Abstract
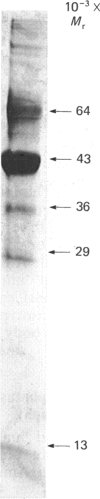
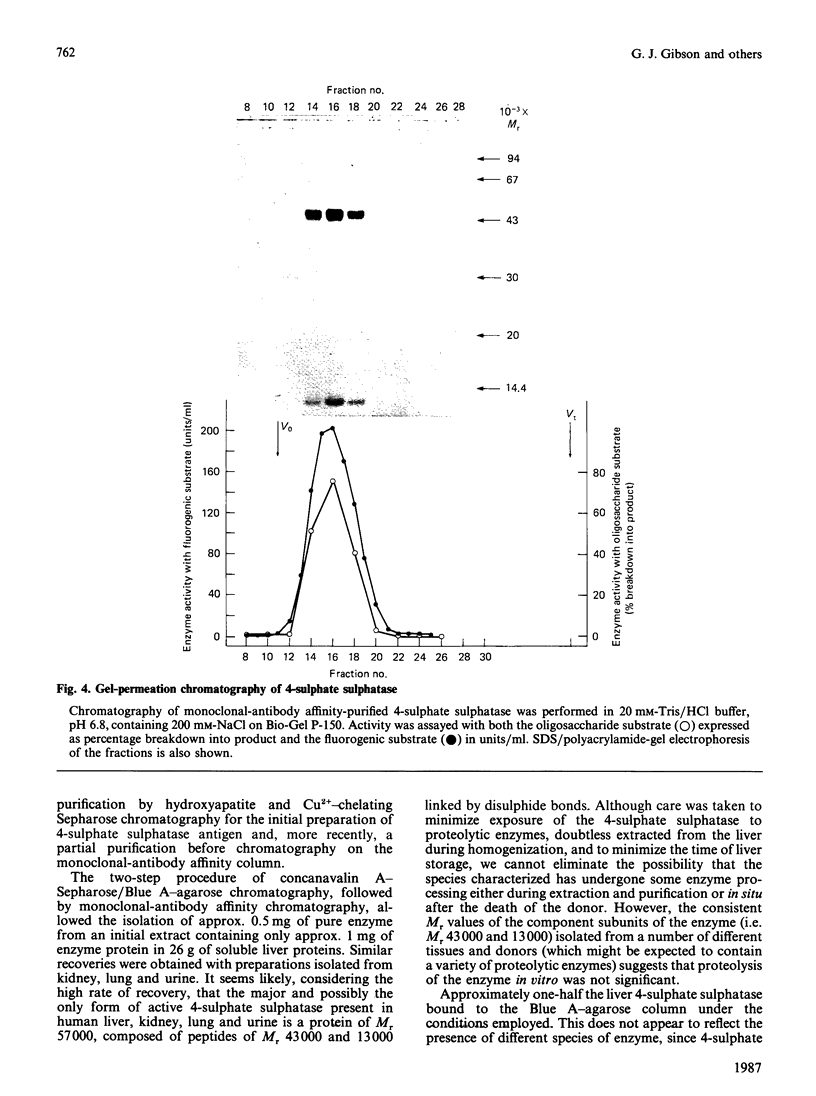
Initial purification of N-acetylgalactosamine-4-sulphate sulphatase from human liver homogenates containing approx. 1 mg of enzyme in 26 g of soluble proteins was achieved by a six-column chromatography procedure and yielded approx. 40 micrograms of a single major protein species. Enzyme thus prepared was used to produce N-acetylgalactosamine-4-sulphate sulphatase-specific monoclonal antibodies. The use of a monoclonal antibody linked to a solid support facilitated the purification of approx. 0.5 mg of N-acetylgalactosamine-4-sulphate sulphatase from a similar liver homogenate. Moreover the enzyme isolated contained a single protein species, shown by SDS/polyacrylamide-gel electrophoresis to have an Mr of 57,000, which dissociated into subunits of Mr 43,000 and 13,000 in the presence of reducing agents. Essentially identical enzyme preparations were isolated from homogenates of human kidney and lung and from concentrated human urine. The native protein Mr of enzyme from human liver and kidney was assessed by gel-permeation chromatography to be 43,000 on Ultrogel AcA and Bio-Gel P-150. The liver N-acetylgalactosamine-4-sulphate sulphatase was shown to have pH optima of approx. 4 and 5.5 with the oligosaccharide substrate (GalNAc4S-GlcA-GalitolNAc4S) and fluorogenic substrate (methylumbelliferyl sulphate) respectively. Km values of 60 microM and 4 mM and Vmax. values of 2 and 20 mumol/min per mg were determined with the oligosaccharide and fluorogenic substrates respectively.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chang P. L., Rosa N. E., Davidson R. G. Differential assay of arylsulfatase A and B activities: a sensitive method for cultured human cells. Anal Biochem. 1981 Nov 1;117(2):382–389. doi: 10.1016/0003-2697(81)90795-8. [DOI] [PubMed] [Google Scholar]
- Clements P. R., Brooks D. A., Saccone G. T., Hopwood J. J. Human alpha-L-iduronidase. 1. Purification, monoclonal antibody production, native and subunit molecular mass. Eur J Biochem. 1985 Oct 1;152(1):21–28. doi: 10.1111/j.1432-1033.1985.tb09158.x. [DOI] [PubMed] [Google Scholar]
- Clements P. R., Mahuran D. J., Hopwood J. J. Improved concanavalin A-Sepharose elution by specific readsorption of glycoproteins. J Chromatogr. 1983 May 20;261(1):77–82. doi: 10.1016/s0021-9673(01)87920-6. [DOI] [PubMed] [Google Scholar]
- Clements P. R., Muller V., Hopwood J. J. Human alpha-L-iduronidase. 2. Catalytic properties. Eur J Biochem. 1985 Oct 1;152(1):29–34. doi: 10.1111/j.1432-1033.1985.tb09159.x. [DOI] [PubMed] [Google Scholar]
- Freeman C., Clements P. R., Hopwood J. J. Human liver N-acetylglucosamine-6-sulphate sulphatase. Purification and characterization. Biochem J. 1987 Sep 1;246(2):347–354. doi: 10.1042/bj2460347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman C., Hopwood J. J. Human liver sulphamate sulphohydrolase. Determinations of native protein and subunit Mr values and influence of substrate agylcone structure on catalytic properties. Biochem J. 1986 Feb 15;234(1):83–92. doi: 10.1042/bj2340083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gacesa P., Savitsky M. J., Dodgson K. S., Olavesen A. H. Modification of functional arginine residues in purified bovine testicular hyaluronidase with butane-2, 3-dione. Biochim Biophys Acta. 1981 Oct 13;661(2):205–212. doi: 10.1016/0005-2744(81)90005-x. [DOI] [PubMed] [Google Scholar]
- Gasa S., Balbaa M., Nakamura M., Yonemori H., Makita A. Phosphorylation of human lysosomal arylsulfatase B by cAMP-dependent protein kinase. Different sites of phosphorylation between normal and cancer tissues. J Biol Chem. 1987 Jan 25;262(3):1230–1238. [PubMed] [Google Scholar]
- Hopwood J. J., Elliott H., Muller V. J., Saccone G. T. Diagnosis of Maroteaux-Lamy syndrome by the use of radiolabelled oligosaccharides as substrates for the determination of arylsulphatase B activity. Biochem J. 1986 Mar 15;234(3):507–514. doi: 10.1042/bj2340507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopwood J. J., Muller V., Smithson A., Baggett N. A fluorometric assay using 4-methylumbelliferyl alpha-L-iduronide for the estimation of alpha-L-iduronidase activity and the detection of Hurler and Scheie syndromes. Clin Chim Acta. 1979 Mar 1;92(2):257–265. doi: 10.1016/0009-8981(79)90121-9. [DOI] [PubMed] [Google Scholar]
- Hopwood J. J. alpha-L-iduronidase, beta-D-glucuronidase, and 2-sulfo-L-iduronate 2-sulfatase: preparation and characterization of radioactive substrates from heparin. Carbohydr Res. 1979 Mar;69:203–216. doi: 10.1016/s0008-6215(00)85765-1. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mahuran D., Clements P., Carrella M., Strasberg P. M. A high recovery method for concentrating microgram quantities of protein from large volumes of solution. Anal Biochem. 1983 Mar;129(2):513–516. doi: 10.1016/0003-2697(83)90585-7. [DOI] [PubMed] [Google Scholar]
- Mahuran D., Clements P., Hopwood J. A rapid four column purification of 2-deoxy-D-glucoside-2-sulphamate sulphohydrolase from human liver. Biochim Biophys Acta. 1983 Jun 9;757(3):359–365. doi: 10.1016/0304-4165(83)90062-4. [DOI] [PubMed] [Google Scholar]
- McGovern M. M., Vine D. T., Haskins M. E., Desnick R. J. Purification and properties of feline and human arylsulfatase B isozymes. Evidence for feline homodimeric and human monomeric structures. J Biol Chem. 1982 Nov 10;257(21):12605–12610. [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- Poulos A., Beckman K. The rapid assay of cerebroside sulphate sulphatase (sulphatidase) using [3H]cerebroside sulphate as substrate. Clin Chim Acta. 1978 Nov 1;89(3):417–420. doi: 10.1016/0009-8981(78)90404-7. [DOI] [PubMed] [Google Scholar]
- Riordan J. F., McElvany K. D., Borders C. L., Jr Arginyl residues: anion recognition sites in enzymes. Science. 1977 Mar 4;195(4281):884–886. doi: 10.1126/science.190679. [DOI] [PubMed] [Google Scholar]
- Rome L. H., Garvin A. J., Neufeld E. F. Human kidney alpha-L-iduronidase: purification and characterization. Arch Biochem Biophys. 1978 Aug;189(2):344–353. doi: 10.1016/0003-9861(78)90221-7. [DOI] [PubMed] [Google Scholar]
- Steckel F., Hasilik A., von Figura K. Biosynthesis and maturation of arylsulfatase B in normal and mutant cultured human fibroblasts. J Biol Chem. 1983 Dec 10;258(23):14322–14326. [PubMed] [Google Scholar]