Abstract
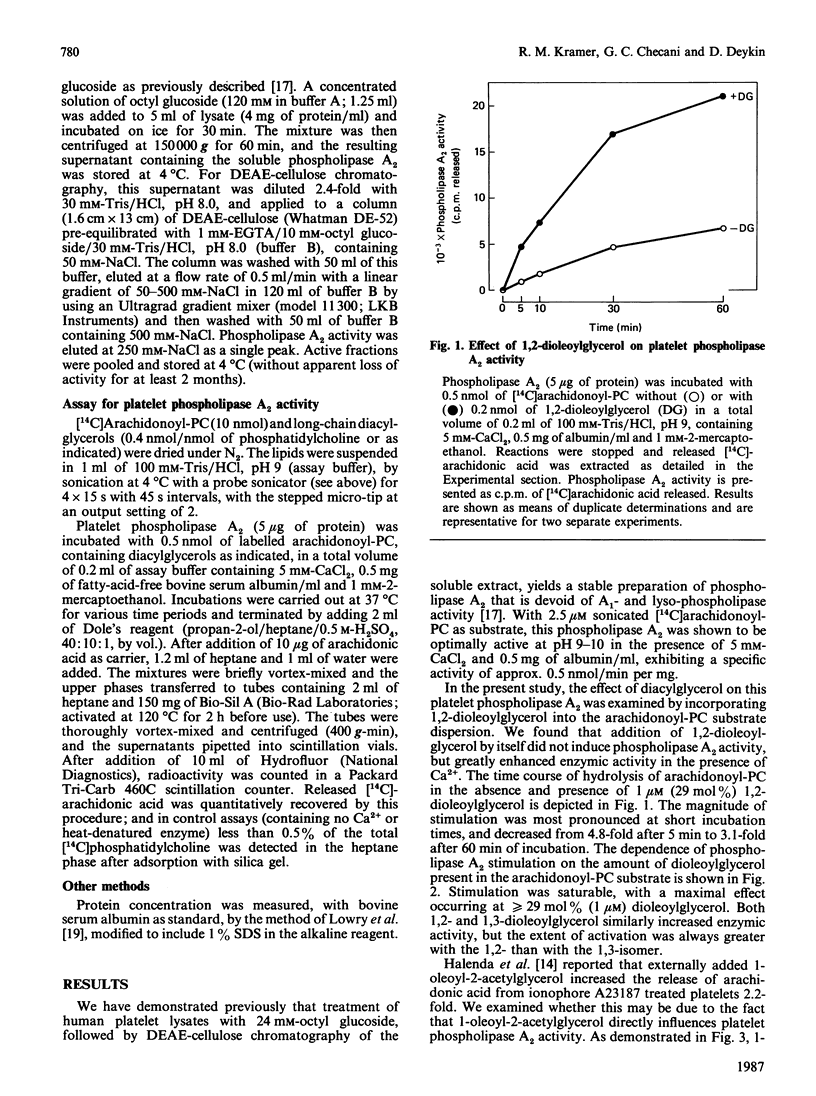
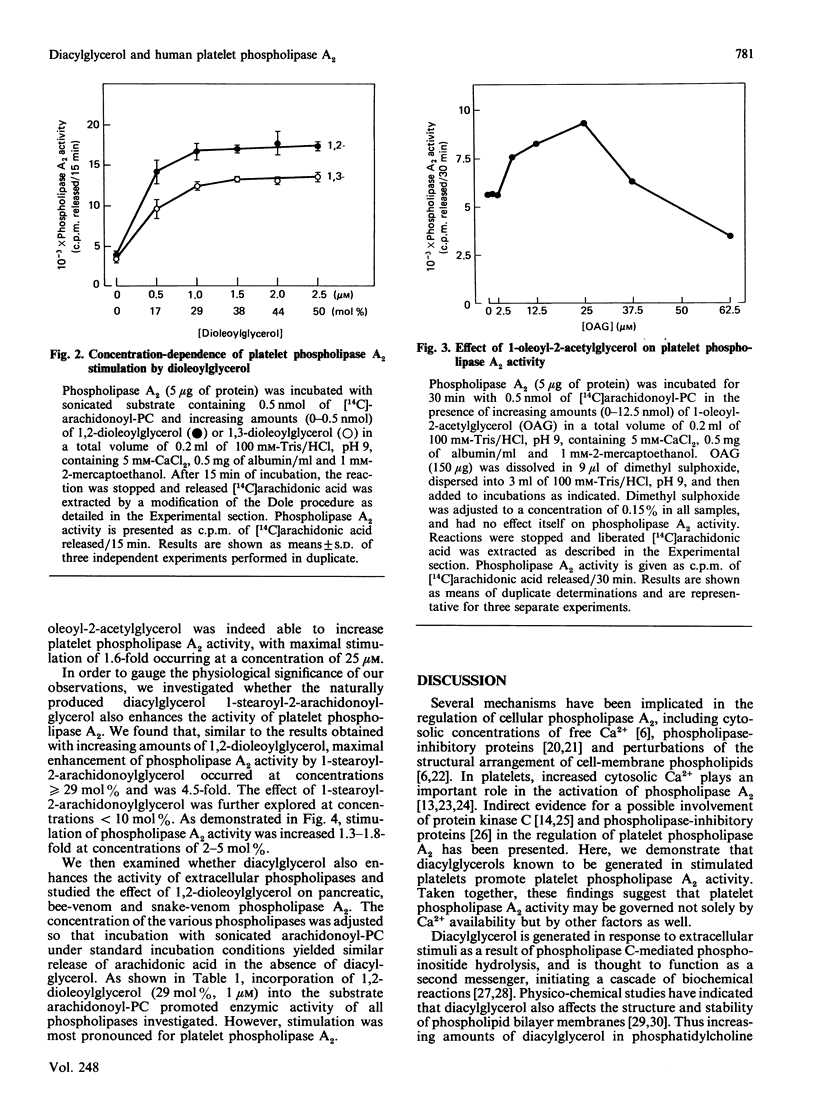
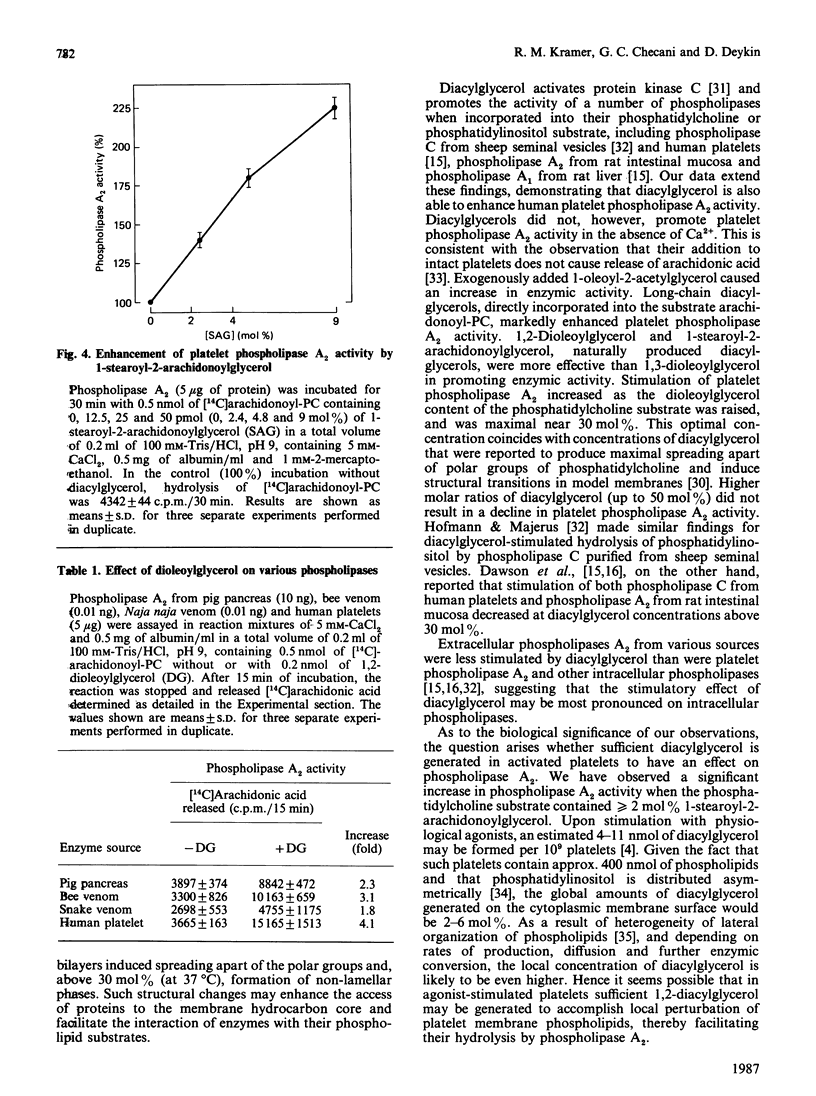
We examined the effect of diacylglycerol on Ca2+-dependent phospholipase A2 from human platelets. Phospholipase A2 was solubilized and partially purified to a stable form in the presence of n-octyl beta-D-glucopyranoside (octyl glucoside), and its enzymic activity was determined with sonicated 2.5 microM-1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine (arachidonoyl-PC) as substrate. Phospholipase A2 activity was increased when diacylglycerol was incorporated into the substrate arachidonoyl-PC. Stimulation was maximal in the presence of greater than or equal to 29 mol% (1 microM) diacylglycerol, and was greater than 4-fold for both 1,2-dioleoylglycerol and 1-stearoyl-2-arachidonoylglycerol. 1-Stearoyl-2-arachidonoylglycerol at concentrations of 2-5 mol% increased phospholipase A2 activity 1.3-1.8-fold. Exogenously added 1-oleoyl-2-acetylglycerol also enhanced phospholipase A2 activity, producing a maximal stimulation of 1.6-fold at a concentration of 25 microM. Comparative studies conducted with pancreatic, bee-venom and snake-venom phospholipase A2 showed that the activity of these extracellular phospholipases towards the arachidonoyl-PC substrate was also increased by diacylglycerol, but stimulation was less than observed for platelet phospholipase A2. Our results suggest that diacylglycerol, known to be generated in stimulated platelets, may enhance Ca2+-activated phospholipase A2.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bass D. A., Gerard C., Olbrantz P., Wilson J., McCall C. E., McPhail L. C. Priming of the respiratory burst of neutrophils by diacylglycerol. Independence from activation or translocation of protein kinase C. J Biol Chem. 1987 May 15;262(14):6643–6649. [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billah M. M., Lapetina E. G. Formation of lysophosphatidylinositol in platelets stimulated with thrombin or ionophore A23187. J Biol Chem. 1982 May 10;257(9):5196–5200. [PubMed] [Google Scholar]
- Billah M. M., Siegel M. I. Phospholipase A2 activation in chemotactic peptide-stimulated HL60 granulocytes: synergism between diacylglycerol and Ca2+ in a protein kinase C-independent mechanism. Biochem Biophys Res Commun. 1987 Apr 29;144(2):683–691. doi: 10.1016/s0006-291x(87)80019-0. [DOI] [PubMed] [Google Scholar]
- Broekman M. J., Ward J. W., Marcus A. J. Phospholipid metabolism in stimulated human platelets. Changes in phosphatidylinositol, phosphatidic acid, and lysophospholipids. J Clin Invest. 1980 Aug;66(2):275–283. doi: 10.1172/JCI109854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch J. W., Majerus P. W. The role of prostaglandins in platelet function. Semin Hematol. 1979 Jul;16(3):196–207. [PubMed] [Google Scholar]
- Chap H. J., Zwaal R. F., van Deenen L. L. Action of highly purified phospholipases on blood platelets. Evidence for an asymmetric distribution of phospholipids in the surface membrane. Biochim Biophys Acta. 1977 Jun 2;467(2):146–164. doi: 10.1016/0005-2736(77)90192-4. [DOI] [PubMed] [Google Scholar]
- Das S., Rand R. P. Diacylglycerol causes major structural transitions in phospholipid bilayer membranes. Biochem Biophys Res Commun. 1984 Oct 30;124(2):491–496. doi: 10.1016/0006-291x(84)91580-8. [DOI] [PubMed] [Google Scholar]
- Das S., Rand R. P. Modification by diacylglycerol of the structure and interaction of various phospholipid bilayer membranes. Biochemistry. 1986 May 20;25(10):2882–2889. doi: 10.1021/bi00358a022. [DOI] [PubMed] [Google Scholar]
- Dawson R. M., Hemington N. L., Irvine R. F. Diacylglycerol potentiates phospholipase attack upon phospholipid bilayers: possible connection with cell stimulation. Biochem Biophys Res Commun. 1983 Nov 30;117(1):196–201. doi: 10.1016/0006-291x(83)91560-7. [DOI] [PubMed] [Google Scholar]
- Dawson R. M., Hemington N., Irvine R. F. The inhibition of diacylglycerol-stimulated intracellular phospholipases by phospholipids with a phosphocholine-containing polar group. A possible physiological role for sphingomyelin. Biochem J. 1985 Aug 15;230(1):61–68. doi: 10.1042/bj2300061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson R. M., Irvine R. F., Bray J., Quinn P. J. Long-chain unsaturated diacylglycerols cause a perturbation in the structure of phospholipid bilayers rendering them susceptible to phospholipase attack. Biochem Biophys Res Commun. 1984 Dec 14;125(2):836–842. doi: 10.1016/0006-291x(84)90615-6. [DOI] [PubMed] [Google Scholar]
- Halenda S. P., Zavoico G. B., Feinstein M. B. Phorbol esters and oleoyl acetoyl glycerol enhance release of arachidonic acid in platelets stimulated by Ca2+ ionophore A23187. J Biol Chem. 1985 Oct 15;260(23):12484–12491. [PubMed] [Google Scholar]
- Hofmann S. L., Majerus P. W. Modulation of phosphatidylinositol-specific phospholipase C activity by phospholipid interactions, diglycerides, and calcium ions. J Biol Chem. 1982 Dec 10;257(23):14359–14364. [PubMed] [Google Scholar]
- Irvine R. F. How is the level of free arachidonic acid controlled in mammalian cells? Biochem J. 1982 Apr 15;204(1):3–16. doi: 10.1042/bj2040003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnovsky M. J., Kleinfeld A. M., Hoover R. L., Klausner R. D. The concept of lipid domains in membranes. J Cell Biol. 1982 Jul;94(1):1–6. doi: 10.1083/jcb.94.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer R. M., Checani G. C., Deykin A., Pritzker C. R., Deykin D. Solubilization and properties of Ca2+-dependent human platelet phospholipase A2. Biochim Biophys Acta. 1986 Oct 3;878(3):394–403. doi: 10.1016/0005-2760(86)90248-1. [DOI] [PubMed] [Google Scholar]
- Kramer R. M., Jakubowski J. A., Vaillancourt R., Deykin D. Effect of membrane cholesterol on phospholipid metabolism in thrombin-stimulated platelets. Enhanced activation of platelet phospholipase(s) for liberation of arachidonic acid. J Biol Chem. 1982 Jun 25;257(12):6844–6849. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Marcus A. J. The role of lipids in platelet function: with particular reference to the arachidonic acid pathway. J Lipid Res. 1978 Sep;19(7):793–826. [PubMed] [Google Scholar]
- McKean M. L., Smith J. B., Silver M. J. Formation of lysophosphatidylcholine by human platelets in response to thrombin. Support for the phospholipase A2 pathway for the liberation of arachidonic acid. J Biol Chem. 1981 Feb 25;256(4):1522–1524. [PubMed] [Google Scholar]
- Mobley A., Tai H. H. Synergistic stimulation of thromboxane biosynthesis by calcium ionophore and phorbol ester or thrombin in human platelets. Biochem Biophys Res Commun. 1985 Jul 31;130(2):717–723. doi: 10.1016/0006-291x(85)90475-9. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Turnover of inositol phospholipids and signal transduction. Science. 1984 Sep 21;225(4668):1365–1370. doi: 10.1126/science.6147898. [DOI] [PubMed] [Google Scholar]
- Rittenhouse-Simmons S., Deykin D. The activation by Ca2+ of platelet phospholipase A2. Effects of dibutyryl cyclic adenosine monophosphate and 8-(N,N-diethylamino)-octyl-3,4,5-trimethoxybenzoate. Biochim Biophys Acta. 1978 Nov 1;543(4):409–422. doi: 10.1016/0304-4165(78)90296-9. [DOI] [PubMed] [Google Scholar]
- Rittenhouse-Simmons S. Production of diglyceride from phosphatidylinositol in activated human platelets. J Clin Invest. 1979 Apr;63(4):580–587. doi: 10.1172/JCI109339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittenhouse-Simmons S., Russell F. A., Deykin D. Mobilization of arachidonic acid in human platelets. Kinetics and Ca2+ dependency. Biochim Biophys Acta. 1977 Sep 28;488(3):370–380. doi: 10.1016/0005-2760(77)90196-5. [DOI] [PubMed] [Google Scholar]
- Rittenhouse S. E. Activation of human platelet phospholipase C by ionophore A23187 is totally dependent upon cyclo-oxygenase products and ADP. Biochem J. 1984 Aug 15;222(1):103–110. doi: 10.1042/bj2220103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittenhouse S. E., Horne W. C. Ionomycin can elevate intraplatelet Ca2+ and activate phospholipase A without activating phospholipase C. Biochem Biophys Res Commun. 1984 Aug 30;123(1):393–397. doi: 10.1016/0006-291x(84)90426-1. [DOI] [PubMed] [Google Scholar]
- Snyder F. Chemical and biochemical aspects of platelet activating factor: a novel class of acetylated ether-linked choline-phospholipids. Med Res Rev. 1985 Jan-Mar;5(1):107–140. doi: 10.1002/med.2610050105. [DOI] [PubMed] [Google Scholar]
- Touqui L., Rothhut B., Shaw A. M., Fradin A., Vargaftig B. B., Russo-Marie F. Platelet activation--a role for a 40K anti-phospholipase A2 protein indistinguishable from lipocortin. Nature. 1986 May 8;321(6066):177–180. doi: 10.1038/321177a0. [DOI] [PubMed] [Google Scholar]
- Waite M. Approaches to the study of mammalian cellular phospholipases. J Lipid Res. 1985 Dec;26(12):1379–1388. [PubMed] [Google Scholar]
- Watson S. P., Ganong B. R., Bell R. M., Lapetina E. G. 1,2-Diacylglycerols do not potentiate the action of phospholipases A2 and C in human platelets. Biochem Biophys Res Commun. 1984 May 31;121(1):386–391. doi: 10.1016/0006-291x(84)90734-4. [DOI] [PubMed] [Google Scholar]


