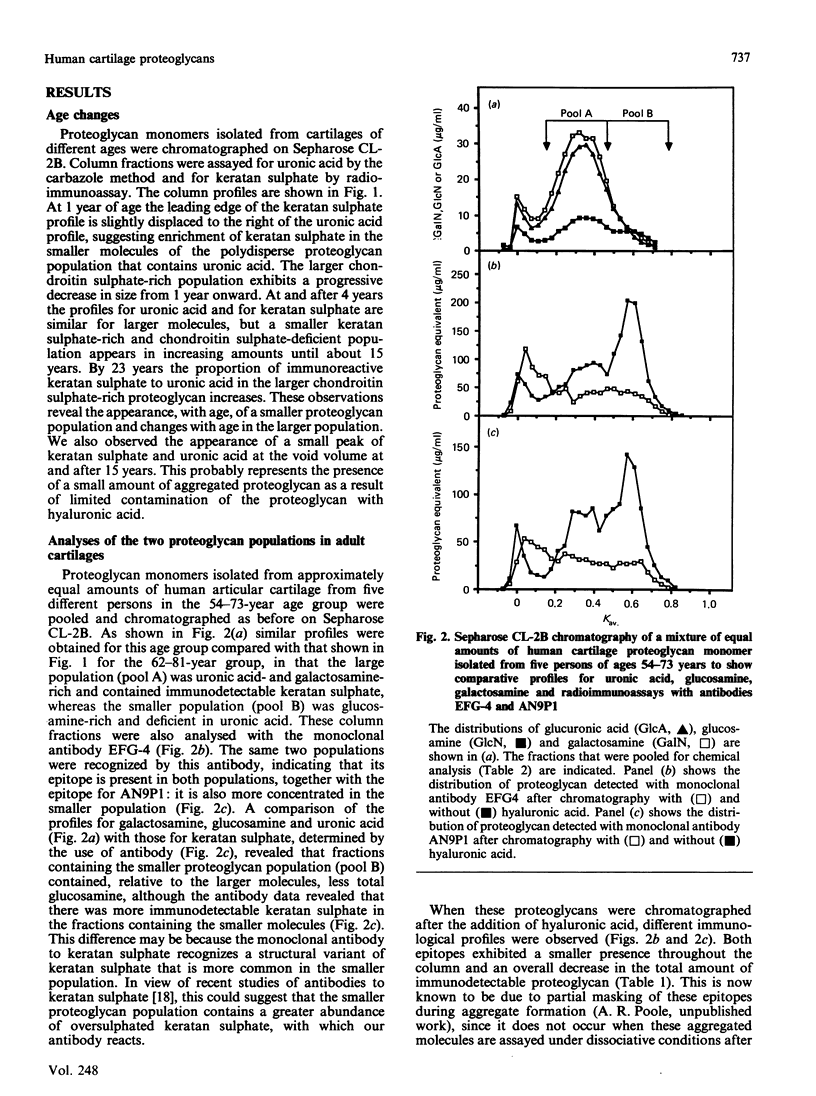
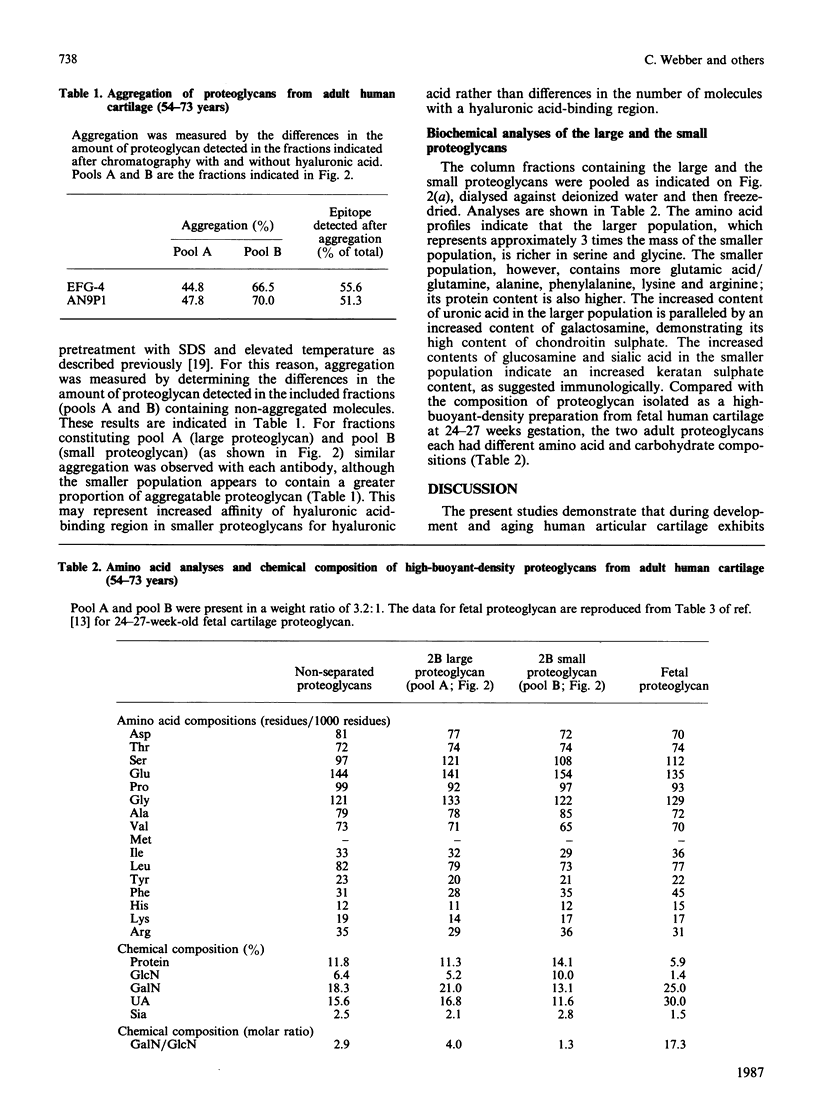
Abstract
After chromatography on Sepharose CL-2B under associative conditions, high-buoyant-density human articular-cartilage proteoglycans were analysed biochemically and by radioimmunoassay with monoclonal antibodies to a core-protein-related epitope and to keratan sulphate. An examination of proteoglycans from individuals of different ages revealed the presence at 1 year of mainly a single polydisperse population containing chondroitin sulphate (uronic acid) and keratan sulphate. From 4 years onwards a smaller keratan sulphate-rich and chondroitin sulphate-deficient population appears in increasing amounts until 15 years. At the same time the larger population shows a progressive decrease in size from 1 year onward. By 23 years and after the proportion of keratan sulphate in the larger chondroitin sulphate-rich proteoglycan increases. Both adult proteoglycan populations are shown immunologically to aggregate with hyaluronic acid, with the smaller showing a greater degree of interaction. The larger population is richer in serine and glycine, and the smaller population contains more glutamic acid/glutamine, alanine, phenylalanine, lysine and arginine; its protein content is also higher. Whether the larger post-natal population represents a different gene product from the single polydisperse population found in the human fetus, which has a different amino acid composition, remains to be established. The smaller population, which represents approximately one-third the mass of the larger population in the adult, may represent a degradation product of the larger population, in which the hyaluronic acid-binding region and keratan sulphate-rich region are conserved.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayliss M. T., Ali S. Y. Age-related changes in the composition and structure of human articular-cartilage proteoglycans. Biochem J. 1978 Dec 15;176(3):683–693. doi: 10.1042/bj1760683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss M. T., Ali S. Y. Isolation of proteoglycans from human articular cartilage. Biochem J. 1978 Jan 1;169(1):123–132. doi: 10.1042/bj1690123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss M. T., Ridgway G. D., Ali S. Y. Delayed aggregation of proteoglycans in adult human articular cartilage. Biosci Rep. 1984 Oct;4(10):827–833. doi: 10.1007/BF01138164. [DOI] [PubMed] [Google Scholar]
- Bayliss M. T., Venn M., Maroudas A., Ali S. Y. Structure of proteoglycans from different layers of human articular cartilage. Biochem J. 1983 Feb 1;209(2):387–400. doi: 10.1042/bj2090387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champion B. R., Reiner A., Roughley P. J., Poole A. R. Age-related changes in the antigenicity of human articular cartilage proteoglycans. Coll Relat Res. 1982 Jan;2(1):45–60. doi: 10.1016/s0174-173x(82)80040-x. [DOI] [PubMed] [Google Scholar]
- Garg H. G., Swann D. A. Age-related changes in the chemical composition of bovine articular cartilage. The structure of high-density proteoglycans. Biochem J. 1981 Feb 1;193(2):459–468. doi: 10.1042/bj1930459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glant T. T., Mikecz K., Poole A. R. Monoclonal antibodies to different protein-related epitopes of human articular cartilage proteoglycans. Biochem J. 1986 Feb 15;234(1):31–41. doi: 10.1042/bj2340031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glant T. T., Mikecz K., Roughley P. J., Buzás E., Poole A. R. Age-related changes in protein-related epitopes of human articular-cartilage proteoglycans. Biochem J. 1986 May 15;236(1):71–75. doi: 10.1042/bj2360071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham T. E., Muir H. The specific interaction of hyaluronic acid with cartillage proteoglycans. Biochim Biophys Acta. 1972 Sep 15;279(2):401–405. doi: 10.1016/0304-4165(72)90160-2. [DOI] [PubMed] [Google Scholar]
- Hascall V. C., Heinegård D. Aggregation of cartilage proteoglycans. I. The role of hyaluronic acid. J Biol Chem. 1974 Jul 10;249(13):4232–4241. [PubMed] [Google Scholar]
- Heinegård D., Axelsson I. Distribution of keratan sulfate in cartilage proteoglycans. J Biol Chem. 1977 Mar 25;252(6):1971–1979. [PubMed] [Google Scholar]
- Heinegård D., Wieslander J., Sheehan J., Paulsson M., Sommarin Y. Separation and characterization of two populations of aggregating proteoglycans from cartilage. Biochem J. 1985 Jan 1;225(1):95–106. doi: 10.1042/bj2250095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inerot S., Heinegård D., Audell L., Olsson S. E. Articular-cartilage proteoglycans in aging and osteoarthritis. Biochem J. 1978 Jan 1;169(1):143–156. doi: 10.1042/bj1690143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inerot S., Heinegård D. Bovine tracheal cartilage proteoglycans. Variations in structure and composition with age. Coll Relat Res. 1983 May;3(3):245–262. doi: 10.1016/s0174-173x(83)80007-7. [DOI] [PubMed] [Google Scholar]
- Mehmet H., Scudder P., Tang P. W., Hounsell E. F., Caterson B., Feizi T. The antigenic determinants recognized by three monoclonal antibodies to keratan sulphate involve sulphated hepta- or larger oligosaccharides of the poly(N-acetyllactosamine) series. Eur J Biochem. 1986 Jun 2;157(2):385–391. doi: 10.1111/j.1432-1033.1986.tb09680.x. [DOI] [PubMed] [Google Scholar]
- Mort J. S., Poole A. R., Roughley P. J. Age-related changes in the structure of proteoglycan link proteins present in normal human articular cartilage. Biochem J. 1983 Jul 15;214(1):269–272. doi: 10.1042/bj2140269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliffe A., Hardingham T. Cartilage proteoglycan binding region and link protein. Radioimmunoassays and the detection of masked determinants in aggregates. Biochem J. 1983 Aug 1;213(2):371–378. doi: 10.1042/bj2130371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughley P. J., White R. J. Age-related changes in the structure of the proteoglycan subunits from human articular cartilage. J Biol Chem. 1980 Jan 10;255(1):217–224. [PubMed] [Google Scholar]
- Roughley P. J., White R. J., Poole A. R. Identification of a hyaluronic acid-binding protein that interferes with the preparation of high-buoyant-density proteoglycan aggregates from adult human articular cartilage. Biochem J. 1985 Oct 1;231(1):129–138. doi: 10.1042/bj2310129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thonar E. J., Sweet M. B. An oligosaccharide component in proteoglycans of articular cartilage. Biochim Biophys Acta. 1979 May 1;584(2):353–357. doi: 10.1016/0304-4165(79)90281-2. [DOI] [PubMed] [Google Scholar]
- Triphaus G. F., Schmidt A., Buddecke E. Age-related changes in the incorporation of [35S]sulfate into two proteoglycan populations from human cartilage. Hoppe Seylers Z Physiol Chem. 1980 Dec;361(12):1773–1779. doi: 10.1515/bchm2.1980.361.2.1773. [DOI] [PubMed] [Google Scholar]
- Witter J., Roughley P. J., Webber C., Roberts N., Keystone E., Poole A. R. The immunologic detection and characterization of cartilage proteoglycan degradation products in synovial fluids of patients with arthritis. Arthritis Rheum. 1987 May;30(5):519–529. doi: 10.1002/art.1780300506. [DOI] [PubMed] [Google Scholar]


