Abstract
Aim: Compare healthcare costs for patients with epidermal growth factor receptor mutated (EGFRm) metastatic non-small-cell lung cancer (mNSCLC) with and without progression and estimate costs of progression.
Materials & methods: Retrospective claims analysis (2015–2020) from adults with EGFRm mNSCLC initiating EGFR tyrosine kinase inhibitors. Adjusted costs for 12 months were compared (with vs without progression) and cumulative costs for early versus late progression were predicted over 36 months.
Results: A total of 228 patients with EGFRm mNSCLC were included. Patients with progression within 12 months incurred significantly higher total costs despite lower treatment costs (vs without progression). Medical costs were significantly higher among early versus late progressors.
Conclusion: These data may aid providers aiming to administer quality care in a cost-efficient way.
Keywords: : epidermal growth factor activating mutation, healthcare costs, lung cancer, progression, tyrosine kinase inhibitors
Plain Language Summary
Lung cancer is the leading cause of cancer death among both men and women in the US. Among US patients with adenocarcinoma histology, approximately 17% have epidermal growth factor activating mutations (EGFRm) that include exon 19 deletions or L858R mutations. These common mutations make up approximately 85% of all EGFR mutations. The aim of this study was to compare healthcare resource utilization and costs for patients with EGFRm metastatic non-small-cell lung cancer with and without disease progression within the first 12 months following first-line treatment initiation using data from insurance claims. The results suggest that patients with EGFRm metastatic non-small-cell lung cancer with disease progression in the first 12 months (after treatment initiation) have significantly higher costs compared with patients without disease progression in the first 12 months (and highest in the first 6 months). These data may help inform oncology providers aiming to administer high quality cancer care in a cost-efficient way.
Plain language summary
Article highlights.
Patients with non-small-cell lung cancer face a high burden of healthcare costs, especially when the disease progresses.
This was a retrospective study using administrative claims data of 228 adult patients with epidermal growth factor receptor mutated (EGFRm) metastatic non-small-cell lung cancer (mNSCLC) that initiated a first-line EGFR tyrosine kinase inhibitor (TKI) between 2015 and 2020.
Healthcare costs were captured during the first 12 months after treatment initiation in patients with and without disease progression, and cumulative costs were predicted over 36 months for patients with early (post initiation months 0–6) versus late (post initiation months 7–12) disease progression.
There were 152 (66.7%) patients whose disease progressed at any time during the 12 months after treatment initiation; 101 (66.4%) patients had early progression and 51 (34.2%) had late progression.
Patients with any disease progression had greater healthcare resource utilization compared with patients whose disease did not progress during follow-up.
Adjusted mean total healthcare costs were statistically significantly higher for patients with any progression compared with those without progression, mainly driven by inpatient costs.
Adjusted monthly predicted cumulative costs were significantly higher among patients who progressed early compared with those that progressed late.
Study limitations include lack of biomarker and histology data from claims data, misclassification of metastatic disease status and specific EGFR mutations based on coding errors and limited generalizability.
This studies provides real-world costs of patients with EGFRm mNSCLC that initiate a TKI whose disease progresses in a 12-month time period; the cost burden was highest among patients with disease progression in the first 6 months after treatment initiation.
This study highlights the importance of delaying EGFRm mNSCLC disease progression in a timely manner.
1. Background
Lung cancer is the leading cause of cancer death among both men and women in the US [1]. Of patients with non-small-cell lung cancer (NSCLC), more than half are diagnosed after the disease has metastasized, and their 5-year survival rate is estimated to be approximately 5% [2]. Among US patients with adenocarcinoma histology, approximately 17% have epidermal growth factor activating mutations (EGFRm) that include exon 19 deletions or L858R mutations. These common mutations make up approximately 85% of all EGFR mutations [3–6].
The recommended first-line treatment for patients with common EGFR mutations include the EGFR-targeted tyrosine kinase inhibitors (TKI) osimertinib, erlotinib, afatinib and gefitinib which have been shown to extend progression-free survival (PFS) when compared with a platinum doublet [7–10]. However, most patients eventually develop resistance to targeted therapies and upon disease progression, subsequent treatment with systemic chemotherapy is recommended [1,11].
Metastatic NSCLC (mNSCLC) is not only burdensome for the patient, but also the healthcare system. Cancer treatment and care is the fastest growing healthcare sector in the US, and it is estimated that lung cancer has the highest last year of life costs [10,12,13]. In a study of patients with mNSCLC, costs in patients with progression were significantly higher compared with similar patients with stable disease, and the authors noted that these incremental costs affect healthcare budgets within a short period of time postprogression [14]. In a more recent study using data through 2014, the incremental costs of metastatic lung cancer progression were estimated over 3 years and varied markedly by the period of time elapsed prior to progression. Patients who progressed in the first month had costs 82.1% higher than nonprogressors, compared with 28.0% higher costs in patients who progressed in month 24 versus nonprogressors [15]. These data highlight the benefits of delaying disease progression, although data examining the economic impact of progression are limited.
The current analysis uses Real World Data to expand on prior research by focusing specifically on an EGFRm mNSCLC population who received targeted therapy as first-line treatment. In addition, we utilize more recent data (January 2015 through December 2020) to capture the current treatment paradigm and reflect increased oncology costs. The aim of this study was to compare healthcare resource utilization (HCRU) and costs for patients with EGFRm mNSCLC with and without disease progression within the first 12 months following first-line treatment initiation and to estimate the incremental costs of early (months 0–12) and late progression (13–36) for up to 36 months after first-line treatment initiation.
These data will increase understanding on the total cost of care in this targeted lung cancer population and how delaying disease advancement may impact medical costs.
2. Materials & methods
2.1. Study design & data source
This retrospective study utilized US administrative claims data from the Merative MarketScan® Commercial Claims and Encounters and Medicare Supplemental and Coordination of Benefits (Medicare) Databases [16]. The databases include the inpatient, outpatient and prescription drug experiences of employees, dependents and retirees covered under a variety of fee-for-service and managed care health plans. Additionally, the National Death Index (NDI) database from the National Center for Health Statistics was linked to the MarketScan databases. The NDI data include all deaths in the US from 1979 forward and provide dates and cause(s) of death for persons who have died. Institutional Review Board (IRB) waiver of authorization approval was obtained from WCG IRB (IRB study #: 1324891; IRB protocol #: 20217016) on 23 December 2021. All variables were defined using International Classification of Diseases, 9th and 10th Revision, Clinical Modification (ICD-9-CM and ICD-10-CM) codes, Current Procedural Terminology 4th edition (CPT-4) codes, Healthcare Common Procedure Coding System (HCPCS) codes and National Drug Codes (NDCs).
2.2. Patient selection
Patients (age 18+) with an eligible diagnosis of lung cancer (two nondiagnostic medical claims at least 30 days apart with an ICD-9-CM [162.2–162.9] or ICD-10-CM [C34.x] code for the condition) during 1 January 2015 through 31 December 2020, were initially selected. Patients were further required to have at least one medical claim with a diagnosis code for secondary malignancy suggesting the presence of metastatic disease (ICD-9: 196.x-198.x; ICD-10: C77.x-C79.x) during the 30 days prior or anytime following the earliest lung cancer diagnosis date (first claim was defined as the metastatic lung cancer diagnosis date). Patients were required to initiate first-line treatment with erlotinib, afatinib, gefitinib, dacomitinib or osimertinib monotherapy (index date) on or following the metastatic lung cancer diagnosis date. Use of an EGFR TKI was also used as a proxy identifier for common EGFR mutations (i.e., exon 19 deletion or L858R mutation) and non-small-cell histology.
Eligible patients were required to have continuous enrollment with medical and pharmacy benefits from index date for a minimum of 60 days following the index date (except if a death). The follow-up period was variable in length until death, database disenrollment, a maximum of 36 months, or study end (31 December 2020) (Figure 1). Patients with evidence of other primary cancers (not including lung) during the 6 months prior to diagnosis of metastatic disease (baseline period) through the day prior to index were excluded. Patients were excluded if they had any claims for the prescription or administration of any systemic antineoplastic therapy (other than the index EGFR TKI) from the metastatic diagnosis date to the day prior to the index date. The final cohort included eligible patients with linkage to the NDI.
Figure 1.
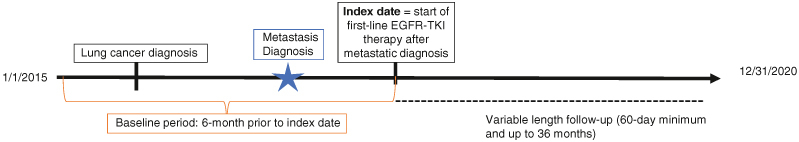
Study design.
EGFR: Epidermal growth factor receptor; TKI: Tyrosine kinase inhibitor.
Demographic characteristics were reported on the index date and included age, sex, payer and index year. The National Cancer Institute (NCI) adapted Charlson Comorbidity Index [17] calculated as an aggregate measure of patient comorbidity was measured during the baseline period and first line EGFR-TKI drug type was reported on the index date.
2.3. Disease progression
A treatment-based claims algorithm was applied to identify antineoplastic lines of therapy (LOT) after the metastatic lung cancer diagnosis date. Combination regimens were defined as any new drug that was filled/administered within 45 days of a previous drug fill or administration. Termination of a LOT was defined as the discontinuation of all agents in a regimen for ≥60 days, or when a new agent was added (triggering advancement to a new LOT). A switch between chemotherapy agents within the same medication class did not advance the LOT. Moving to a next line therapy was defined the introduction of a new agent (that was not included in the prior LOT). Disease progression was defined as advancing to a next line of therapy, having medical claims with evidence of hospice care, or death. If multiple events occurred during follow-up, the date of progression was set to the date of the earliest event. Time to first progression was calculated from index date to the earlier of progression (among those with progression). Follow-up was censored at disenrollment or end of study period (among those without progression). The progression triggering event (advancement to a subsequent line of therapy, hospice care or death) was reported. Patients with progression at anytime during follow-up were stratified by early (months 0–12 for first progression triggering event) and late progression (months 13–36 for first progression triggering event).
2.4. Outcomes
Healthcare costs were identified based on paid amounts of adjudicated claims, including insurer and health plan payments as well as patient cost-sharing in the form of copayment, deductible and coinsurance. All dollar estimates were inflated to 2020 dollars using the Medical Care Component of the Consumer Price Index. HCRU and costs were measured per-patient per month (PPPM) during the first 12 months of follow-up for patients with and without progression (stratified by those with progression during months 0–6 and months 7–12). HCRU and costs were reported by type of service (inpatient, outpatient [emergency room, office administered treatment, office visits, laboratory, radiology inclusive of diagnostic and therapeutic radiology services, other] and pharmacy). In addition, healthcare costs were evaluated with and without the costs of antineoplastic treatment (both medical and pharmacy treatment costs).
2.5. Statistical analysis
Descriptive analyses were conducted using the latest version of World Programming System, which is a software platform for working with data and statistics. Categorical variables were presented as the count and percentage of patients in each category, and continuous variables were summarized by mean and standard deviation (SD). General linear regression models were used to adjust PPPM costs for patients with versus without disease progression during the first 12 months post index.
Weighted marginal effects generalized estimating equations (GEE) (normal distributions with identity link) with robust standard errors adjusting for within patient correlations over month for the patient level parameter estimates were used to predict adjusted cumulative monthly costs (total, medical and antineoplastic) for patients with early vs late progression over 36 months. The GEE model was utilized as it provides individual month costs (allowing for a clearer graphical representation of the data) for each month and extending out over 36 months over a more simplified regression model that provides a single estimate of cumulative costs at 36 months. The individual patient’s weights were calculated as the inverse of the probability of disenrollment for a patient's month of disenrollment. Post the fitting of the GEE model, cumulative observed and predicted monthly costs were calculated from the summed observed and predicted (from the fitted model) monthly costs.
Baseline covariates included in all adjusted analyses (to account for known potential confounding of the relationship between healthcare costs and disease progression) were: age, sex, region, insurance plan type, urbanicity, payer type, index year, NCI score and first-line TKI drug type. Diagnostics were assessed to examine the variance inflation factor to measure the impact of collinearity among the variables included in the models. All variables had a variance inflation factor of 1–4 suggesting no to moderate correlation and therefore coefficient estimates and p-values estimates in the regressions are reliable.
3. Results
3.1. Patient characteristics
There were 286 patients with EGFRm mNSCLC who met the study eligibility criteria and of those 228 (79.7%) were linked to the NDI and comprised the final sample for analysis (Figure 2). After first-line EGFR-TKI initiation, there were 152 (66.7%) patients with disease progression during follow-up, with a mean (SD) time to progression of 311.1 (219.2) days. Of the patients with progression (n = 152), 66.4% (n = 101) progressed early (first 12 months) while 34.2% (n = 51) progressed late (13–36 months). The mean number of LOT during follow-up was 2.2 (median 2.0) for both early and late progressors. Among patients with progression in the first 12 months (n = 101) 59.4% progressed due to line of therapy advancement, followed by 24.8% having hospice care and 15.8% with death. Patients who progressed most quickly in months 0–6, had a larger proportion with progression due to hospice and death (32.6 and 17.4% respectively, versus those who progressed in months 7–12 (18.2% with hospice and 17.4% with death Figure 3).
Figure 2.
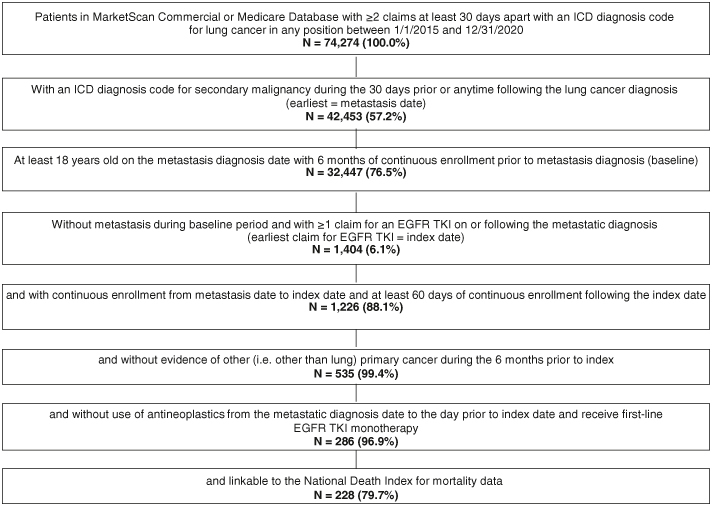
Patient identification.
EGFR: Epidermal growth factor receptor; ICD: International classification of diseases; TKI: Tyrosine kinase inhibitor.
Figure 3.
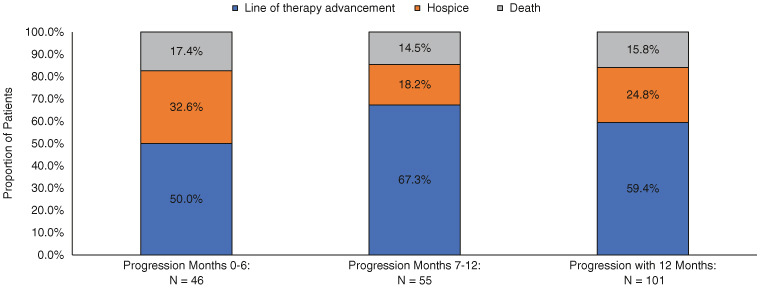
First progression event in first 12 months of follow-up among patients with metastatic non-small-cell lung cancer epidermal growth factor receptor activating mutation following initiation of first-line treatment on an epidermal growth factor receptor tyrosine kinase inhibitor.
EGFRm: Epidermal growth factor receptor activating mutation; mNSCLC: Metastatic non-small-cell lung cancer; TKI: Tyrosine kinase inhibitor.
In the full cohort, mean (SD) age was 63.0 (12.4) years, 66.2% were female, and the mean (SD) NCI score was 0.4 (0.4). The average duration of follow-up was 551.0 days and was shorter for patients with (without) disease progression in the first 12 months (444.5 vs 563.9 days, p = 0.004 Table 1). The most common EGFK-TKI prescribed in first-line was erlotinib (43.4%, n = 99), followed by osimertinib (33.8%, n = 77), afatinib (21.5%, n = 49) and gefitinib (1.3%, n = 3 Table 1). Patients initiating treatment on erlotinib or afatinib were more likely to progress during the first 12 months (58.6 and 46.9%) compared with patients starting their first-line of therapy with osimertinib (26.0% Figure 4).
Table 1.
Characteristics of patients with metastatic non-small cell lung cancer epidermal growth factor receptor activating mutation with and without disease progression in the first 12 months following initiation of first-line of an epidermal growth factor receptor tyrosine kinase inhibitor.
| All patients (n = 228) | No disease progression in the first 12 Months (n = 127) | With disease progression in the first 12 months (n = 101) | p-value1 | ||||
|---|---|---|---|---|---|---|---|
| Age: mean, SD | 63.0 | 12.4 | 61.6 | 11.6 | 64.7 | 13.2 | 0.062 |
| Female: n, % | 151 | 66.2% | 81 | 63.8% | 70 | 69.3% | 0.381 |
| Payer: n, % | |||||||
| Commercial insurance | 140 | 61.4% | 84 | 66.1% | 56 | 55.4% | 0.099 |
| Medicare supplemental insurance | 88 | 38.6% | 43 | 33.9% | 45 | 44.6% | |
| Insurance plan type: n,% | |||||||
| Comprehensive/indemnity | 38 | 16.7% | 17 | 13.4% | 21 | 20.8% | 0.084 |
| EPO/PPO | 123 | 53.9% | 69 | 54.3% | 54 | 53.5% | |
| POS/POS with capitation | 11 | 4.8% | 4 | 3.1% | 7 | 6.9% | |
| HMO | 24 | 10.5% | 14 | 11.0% | 10 | 9.9% | |
| CDHP/HDHP | 31 | 13.6% | 23 | 18.1% | 8 | 7.9% | |
| Other/Unknown | 1 | 0.4% | 0 | 0.0% | 1 | 1.0% | |
| Index year: n, % | |||||||
| 2015–2017 | 154 | 67.5% | 70 | 55.1% | 84 | 83.2% | 0.000 |
| 2018–2020 | 74 | 32.5% | 57 | 44.9% | 17 | 16.8% | 0.000 |
| National Cancer Institute Index (NCI): mean, SD | 0.4 | 0.4 | 0.4 | 0.4 | 0.5 | 0.5 | 0.090 |
| Duration of follow-up (days): mean, SD | 551.0 | 316.4 | 563.9 | 335.0 | 444.5 | 279.1 | 0.004 |
| First line TKI (N, %) | |||||||
| Erlotinib | 99 | 43.4% | 41 | 32.3% | 58 | 57.4% | 0.000 |
| Osimertinib | 77 | 33.8% | 57 | 44.9% | 20 | 19.8% | 0.000 |
| Afatinib | 49 | 21.5% | 26 | 20.5% | 23 | 22.8% | 0.675 |
| Gefitinib | 3 | 1.3% | 3 | 2.4% | 0 | 0.0% | 0.120 |
Bolded indicates p < 0.05.
CDHP: Consumer-driven health plan; EGFRm: Epidermal growth factor receptor activating mutation; EPO: Exclusive provider organization; HDHP: High deductible health plan; HMO: Health maintenance organization; mNSCLC: Metastatic non-small-cell lung cancer; POS: Point of service; PPO: Preferred provider organization; SD: Standard deviation; TKI: Tyrosine kinase inhibitor.
Figure 4.
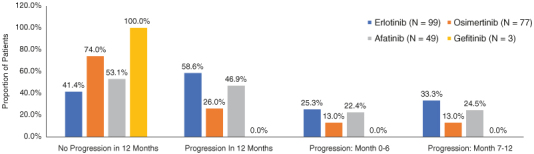
First-line epidermal growth factor receptor tyrosine kinase inhibitor by progression status in the first 12 months.
3.2. Healthcare resource utilization & costs
Patients with progression in the first 12 months of follow-up had greater utilization of healthcare resources compared with those with no progression during the same time period. Patients with progression (vs without progression) were significantly more likely to have at least one inpatient admission (54.5 vs 18.1%, p < 0.0001) and at least one ER visit (61.4 vs 32.3%, p < 0.0001). In addition, patients with progression had a significantly (p < 0.001) higher mean number of inpatient admissions, ER visits, radiology services and other outpatient services compared with patients without progression (Table 2). Patients without progression (vs with progression) had a larger mean (SD) number of pharmacy prescriptions for antineoplastic treatments PPPM (1.1 [0.2] vs 0.8 [0.3], p < 0.001) during the first 12 months of follow-up due to longer continuation on their index EGFR-TKI. Utilization of office-administered antineoplastic treatments were therefore higher among those with progression (vs without progression) due to line of therapy advancement (i.e., either adding or switching to infused agents in the second line for ~40% of patients Table 2).
Table 2.
Healthcare resource utilization measured per patient per month among patients with metastatic non-small-cell lung cancer epidermal growth factor receptor activating mutation with and without disease progression within 12 months after first-line initiation on an epidermal growth factor receptor activating tyrosine kinase inhibitor.
| No disease progression in the first 12 months | With disease progression in the first 12 months | p-value (w/progression vs no progression) | Disease progression in months 0–6 | p-value (w/progression in months 0–6 vs no progression) | Disease progression in months 7–12 | p-value (w/progression in months 7–12 vs no progression) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n = 127 | n = 101 | n = 46 | n = 55 | ||||||||
| Inpatient | |||||||||||
| Patients with ≥1 admission: n, % | 23 | 18.1% | 55 | 54.5% | 0.000 | 24 | 52.2% | 0.000 | 31 | 56.4% | 0.000 |
| Number of admissions, PPPM: mean, SD | 0.03 | 0.07 | 0.11 | 0.14 | 0.000 | 0.13 | 0.16 | 0.000 | 0.09 | 0.11 | 0.000 |
| Length of stay (in days) per admission: mean, SD | 0.08 | 0.21 | 0.40 | 0.68 | 0.000 | 0.53 | 0.87 | 0.000 | 0.30 | 0.45 | 0.000 |
| Outpatient | |||||||||||
| Emergency room visitis | |||||||||||
| Patients with ≥1 emergency room visit: n, % | 41 | 32.3% | 62 | 61.4% | 0.000 | 25 | 54.3% | 0.008 | 37 | 67.3% | 0.000 |
| Number of emergency room visits, PPPM: mean, SD | 0.04 | 0.08 | 0.13 | 0.17 | 0.000 | 0.16 | 0.22 | 0.000 | 0.11 | 0.12 | 0.000 |
| Office-administered antineoplastics | |||||||||||
| Patients with ≥1 administration: n, % | 0 | 0.0% | 31 | 30.7% | 0.000 | 12 | 26.1% | 0.000 | 19 | 34.5% | 0.000 |
| Number of administrations, PPPM: mean, SD | 0.00 | 0.00 | 0.18 | 0.37 | 0.000 | 0.19 | 0.44 | 0.000 | 0.17 | 0.31 | 0.000 |
| Outpatient office visits | |||||||||||
| Patients with ≥1 office visit: n, % | 126 | 99.2% | 98 | 97.0% | 0.324 | 43 | 93.5% | 0.058 | 55 | 100.0% | 1.000 |
| Number of office visits, PPPM: mean, SD | 1.87 | 1.08 | 1.90 | 1.08 | 0.844 | 1.80 | 1.13 | 0.712 | 1.98 | 1.04 | 0.523 |
| Outpatient radiology | |||||||||||
| Patients with ≥1 radiology service: n, % | 125 | 98.4% | 98 | 97.0% | 0.657 | 44 | 95.7% | 0.288 | 54 | 98.2% | 1.000 |
| Number of radiology services, PPPM: mean, SD | 2.01 | 2.57 | 3.09 | 2.90 | 0.003 | 3.61 | 3.69 | 0.002 | 2.66 | 1.96 | 0.098 |
| Outpatient laboratory services | |||||||||||
| Patients with ≥1 laboratory service: n, % | 123 | 96.9% | 94 | 93.1% | 0.222 | 40 | 87.0% | 0.023 | 54 | 98.2% | 1.000 |
| Number of laboratory services, PPPM: mean, SD | 3.74 | 3.32 | 3.66 | 2.91 | 0.860 | 2.88 | 2.53 | 0.111 | 4.32 | 3.06 | 0.265 |
| Other outpatient services† | |||||||||||
| Patients with ≥1 other outpatient service: n, % | 125 | 98.4% | 101 | 100.0% | 0.504 | 46 | 100.0% | 1.000 | 55 | 100.0% | 1.000 |
| Number of other outpatient services, PPPM: mean, SD | 2.30 | 1.72 | 3.66 | 3.54 | 0.000 | 3.65 | 3.57 | 0.001 | 3.67 | 3.55 | 0.001 |
| Outpatient pharmacy | |||||||||||
| Patients with a ≥1 prescription (any): n, % | 127 | 100.0% | 101 | 100.0% | 1.000 | 46 | 100.0% | 1.000 | 55 | 100.0% | 1.000 |
| Number of prescriptions, PPPM: mean, SD | 4.15 | 2.31 | 3.93 | 1.91 | 0.448 | 4.07 | 1.93 | 0.827 | 3.82 | 1.90 | 0.355 |
| Patients with ≥1 prescription (antineoplastics): n, % | 127 | 100.0% | 101 | 100.0% | 1.000 | 46 | 100.0% | 1.000 | 55 | 100.0% | 1.000 |
| Number of prescriptions, PPPM: mean, SD | 1.07 | 0.24 | 0.75 | 0.33 | 0.000 | 0.71 | 0.33 | 0.000 | 0.79 | 0.33 | 0.000 |
| Hospice Care | |||||||||||
| Patients with any hospice care: n, % | 38 | 37.6% | n/a | 21 | 45.7% | n/a | 17 | 30.9% | n/a | ||
| Patients with inpatient hospice care: n, % | 16 | 15.8% | n/a | 10 | 21.7% | n/a | 6 | 10.9% | n/a | ||
| Patients with outpatient hospice care: n, % | 29 | 28.7% | n/a | 16 | 34.8% | n/a | 13 | 23.6% | n/a | ||
Inclusive of all other outpatient services not reported out individually.
EGFRm: Epidermal growth factor receptor activating mutation; mNSCLC: Metastatic non-small-cell lung cancer; PPPM: Per patient per month; SD: Standard deviation; TKI: Tyrosine kinase inhibitor.
Bolded indicates p < 0.05.
n/a: Not applicable.
Adjusted mean (SD) total healthcare costs PPPM were significantly higher for patients with (vs without) progression in the first 12 months of follow-up ($19,927 [$5,885] vs $17,527 [$5,737]; p = 0.002). The cost difference was more pronounced after excluding antineoplastic treatment costs ($11,480 [SD $4,752] vs $4,996 [SD $1,974]; p < 0.001 Figure 5). Unadjusted costs were significantly higher among patients with progression across most services categories (vs without progression) with those progressing in months 0–6 the most costly (Table 3 & Figure 6). Inpatient admissions were the most notable driver of cost differences with mean (SD) costs PPPM $808 ($2872) for nonprogressors, compared with $7508 ($19,080) and $3,882 ($9792) for those who progressed in months 0–6 and 7–12, respectively (Table 3 & Figure 6).
Figure 5.
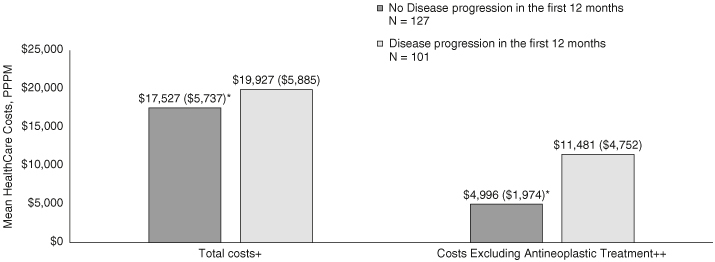
Adjusted total healthcare costs measured per patient per month among patients with metastatic non-small-cell lung cancer epidermal growth factor receptor activating mutation with and without disease progression within 12 months after first-line initiation on an epidermal growth factor receptor activating tyrosine kinase inhibitor.
*Comparison p ≤ 0.05 versus no disease progression.
+ Total costs include medical costs (inpatient and outpatient services) as well as pharmacy costs.
++ Both medical (for office administrated treatments) and pharmacy (for treatments filled at a pharmacy) costs for antineoplastic therapy are removed.
EGFRm: Epidermal growth factor receptor activating mutation; mNSCLC: Metastatic non-small-cell lung cancer; PPPM: Per patient per month; TKI: Tyrosine kinase inhibitor.
Table 3.
Unadjusted healthcare costs measured per patient per month among patients with metastatic non-small-cell lung cancer epidermal growth factor receptor activating mutation with and without disease progression within 12 months after first-line initiation on an epidermal growth factor receptor tyrosine kinase inhibitor.
| PPPM healthcare costs; Mean (SD) | No disease progression in the first 12 months (n = 127) | Disease progression in the first 12 months (n = 101) | Disease progression in months 0–6 (n = 46) | Disease progression in months 7–12 (n = 55) |
|---|---|---|---|---|
| Inpatient | $808 ($2872) | $5533 ($14,795)§ | $7508 ($19,080)§ | $3882 ($9792)§ |
| Outpatient medical† | $3923 ($4299) | $6633 ($7004)§ | $7374 ($6518)§ | $6,013 ($7387)§ |
| Emergency room | $56 ($160) | $362 ($1146)§ | $485 ($1548)§ | $259 ($642)§ |
| Office administered antineoplastics | $2 (22) | $1106 ($2482)§ | $1205 ($2847)§ | $1024 ($2152)§ |
| Infusion costs (i.e., administration costs for antineoplastics)† | $0.3 ($3) | $60 ($153)§ | $75 ($193)§ | $48 ($108)§ |
| Office visits | $310 ($253) | $265 ($177) | $254 ($196) | $273 ($161) |
| Radiology services | $1689 ($2658) | $2498 ($5240) | $2838 ($3966)§ | $2213 ($6128) |
| Laboratory services | $238 ($378) | $324 ($564) | $383 ($736) | $274 ($365) |
| Other outpatient services‡ | $1630 ($1936) | $3185 ($3488)§ | $3414 ($3796)§ | $2993 ($3231)§ |
| Total medical (inpatient + outpatient services + outpatient treatment) | $4731 ($5333) | $12,167 ($16,944)§ | $14,883 ($20,152)§ | $9895 ($13,480)§ |
| Outpatient pharmacy | $12,639 ($5077) | $8017 ($4593)§ | $7990 ($5233)§ | $8040 ($4030)§ |
| Antineoplastic pharmacy prescription costs | $12,319 ($5,080) | $7652 ($4522)§ | $7718 ($5214)§ | $7597 ($3899)§ |
| Other pharmacy prescription costs | $319 ($433) | $365 ($778) | $271 ($373) | $443 ($996) |
| Total (medical + pharmacy) | $17,370 ($8285) | $20,184 ($18,068) | $22,872 ($21,848)§ | $17,936 ($13,982) |
| Total (excluding all antineoplastic treatment costs both medical and pharmacy) | $5049 ($5401) | $11,425 ($16,810)§ | $13,949 ($20,004)§ | $9314 ($13,410)§ |
Procedure codes for antineoplastic administration.
Inclusive of all other outpatient services not reported out individually.
Comparison p ≤ 0.05 versus no disease progression.
EGFRm: Epidermal growth factor receptor activating mutation; mNSCLC: Metastatic non-small-cell lung cancer; PPPM: Per patient per month; SD: Standard deviation; TKI: Tyrosine kinase inhibitor.
Figure 6.
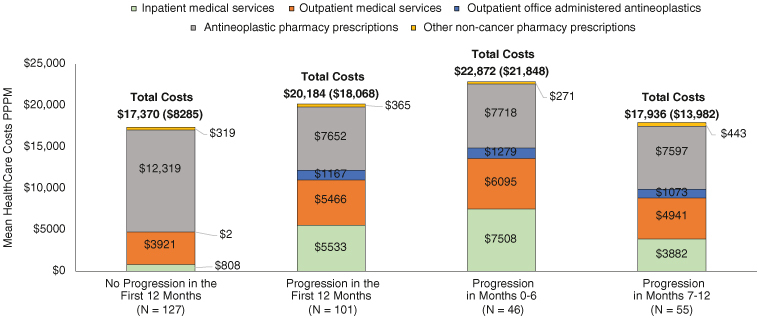
Unadjusted healthcare costs by service category measured per patient per month among metastatic non-small-cell lung cancer epidermal growth factor receptor activating mutation patients with and without disease progression within 12 months after first-line initiation on an epidermal growth factor receptor tyrosine kinase inhibitor.
EGFRm: Epidermal growth factor receptor activating mutation; mNSCLC: Metastatic non-small-cell lung cancer; PPPM: Per patient per month; TKI: Tyrosine kinase inhibitor.
Adjusted monthly predicted cumulative costs for medical services (excluding antineoplastic costs) were significantly higher among patients who progressed early (months 0–12) compared with patients who progressed late (months 13–36). Total costs (medical services + antineoplastic treatment costs) trended higher for early progressors (Figure 7), however, between group differences were attenuated by the higher treatment costs among those with late progression who remained on first-line EGFR TKIs for a more extended period of time.
Figure 7.
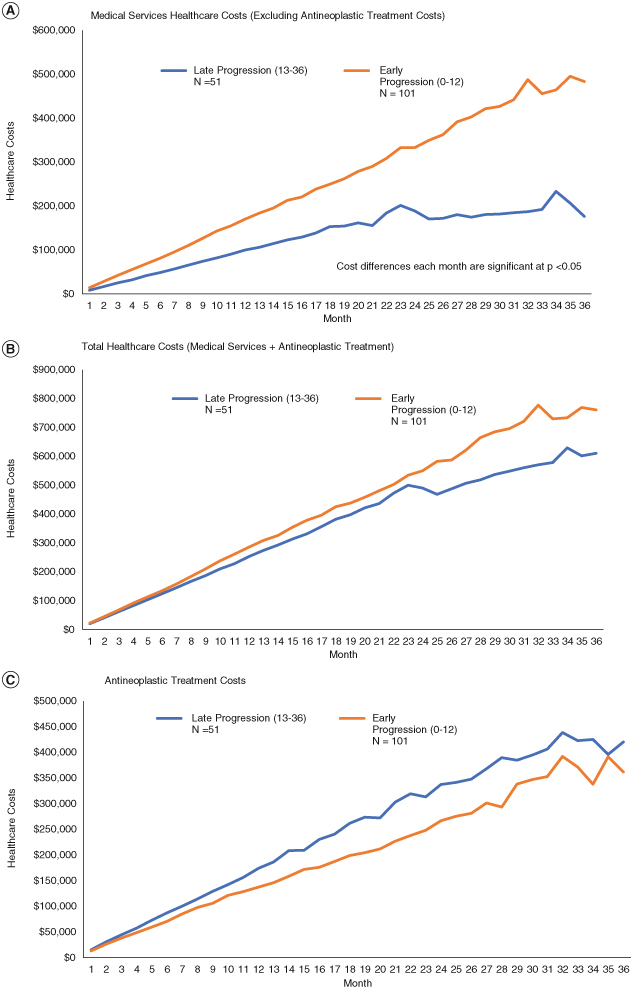
Adjusted* cumulative weighted predicted costs for among patients with metastatic non-small-cell lung cancer epidermal growth factor receptor activating mutation that progress early (months 0–12) versus late (months 13–36) after initiation on a first-line epidermal growth factor receptor tyrosine kinase inhibitor. (A) Medical services healthcare costs (excluding antineoplastic treatment costs). (B) Total healthcare costs (medical services + antineoplastic treatment). (C) Antineoplastic treatment costs.
*Models adjusted for age, gender, region, health plan type, year of first line treatment initiation, comorbidity index score and first line EGFR TKI.
**Statistical significance reported for each month has some variation, p-values are less than 0.05 for all months in model a.
EGFRm: Epidermal growth factor receptor activating mutation; mNSCLC: Metastatic non-small-cell lung cancer; TKI: Tyrosine kinase inhibitor.
4. Discussion
This real-world analysis is among the first to examine the economic burden of early disease progression among US patients with EGFRm NSCLC who received an EGFR TKI as first-line treatment for metastatic disease. This study augments prior literature that included a broad cohort of patients with metastatic lung cancer, irrespective of disease histology or genomic mutational status, with more recent data specific to patients with a common EGFR mutation and treated with targeted therapy [2,15,18]. In addition, by linking our population to the NDI to accurately capture patient mortality in the real-world setting this analysis further highlights the importance of PFS beyond what is available from clinical trials. Of the 228 patients with EGFRm mNSCLC, 66.7% (n = 152) experienced disease progression over 36 months, and (n = 101) 44.3% progressed within 12 months of initiating treatment with an EGFR TKI. This is consistent with clinical trials which have shown that first-line treatment with EGFR-TKIs (osimertinib, erlotinib, afatinib and gefitinib) extend PFS to 10–18 months [7–10].
In the adjusted analysis, our results showed a significant increase in total PPPM costs for patients with progression in the first 12 months after first-line treatment initiation, compared with those with no progression. However, the cost difference was attenuated by the antineoplastic agents prescribed. All patients without progression were treated with EGFR TKIs, whereas upon progression, infused agents were incorporated into treatment regimens. When antineoplastics were excluded from the analysis, costs for medical services were more than two-times higher for progressors versus nonprogressors within 12 months. Inpatient stays accounted for 4.7% of total PPPM costs for patients with no progression, and 27.4/32.8% for those with progression in the first 12/6 months of follow-up. These data are consistent with prior studies among patients with advanced NSCLC that have shown that hospitalization and anticancer treatment together account for the majority of total mean monthly costs [19]. The reduced number of hospitalizations, shorter length of inpatient stays, and lower hospitalization costs during first-line TKI therapy (e.g., prior to progression vs following progression) suggest treatments that delay progression may offer improved quality of care for lower medical costs for patients with EGFRm NSCLC.
Patients with progression in months 0–6 after first-line treatment initiation had higher PPPM medical costs than those who progressed in months 7–12, primarily driven by inpatient stays. It is possible that these individuals had more advanced disease upon metastatic diagnosis or a poor response to first-line EGFR TKIs compared with those who progressed later [20]. A poor prognosis is suggested by our data. Among progressors in the first 6 months, 50% of the events were attributable to hospice care or death compared with only 33% for progressors in months 7–12. In the analysis of early versus late progression over 36 months, results remained consistent. Patients with early progression had significantly higher cumulative predicted medical costs each month compared with those with late progression. The inverse was true for antineoplastics with late progressors having higher drug costs attributable to a longer duration of treatment with EGFR TKIs.
These data highlight the need for better treatment options to delay progression. Reyes et al. conducted a similar analysis reporting the costs of disease progression among patients with metastatic breast, colorectal and lung cancer using data from 2007–2014. Metastatic lung cancer was shown to have the highest rate of progression within 12 months (compared with breast and colorectal). The current analysis identified a specific cohort of EGFRm mNSCLC patients with progression in months 0–6, 7–12 (early progressors) compared with those who progressed later (months 13–36) and found that those who progressed in the earliest months had the highest costs providing further support suggesting that delaying disease advancement (through availability of more effective treatment options) may reduce medical costs. These findings are relevant both for payers (as lower medical costs incurred by delaying disease advancement may offset treatment costs) and for clinicians of whom many of their patients are covered under Medicare plans. The Enhancing Oncology Model announced in June 2022 (from the Center of Medicare and Medicaid Innovation) provides financial incentives for employing cost-effective practices. Understanding costs overtime (and the impact of early progression) may help payers anticipate their annual budgets and help providers participating in value-based programs manage the total cost of care for their patients [21]. New treatment options are needed for patients with EGFRm mNSCLC that have a longer duration of disease control both in the first line setting and also in later line settings where options are further limited.
This study is subject to several limitations. While administrative claims data provide detailed cost information for a diverse group of real-world patients, not all clinical information is recorded. Biomarker results and lung cancer histology were not available, therefore, treatment with afatinib, erlotinib, gefitinib or osimertinib was used as a proxy to identify EGFRm NSCLC. Although afatinib, erlotinib, gefitinib and osimertinib are most often prescribed to address common EGFR mutations, using these targeted agents as a proxy for the identification of patients with an exon 19 deletion or L858R mutation may result in some misclassification. Metastatic disease was determined based on claims evidence of a secondary neoplasm and may be subject to coding inaccuracies. Physician documented disease progression was unavailable, therefore, an algorithm comprised of line of therapy advancement, evidence of hospice care coded on medical claims, and death was used to identify disease progression. Of note, some patients who advanced to a subsequent line of therapy may have done so for reasons other than disease progression and therefore may have been misclassified. In addition, the MarketScan commercial and Medicare databases are convenience samples of employees, retirees and dependents with US commercial and Medicare health insurance coverage. Future analyses in alternative datasets and with a larger sample size are needed to augment study findings and improve generalizability to patients with other insurance types (e.g., Medicaid) or who lack healthcare coverage needs to be confirmed in future studies.
5. Conclusion
Given that the cost of oncology care has been increasing dramatically over the past decade and with increases expected to continue [13] the need for data quantifying how costs for both medical services and treatments are impacted by disease progression is paramount. The results from this analysis suggest that patients with EGFRm mNSCLC with disease progression in the first 12 months following initiation of an EGFR TKI incurred significantly higher total costs despite having lower treatment costs compared with patients without disease progression in the first 12 months. Cost burden was highest among those with progression in the first 6 months, driven primarily by hospitalizations substantiating the need for improved treatment options to delay disease advancement. These data may help inform oncology providers aiming to administer high quality cancer care in a cost-efficient way.
Funding Statement
N Princic, D McMorrow and H Schwartz are employees of Merative currently or at the time the analysis was conducted and Merative was funded by Daiichi Sankyo to conduct this study. E Marrett and WJ Kwong are employees of Daiichi Sankyo, Inc. currently or at the time the analysis was conducted. J Subramanian has served in an advisory role and/or speakers bureau for Astra Zeneca, Boehringer Ingelheim, Cardinal Health, Daiichi Sankyo, G1 Therapeutics, GLG, Guidepoint, Jazz Pharmaceuticals, Janssen. Oncohost and Pfizer. Programming services were provided George Shrady of Merative. These services were paid for by Daiichi Sankyo, Inc.
Author contributions
N Princic, E Marrett, WJ Kwong, D McMorrow and H Schwartz contributed to conception, design, and planning of the study, analysis of the data and interpretation of the results and drafting of the manuscript. J Subramanian contributed to interpretation of the results, as well as critically reviewing the manuscript for important intellectual content.
Financial disclosure
N Princic, D McMorrow and H Schwartz are employees of Merative currently or at the time the analysis was conducted and Merative was funded by Daiichi Sankyo to conduct this study. E Marrett and WJ Kwong are employees of Daiichi Sankyo, Inc. currently or at the time the analysis was conducted. J Subramanian has served in an advisory role and/or speakers bureau for Astra Zeneca, Boehringer Ingelheim, Cardinal Health, Daiichi Sankyo, G1 Therapeutics, GLG, Guidepoint, Jazz Pharmaceuticals, Janssen. Oncohost and Pfizer. Programming services were provided George Shrady of Merative. These services were paid for by Daiichi Sankyo, Inc. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Competing interests disclosure
The authors have no competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Writing disclosure
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
All database records are statistically de-identified and certified to be fully compliant with US patient confidentiality requirements set forth in the Health Insurance Portability and Accountability Act of 1996. Institutional Review Board (IRB) waiver of authorization approval was obtained from WCG IRB (IRB study #: 1324891; IRB protocol #: 20217016) on December 23, 2021
Data availability statement
The data that support the findings of this study are available from Merative. Restrictions apply to the availability of these data, which were used under license for this study.
References
Papers of special note have been highlighted as: • of interest
- 1.American Cancer Society . Key statistics for lung cancer. https://www.cancer.org/cancer/non-small-cell-lung-cancer/about/key-statistics.html
- 2.U.S. National Institute of Health, National Cancer Institute . SEER Cancer Statistics Review, 1975–2018. https://seer.cancer.gov/archive/csr/1975_2015/index.html ; • Provides comprehensive statistics of survival rates among patients with non-small-cell lung cancer (NSCLC).
- 3.Chen F, Chen N, Yu Y, et al. Efficacy and safety of epidermal growth factor receptor (EGFR) inhibitors plus antiangiogenic agents as first-line treatments for patients with advanced EGFR-mutated non-small cell lung cancer: a meta-analysis. Front Oncol. 2020;10:904. doi: 10.3389/fonc.2020.00904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Papadimitrakopoulou VA, Mok TS, Han JY, et al. Osimertinib versus platinum-pemetrexed for patients with EGFR T790M advanced NSCLC and progression on a prior EGFR-tyrosine kinase inhibitor: AURA3 overall survival analysis. Ann Oncol. 2020;31(11):1536–1544. doi: 10.1016/j.annonc.2020.08.2100 [DOI] [PubMed] [Google Scholar]
- 5.Jorge SE, Kobayashi SS, Costa DB. Epidermal growth factor receptor (EGFR) mutations in lung cancer: preclinical and clinical data. Braz J Med Biol Res. 2014;47(11):929–939. doi: 10.1590/1414-431X20144099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kitadai R, Okuma Y. Treatment strategies for non-small cell lung cancer harboring common and uncommon EGFR mutations: drug sensitivity based on exon classification, and structure-function analysis. Cancer. 2022;14(10):2519. doi: 10.3390/cancers14102519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378(2):113–125. doi: 10.1056/NEJMoa1713137 [DOI] [PubMed] [Google Scholar]
- 8.Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised Phase III trial. Lancet Oncol. 2012;13(3):239–246. doi: 10.1016/S1470-2045(11)70393-X [DOI] [PubMed] [Google Scholar]
- 9.Yang JC, Sequist LV, Geater SL, et al. Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: a combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol. 2015;16(7):830–838. doi: 10.1016/S1470-2045(15)00026-1 [DOI] [PubMed] [Google Scholar]
- 10.Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31(27):3327–3334. doi: 10.1200/JCO.2012.44.2806 [DOI] [PubMed] [Google Scholar]
- 11.Wu SG, Shih JY. Management of acquired resistance to EGFR TKI–targeted therapy in advanced non-small cell lung cancer. Mol Cancer. 2018;17(1):38. doi: 10.1186/s12943-018-0777-1 [DOI] [PMC free article] [PubMed] [Google Scholar]; • Emphasizes the need to explore subsequent treatment management for patients after acquiring resistance to first-line epidermal growth factor receptor tyrosine kinase inhibitor therapy.
- 12.Chastek B, Harley C, Kallich J, et al. Health care costs for patients with cancer at the end of life. J Oncol Pract. 2012;8(6):75S–80S. doi: 10.1200/JOP.2011.000469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mariotto AB, Enewold L, Zhao J, et al. Medical care costs associated with cancer survivorship in the United States. Cancer Epidemiol Biomarkers Prev. 2020;29(7):1304–1312. doi: 10.1158/1055-9965.EPI-19-1534 [DOI] [PMC free article] [PubMed] [Google Scholar]; • Suggests that due to the significant and ongoing rise of cost of oncology care, phase of cancer-attributable medical costs are critical factors to consider for cost–effectiveness analyses.
- 14.Fox KM, Brooks JM, Kim J. Metastatic non-small cell lung cancer: costs associated with disease progression. Am J Manag Care. 2008;14(9):565–571. [PubMed] [Google Scholar]; • A retrospective study that showed that patients with metastatic NSCLC with disease progression incurred significantly higher costs compared to similar patients with stable disease.
- 15.Reyes C, Engel-Nitz NM, Byfield SD, et al. Cost of disease progression in patients with metastatic breast, lung and colorectal cancer. Oncologist. 2019;24(9):1209–1218. doi: 10.1634/theoncologist.2018-0018 [DOI] [PMC free article] [PubMed] [Google Scholar]; • Retrospective claims-based study that found that delay in progression in metastatic lung cancer was associated with lower healthcare costs.
- 16.Merative. Merative MarketScan Research Databases. 2022. https://www.merative.com/content/dam/merative/documents/brief/Marketscan_explainer_general.pdf
- 17.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8 [DOI] [PubMed] [Google Scholar]
- 18.Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin. 2018;68(1):7–30. [DOI] [PubMed] [Google Scholar]
- 19.Skinner KE, Fernandes AW, Walker MS, et al. Healthcare costs in patients with advanced non-small cell lung cancer and disease progression during targeted therapy: a real-world observational study. J Med Econ. 2018;21(2):192–200. doi: 10.1080/13696998.2017.1389744 [DOI] [PubMed] [Google Scholar]; • Study that found hospitalization and anticancer treatment together account for the majority of total mean monthly costs in patients with advanced NSCLC.
- 20.Hung MS, Fang YH, Lin YC, et al. Survival-associated factors of first-line EGFR-tyrosine kinase inhibitor responders and non-responders in lung adenocarcinoma patients with common EGFR mutations. Mol Clin Oncol. 2018; 8(3):421–428. doi: 10.3892/mco.2018.1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kocher RP, Adashi EY. A new approach to cancer bundled payments in medicare—the enhancing oncology model. JAMA Health Forum. 2023;4(1):e224904. doi: 10.1001/jamahealthforum.2022.4904 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from Merative. Restrictions apply to the availability of these data, which were used under license for this study.


