Abstract
Aim: The design, synthesis, docking studies and evaluation of the in vitro antifungal and cytotoxic properties of eugenol (EUG) containing 1,2,3-triazole derivatives are reported. Most of the derivatives have not been reported.
Materials & methods: The EUG derivatives were synthesized, molecular docked and tested for their antifungal activity.
Results: The compounds showed potent antifungal activity against Trichophyton rubrum, associated with dermatophytosis. Compounds 2a and 2i exhibited promising results, with 2a being four-times more potent than EUG. The binding mode prediction was similar to itraconazole in the lanosterol-14-α-demethylase wild-type and G73E mutant binding sites. Additionally, the pharmacokinetic profile prediction suggests good gastrointestinal absorption and potential oral administration.
Conclusion: Compound 2a is a promising antifungal agent against dermatophytosis caused by T. rubrum.
Keywords: : antifungal activity, dermatophytes, eugenol, triazole compounds, Trichophyton
Graphical Abstract

Plain language summary
Article highlights.
This study describes the synthesis, antifungal activity evaluation and docking studies of a series of eugenol (EUG)-containing 1,2,3-triazole derivatives.
The synthesis of the compounds was achieved in two steps, namely propargylation of EUG followed by the Cu(I)-catalyzed azide–alkyne cycloaddition reactions between the propargylated EUG and different commercially avaialable phenyl azides.
The identities of the compounds were confirmed by 1H and 13C nuclear magnetic resonance and Infrared spectroscopies along with Mass spectrometry.
The EUG derivatives were evaluated against Aspergillus flavus ATCC 204304, A. fumigatus ATCC 16913, Trichophyton rubrum CCT 5506 and Candida albicans ATCC 10231.
The best results concerning antifungal activity were noted for compounds 4-((4-allyl-2-methoxyphenoxy)methyl)-1-(4-bromophenyl)-1H-1,2,3-triazole (2a) and 4-((4-allyl-2-methoxyphenoxy)methyl)-1-(2-fluorophenyl)-1H-1,2,3-triazole (2i), which demonstrated significant activity against the dermatophyte T. rubrum, with MIC values of 16 and 32 μg ml-1, respectively.
The docking study involving compounds 2a and 2i revealed a behavior similar to itraconazole in the lanosterol-14α-demethylase wild-type and G73E mutant binding site.
In addition, the pharmacokinetic profile prediction suggests good gastrointestinal aborption and potential oral administration for these compunds.
The prevalence of fungal infections has increased dramatically in the past three decades and this has been associated with the rising number of immunocompromised patients. Globally, it is estimated that over 6 million species of fungi exist; however, only a small fraction has the potential to induce severe diseases in humans, such as Cadydemia, Aspergillosis and Cryptococcosis [1,2]. The limited therapeutic options for the management of fungal infections, when compared with bacterial infections, contribute to the increased mortality rate [3]. Currently, only four commercial antifungal groups – polyenes (amphotericin b), triazoles (fluconazole [FCZ] and itraconazole [ITZ]), echinocandins (micafungin and anidulafingin) and allylamines such as terbinafine – are available [4,5].
The triazole class, characterized by a heterocyclic ring of either 1,2,3-triazole or 1,2,4-triazole, exhibits a broad spectrum for antifungal activity [6,7]. Its primary mechanism of action involves inhibiting the enzyme lanosterol 14α-demethylase by covalently binding to the heme iron of the enzyme, thereby inhibiting the biosynthesis of ergosterol – a vital sterol for fungal cell membrane fluidity [5,8]. FCZ, for instance, binds to the heme iron through the nitrogen atom in the triazole moiety, with a 2.13 Å Fe-N distance in the active site of the lanosterol 14α-demethylase. This drug is encircled by a hydrophobic pocket, as anticipated from the nature of the lanosterol substrate. Noteworthy amino acids in the catalytic domain, positioned at a desirable distance from FCZ for hydrogen bonding, include Tyr126, Phe134, Ile139, Tyr140, Phe236, Gly310, Val311, Gly314, Gly315, Thr318, Leu380 and Met509 [9,10]. The 2,4-difluorophenyl segment of this azole aligns with Gly310, while the coordinated triazole heterocyclic is adjacent to Gly314 in the consensus pocket of sterol binding on helix-I. Water molecules W743 and W790 contribute to a hydrogen bond network with this azole. Specifically, W743 acts as a bridge between the hydroxyl groups of the azole, Tyr140 and the propionate of the heme cofactor, while W790 engages in hydrogen bonds with the carbonyl group of Ser382, the hydroxyl of Tyr126 and N4 of the second triazole ring which extends away from the heme [9,10].
Although azoles exhibit a broad spectrum for antifungal therapy, the treatment of fungal infections is raising concerns due to the emergence of fungal resistance to commercial drugs. The resistance is developed due to prolonged exposure or disordered use of antifungals [11–13]. As established in the literature, mutated proteins can characterize resistance to azole drugs, as seen in pathogenic fungi. Consequently, these targets show promise for the development of new antifungals. Therefore, an overview of the lanosterol 14α-demethylase Gly73Glu (G73E) mutation (PDB ID 5ESG), with a resolution of 1.98 Å, in complex with the linker ITZ, can suggest avenues for the discovery of new drugs [10]. The significance is illustrated by the lower minimum inhibitory concentration (MIC) of azole drugs FCZ and voriconazole reduced by up to 2.5-fold against Glu73 mutants compared with the wild-type protein. The mutant structure reveals a different conformation of ITZ observed in the lanosterol 14α-demethylase of the wild-type. The piperazine moiety of this drug acts as a hinge, changing from a chair to a twisted boat conformation. In the wild-type, this ring presents a chair conformation, allowing an extended conformation of the ITZ structure. In the G73E mutant, the twisted boat shape of the piperazine nucleus permits the bending of the drug tail away from E73. Additionally, the 1,2,4-triazolin-3-one moiety of this drug can be involved in π-anion interaction with the carboxylate of Glu73 [14]. Finally, a hydrogen-bonding network comprising Pro379, His381, Ser382, Asp504, Ser508 and Met509 residues is observed interacting with three water molecules [15,16].
In the pursuit of new models for the development of a diversity of drugs, natural products derived from plants and microorganisms have been playing a crucial role, as illustrated by several works [17–20]. Among the diversity of such natural products, many can be employed directly as fungicides or subjected to chemical modifications to generate derivatives endowed with antifungal properties [21–23]. One such natural product is eugenol (EUG; 4-allyl-2-methoxy phenol) a well-known and extensively-studied phenylpropanoid found in essential oils of plants from the Lamiaceae, Lauraceae, Myrtaceae and Myristaceae families. Clove oil (Syzygium aromaticum), representing up to 1.5% w/w of fresh plant material, serves as a primary source of this compound [24]. This natural product, widely utilized as a raw material in the pharmaceutical industry, contains an aromatic ring as well as hydroxyl and an allyl group, structural features amenable to chemical modifications that allow the synthesis of new compounds promising for the treatment of various diseases. The antifungal activity of EUG is evident due to its lipid solubility, enabling penetration into the fungal cells, where it induces fungicidal effects through disturbances, distortions and depletions in essential components such as ergosterol [25,26]. Previous studies have demonstrated promising anti-Candida activity in some EUG derivatives [27–29]. Considering the premises just presented, the objective of this study was to synthesize and evaluate the antifungal and cytotoxic activities of triazole-containing compounds derived from EUG. Additionally, computational studies using the crystallographic structure of the mutant enzyme lanosterol 14α-demethylase G73E were conducted to predict the binding mode.
1. Material & methods
1.1. Generalities
The reagents and high-purity solvents were procured from Sigma-Aldrich (MO, USA), Êxodo Científica (Sumaré, SP, Brazil) and Química Moderna (Barueri, SP, Brazil) and used without prior purification. Thin layer chromatography (TLC) analyses were carried out with silica gel plates on aluminum support using different solvent systems. TLC plates were visualized under ultraviolet light (λ = 254 nm) and/or a potassium permanganate aqueous solution. Column chromatography separations were carried out using silica gel (70–230 mesh, Sigma-Aldrich) as the stationary phase.
IR spectra were recorded using the attenuated total reflectance technique on a Varian 660 instrument (Varian, CA, USA) equipped with a GladiATr accessory, within the range of 4000–500 cm-1. Hydrogen nuclear magnetic resonance (NMR) spectra (1H NMR, 300, 400 and 600 MHz) and carbon NMR spectra (13C NMR, 75 , 100 and 150 MHz) were recorded using three spectrometers: VARIAN MERCURY 300 (Varian), Bruker Avance III 400 MHz (Bruker, MA, USA) and Premium Compact 600 MHZ (Bruker, MA, USA). Deuterated chloroform (CDCl3) and dimethyl sulfoxide (DMSO-d6) were employed. NMR data are presented as follows: chemical shift (δ) in ppm, multiplicity, number of hydrogens and scalar coupling constants (J) expressed in Hertz (Hz). Multiplicities are indicated by the following abbreviations: s (singlet), d (doublet), dd (doublet of doublet), t (triplet), td (triplet of doublet), ddt (doublet of doublet of triplet) and m (multiplet).
Melting points were determined using an MQAPF-302 instrument (Micro Química, Cotia, Brazil) and were not corrected. Chromatograms of samples 2a–2i were acquired on the Vanquish Flex ultra-high-performance liquid chromatography system (Thermo Scientific, Bremen, Germany) coupled with the LTQ-XL mass spectrometer (Thermo Scientific). A C18 column (100 A, 150 × 2.1 mm, Luna Omega 1.6 μm, Phenomenex, São Paulo, Brazil) was used for chromatographic separation, by injecting 2 μl of the sample at a flow rate of 350 μl min-1. The gradient employed was 5–95% over 7 min at 60°C, using water and methanol, both with 0.1% formic acid. Mass spectra were acquired in the m/z range of 100–1500 in positive ionization mode. Other parameters of the electron-spray ionization (ESI) source were: heater temperature – 350°C; sheath gas flow rate (μl min-1) – 30; aux. gas flow rate (μl min-1) – 10; spray voltage – 4 kV; and capillary voltage – 44.00 V. Calibration of the LTQ-XL equipment was performed using a CalMix LTQ solution, positive mode, over a mass range of m/z 100–2000, ion accumulation time of 0.005 s and capillary voltage of 4.0 kV. Mass spectra obtained were processed using the Xcalibur program, version 2.2 (Thermo Scientific). Finally, MS/MS experiments were conducted using 20% normalized energy.
1.2. Synthetic procedures
1.2.1. Synthesis of terminal alkyne 4-allyl-2-methoxy-1-(prop-2-yn-1-yloxy)benzene (1)
To a 50 ml round-bottom flask, sodium hydroxide (0.313 g; 7.38 mmol), EUG (1.20 g; 7.32 mmol) and methanol (25.0 ml) were added. The resulting mixture was stirred at 40°C for 30 min. Subsequently, methanol was removed under reduced pressure and absolute ethanol (10.0 ml) was added to eliminate residual water. Ethanol was evaporated and to the flask, under a nitrogen atmosphere, acetonitrile (25.0 ml) and propargyl bromide (800 μl; 8.79 mmol) were added slowly. The system was stirred at room temperature for 18 h. After this period, the reaction solvent was removed under reduced pressure in a rotary evaporator, followed by the addition of an aqueous solution of sodium hydroxide (25 ml; 0.1 mol L-1). The mixture was then transferred to a separating funnel and the aqueous phase was extracted with dichloromethane (3 × 25.0 ml). The combined organic phase was washed with a saturated aqueous solution of sodium chloride (25.0 ml), dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure. The crude product, purified by silica gel column chromatography eluted with hexane-ethyl acetate (4:1 v/v), afforded compound 1 as a yellow oil with a yield of 81% (1.20 g; 7.30 mmol). The structure of alkyne 1 is supported by the following data.
TLC: Rf = 0.65 (hexane-ethyl acetate 4:1 v/v). IR (ῡ/cm-1): 3291, 3076, 3002, 2935, 2905, 2834, 1638, 1594, 1507, 1452, 1419, 1374, 1334, 1257, 1214, 1138, 1023, 995, 914, 851, 802, 750, 639, 549, 457. 1H NMR (300 MHz, CDCl3) δ: 2.49 (t, 1H, J = 2.4 Hz), 3.35 (d, 2H, J = 6.7 Hz), 3.86 (s, 3H), 4.73 (d, 2H, J = 2.4 Hz), 5.06–5.12 (m, 2H), 5.96 (ddt, 1H, J = 16.8 Hz, J = 10.2 Hz and J = 6.7 Hz), 6.72–6.74 (m, 2H), 7.00 (d, 1H, J = 8.6 Hz); 13C NMR (75 MHz, CDCl3) δ: 39.8, 55.8, 56.9, 75.5, 78.7, 112.3, 114.6, 115.7, 120.3, 134.2, 137.4, 145.0, 149.6.
1.2.2. General procedure for the synthesis of triazolic derivatives of EUG 2a–2i
To a round-bottom flask, azide of interest (1.0 equivalent), alkyne 1 (1.0 equivalent), sodium ascorbate (0.4 equivalent), distilled water (2.0 ml) and ethanol (2.0 ml) were added. Subsequently, CuSO4·5H2O (0.2 equivalent) was added. The reaction mixture was vigorously stirred for 24/48 h at room temperature. The reaction was monitored by TLC analysis and upon completion, it was quenched by the addition of a saturated solution of Na2CO3, followed by the extraction of the product with dichloromethane (3 × 20.0 ml). The combined organic extract was dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure. The resulting material was purified by silica gel column chromatography eluting with a hexane-ethyl acetate mixture (4:1 or 3:2 v v-1). The structures of compounds 2a–2i are supported by the following data.
1.2.2.1. 4-((4-allyl-2-methoxyphenoxy)methyl)-1-(4-bromophenyl)-1H-1,2,3-triazole (2a)
Brown solid obtained with a yield of 80% (0.157 g, 0.395 mmol), m.p. 124.3–124.8°C, TLC: Rf = 0.30 (hexane-ethyl acetate 4:1 v/v). IR (ῡ/cm-1): 3128, 3094, 2957, 2924, 2871, 1638, 1590, 1510, 1493, 1462, 1259, 1230, 1138, 1068, 1034, 1025, 987, 926, 824, 804, 749, 650, 605, 517, 453. 1H NMR (600 MHz, DMSO-d6) δ: 3.29 (d, 2H, J = 6.6 Hz), 3.73 (s, 3H), 5.08–5.01 (m, 2H), 5.16 (s, 2H), 5.96 (ddt, 1H, J = 16.8 Hz, J = 9.6 Hz and J = 6.6 Hz), 6.70–6.69 (m, 1H), 6.81–6.80 (m, 1H), 7.07 (d, 1H, J = 7.8 Hz), 7.80 (d, 2H, J = 9.0 Hz), 7.90 (d, 2H, J = 9.0 Hz), 8.93 (s, 1H). 13C NMR (150 MHz, DMSO-d6) δ: 39.5, 55.8, 62.2, 113.0, 114.6, 116.0, 120.6, 121.8, 122.4, 123.3, 133.2, 133.6, 136.2, 138.2, 144.6, 146.1, 149.5. LC-MS (ESI) m/z: calculated for C19H18BrN3O2Na [M+Na]+: 422.05; found: 422.07.
1.2.2.2. 4-((4-allyl-2-methoxyphenoxy)methyl)-1-(2-bromophenyl)-1H-1,2,3-triazole (2b)
White solid, obtained with a yield of 73.0% (0.143 g, 0.360 mmol), m.p. 64.6–65.4°C, TLC: Rf = 0.72 (hexane-ethyl acetate 3:2 v/v). IR (ῡ/cm-1): 3135, 3072, 2935, 2868, 2838, 1634, 1588, 1508, 1456, 1378, 1256, 1222, 1139, 1021, 1009, 995, 913, 845, 802, 753, 640, 605, 553, 450. 1H NMR (600 MHz, DMSO-d6) δ: 3.30 (d, 2H, J = 6.6 Hz), 3.74 (s, 3H), 5.02–5.09 (m, 2H), 5.17 (s, 2H), 5.96 (ddt, 1H, J = 16.8 Hz, J = 10.2 Hz and J = 6.6 Hz), 6.71 (dd, 1H, J = 1.8 Hz, J = 8.4 Hz), 6.81–6.82 (m, 1H), 7.08 (d, 1H, J = 8.4 Hz), 7.56 (td, 1H, J = 1.8 Hz and J = 7.8 Hz), 7.63 (td, 1H, J = 1.2 and J = 7.8 Hz), 7.65 (dd, 1H, J = 1.8 and J = 7.8 Hz), 7.91 (d, 1H, J = 9.0 Hz), 8.62 (s, 1H). 13C NMR (150 MHz, DMSO-d6) δ: 39.5, 55.8, 62.2, 113.0, 114.9, 115.9, 119.2, 120.6, 127.2, 129.1, 129.3, 132.4, 133.6, 134.0, 136.5, 138.3, 143.3, 146.1, 149.6. LC-MS (ESI) m/z: calculated for C19H18BrN3O2Na [M+Na]+: 422.05; found: 422.05.
1.2.2.3. 4-((4-allyl-2-methoxyphenoxy)methyl)-1-(3-bromophenyl)-1H-1,2,3-triazole (2c)
Brown solid, obtained with a yield of 70.0% (0.138 g, 0.346 mmol), m.p. 102.2–102.6°C, TLC: Rf = 0.50 (hexane-ethyl acetate 4:1 v/v). IR (ῡ/cm-1): 3139, 3076, 2991, 2916, 2849, 1679, 1634, 1588, 1513, 1493, 1460, 1423, 1337, 1258, 1235, 1211, 1136, 1046, 1027, 992, 911, 865, 780, 675, 646, 598, 542, 438. 1H NMR (600 MHz, DMSO-d6) δ: 3.28 (d, 2H, J = 6.6 Hz), 3.71 (s, 3H), 5.99–5.06 (m, 2H), 5.14 (s, 2H), 5.88–5.95 (m, 1H), 6.68 (d, 1H, J = 8.4 Hz), 6.78 (s, 1H), 7.04 (d, 1H, J = 8.4 Hz), 7.54 (t, 1H, J = 8.1 Hz), 7.68 (d, 1H, J = 8.4 Hz), 7.94 (d, 1H, J = 8.4 Hz), 8.15 (s, 1H), 8.96 (s, 1H). 13C NMR (150 MHz, DMSO-d6) δ: 39.5, 55.8, 62.2, 113.0, 114.7, 116.0, 119.5, 120.6, 122.8, 123.1, 123.5, 131.9, 132.3, 133.6, 138.1, 138.3, 144.6, 146.1, 149.6. LC-MS (ESI) m/z: calculated for C19H18BrN3O2Na [M+Na]+: 422.05; found: 422.13.
1.2.2.4. 4-((4-allyl-2-methoxyphenoxy)methyl)-1-(4-chlorophenyl)-1H-1,2,3-triazole (2d)
White solid, obtained with a yield of 83.0% (0.145 g, 0.410 mmol), m.p. 124.8–125.3°C, TLC: Rf = 0.48 (hexane-ethyl acetate 4:1 v/v). IR (ῡ/cm-1): 3132, 3098, 2998, 2924, 2875, 2834, 1638, 1590, 1510, 1497, 1463, 1404, 1337, 1260, 1231, 1218, 1140, 1090, 1036, 1026, 992, 926, 826, 751, 648, 605, 520, 470. 1H NMR (600 MHz, DMSO-d6) δ: 3.29 (d, 2H, J = 6.6 Hz), 3.73 (s, 3H), 5.01–5.08 (m, 2H), 5.16 (s, 2H), 5.96 (ddt, 1H, J = 16.8 Hz, J = 10.2 Hz and J = 6.6 Hz), 6.69–6.70 (m, 1H), 6.80–6.81 (m, 1H), 7.07 (d, 1H, J = 7.8 Hz), 7.67 (d, 2H, J = 9.0 Hz), 7.95 (d, 2H, J = 9.0 Hz), 8.94 (s, 1H). 13C NMR (150 MHz, DMSO-d6) δ: 39.5, 55.8, 62.2, 113.0, 114.7, 116.0, 120.6, 122.2, 123.4, 130.3, 133.4, 133.6, 135.8, 138.3, 144.6, 146.1, 149.5. LC-MS (ESI) m/z: calculated for C19H18ClN3O2Na [M+Na]+: 378.10; found: 378.09.
1.2.2.5. 4-((4-allyl-2-methoxyphenoxy)methyl)-1-(3-chlorophenyl)-1H-1,2,3-triazole (2e)
White solid, obtained with a yield of 76.0% (0.133 g, 0.375 mmol), m.p. 104.2–104.8°C, TLC: Rf = 0.46 (hexane-ethyl acetate 4:1 v/v). IR (ῡ/cm-1): 3143, 3076, 2991, 2912, 2831, 1638, 1594, 1514, 1496, 1460, 1423, 1259, 1235, 1211, 1136, 1030, 993, 911, 866, 781, 753, 781, 676, 646, 598, 546, 442; 1H NMR (600 MHz, DMSO-d6) δ: 3.30 (d, 2H, J = 7.2 Hz), 3.73 (s, 3H), 5.01–5.08 (m, 2H), 5.16 (s, 2H), 5.96 (ddt, 1H, J = 16.8 Hz, J = 9.6 Hz and J = 6.6 Hz), 6.70 (dd, 1H, J = 2.4 Hz and J = 8.4 Hz), 6.80–6.81 (m, 1H), 7.06 (d, 1H, J = 8.4 Hz), 7.56–7.57 (m, 1H), 7.63 (t, 1H, J = 8.1 Hz), 7.93–7.94 (m, 1H), 8.05–8.06 (m, 1H), 8.99 (s, 1H). 13C NMR (150 MHz, DMSO-d6) δ: 41.7, 58.0, 64.4, 115.2, 116.9, 118.1, 121.3, 122.5, 122.7, 125.7, 131.1, 134.2, 135.8, 136.8, 140.2, 140.4, 146.8, 148.2, 151.7. LC-MS (ESI) m/z: calculated for C19H18ClN3O2Na [M+Na]+: 378.10; found: 378.16.
1.2.2.6. 4-((4-allyl-2-methoxyphenoxy)methyl)-1-(2-chlorophenyl)-1H-1,2,3-triazole (2f)
White solid, obtained with a yield of 68.0% (0.120 g, 0.336 mmol), m.p. 56.1–56.8°C, TLC: Rf = 0.54 (hexane-ethyl acetate 3:2 v/v). IR (ῡ/cm-1): 3143, 3076, 3002, 2935, 2871, 2834, 1716, 1638, 1590, 1508, 1496, 1459, 1419, 1257, 1224, 1138, 1035, 1017, 911, 847, 804, 756, 647, 602, 546, 459. 1H NMR (400 MHz, CDCl3) δ: 3.34 (d, 2H, J = 6.4 Hz), 3.86 (s, 3H), 5.06–5.10 (m, 2H), 5.38 (s, 2H), 5.90–6.00 (m, 1H), 6.71–6.73 (m, 2H), 7.01 (d, 1H, J = 8.0 Hz), 7.44–7.46 (m, 2H), 7.56–7.62 (m, 2H), 8.08 (s, 1H). 13C NMR (100 MHz, CDCl3) δ: 40.0, 56.0, 63.5, 112.6, 115.2, 115.9, 120.7, 125.3, 128.0, 128.1, 131.0, 131.0, 134.3, 137.7, 144.4, 146.0, 149.9. LC-MS (ESI) m/z: calculated for C19H18ClN3O2Na [M+Na]+: 378.10; found: 378.10.
1.2.2.7. 4-((4-allyl-2-methoxyphenoxy)methyl)-1-phenyl-1H-1,2,3-triazole (2g)
White solid, obtained with a yield a yield of 62% (0.098 g, 0.305 mmol), m.p. 78.3–78.9°C, TLC: Rf = 0.32 (hexane-ethyl acetate 4:1 v/v). IR (ῡ/cm-1): 3135, 3080, 2994, 2935, 2905, 2827, 1638, 1592, 1501, 1463, 1423, 1385, 1330, 1258, 1222, 1139, 1033, 1001, 983, 913, 834, 762, 689, 650, 598, 518, 464. 1H NMR (300 MHz, CDCl3) δ: 3.33 (d, 2H, J = 6.9 Hz), 3.86 (s, 3H), 5.04–5.10 (m, 2H), 5.35 (s, 2H), 5.98 (ddt, 1H, J = 16.8 Hz, J = 10.2 Hz and J = 6.9 Hz), 6.70–6.73 (m, 2H), 7.00 (d, 1H, J = 7.8 Hz), 7.40–7.54 (m, 3H), 7.71 (d, 2H, J = 8.1 Hz), 8.07 (s, 1H). 13C NMR (75 MHz, CDCl3) δ: 39.8, 55.8, 63.3, 112.3, 114.4, 115.7, 120.5, 120.5, 121.0, 128.8, 129.7, 133.9, 136.9, 137.4, 145.2, 145.8, 149.5. LC-MS (ESI) m/z: calculated for C19H18N3O2Na [M+Na]+: 344.14; found: 344.22.
1.2.2.8. 4-((4-allyl-2-methoxyphenoxy)methyl)-1-(4-fluorophenyl)-1H-1,2,3-triazole (2h)
White solid, obtained with a yield of 69% (0.073 g, 0.215 mmol), m.p. 80.4–81.2°C, TLC: Rf = 0.46 (hexane-ethyl acetate 4:1 v/v). IR (ῡ/cm-1): 3124, 3065, 3005, 2935, 2879, 2831, 1638, 1592, 1510, 1463, 1420, 1378, 1222, 1138, 1051, 1031, 1003, 995, 911, 836, 799, 761, 698, 646, 602, 518, 479. 1H NMR (600 MHz, DMSO-d6) δ: 3.30 (d, 2H, J = 7.2 Hz), 3.73 (s, 3H), 5.01–5.08 (m, 2H), 5.16 (s, 2H), 5.96 (ddt, 1H, J = 16.8 Hz, J = 10.2 Hz and J = 7.2 Hz), 6.70 (dd, 1H, J = 1.8 and J = 7.8 Hz), 6.80–6.81 (m, 1H), 7.07 (d, 1H, J = 8.4 Hz), 7.46 (t, 2H, J = 8.7 Hz), 7.93 (dd, 2H, J = 4.8 and J = 9.0 Hz), 8.89 (s, 1H). 13C NMR (150 MHz, DMSO-d6) δ: 39.5, 55.8, 62.2, 113.0, 114.6, 116.0, 117,1 (d, J = 9.0 Hz), 120.6, 122.9 (d, J = 9.0 Hz), 123.5, 133.5, 133.6, 138.3, 144.5, 146.1, 149.5, 162.1 (d, J = 244.5 Hz). LC-MS (ESI) m/z: calculated for C19H18FN3O2Na [M+Na]+: 362.13; found: 362.15.
1.2.2.9. 4-((4-allyl-2-methoxyphenoxy)methyl)-1-(2-fluorophenyl)-1H-1,2,3-triazole (2i)
White solid, obtained with a yield of 77% (0.193 g, 0.568 mmol), TLC: Rf = 0.22 (hexane-ethyl acetate 4:1 v/v). IR (ῡ/cm-1): 3143, 3083, 2998, 2935, 2834, 1638, 1588, 1508, 1463, 1404, 1334, 1258, 1228, 1136, 1108, 1050, 1035, 1018, 991, 906, 851, 806, 704, 646, 553, 475. 1H NMR (600 MHz, DMSO-d6) δ: 3.30 (d, 2H, J = 6.6 Hz), 3.73 (s, 3H), 5.02–5.09 (m, 2H), 5.17 (s, 2H), 5. 96 (ddt, 1H, J = 16.8 Hz, J = 10.2 Hz and J = 6.6 Hz), 6.71 (dd, 1H, J = 1.8 Hz and J = 8.4 Hz), 6.80–6.81 (m, 1H), 7.09 (d, 1H, J = 8.4 Hz), 7.43–7.46 (m, 1H), 7.59–7.64 (m, 2H), 7.83–7.86 (m, 1H), 8.69–8.70 (m, 1H). 13C NMR (150 MHz, DMSO-d6) δ: 39.5, 55.8, 62.0, 113.0, 114.6, 116.0, 117.5 (d, J = 19.5 Hz), 120.6, 125.1 (d, J = 10.5 Hz), 126.0 (d, J = 3.0 Hz), 126.4, 126.6 (d, J = 4.5 Hz), 131.7 (d, J = 9.0 Hz), 133.5, 138.3, 143.9, 146.1, 149.5, 154.3 (d, J = 249.0 Hz). LC-MS (ESI) m/z: calculated for C19H18FN3O2Na [M+Na]+: 362.13; found: 362.16.
1.3. Antifungal activity
1.3.1. Determination of MIC
Strains of Aspergillus flavus ATCC 204304, A. fumigatus ATCC 16913, Trichophyton rubrum CCT 5506 and Candida albicans ATCC 10231 were employed for this study. The susceptibility test followed the broth microdilution method as outlined in reference documents M27-A3 and M38-A [30,31]. The standards ITZ, FCZ and EUG, along with the compounds 2a–2i, were dissolved in DMSO and subsequently serially diluted in RPMI-1640 broth at concentrations of 0.03–256 μg ml-1. The final concentration of DMSO did not exceed 1%. The inoculum was prepared using the spectrophotometric method (OD 0.09–0.13 at 530 nm) to obtain final concentrations between 0.4 and 5 × 104 CFU ml-1. Microplates were incubated in a microbiological oven at 35°C for 24 h for C. albicans, 48 h for Aspergillus and 96 h for T. rubrum. The MIC was identified as the lowest concentration where no growth was observed when compared with the control. To validate the method, a reference strain Candida parapsilosis ATCC 22019 was utilized following the quality standards outlined in the CLSI document. All experiments were performed in triplicates.
1.3.2. Determination of minimum fungicidal concentration
The minimum fungicidal concentration determination followed adaptations to the protocol outlined by Espinel-Ingroff et al. [32]. In brief, 20 μl from the wells corresponding to the MIC, 2×MIC and 4×MIC values were applied to the culture plate containing sabourad dextrose agar. The plate was then incubated at 35°C for 24 h for Candida, 48 h for Aspergillus and 96 h for T. rubrum. Minimal fungicide concentration (MFC) was defined as the lowest concentration at which no visual colony growth was observed [29]. All experiments were conducted in triplicates.
1.3.3. Time-kill curve studies
The test was conducted according to the adaptations proposed by Ghannoum et al. [33] based on the method established by Klepser et al. [34]. In summary, the suspension of the test microorganism (Trychophyton rubrum) was adjusted within the range specified for dermatophytes in the CLSI protocol [31] to achieve a final inoculum concentration corresponding to 1–3 × 103 CFU ml-1 in RPMI broth. This was done in conjunction with the test concentrations (MIC, 2 × MIC and 4 × MIC) of the antifungals FCZ, EUG and compounds 2a and 2i. A positive control without the presence of a drug was also prepared. Then, at times 0, 6, 12, 24, 36 and 48 h, an aliquot of 100 μl was removed from the test concentrations and subsequently diluted in 900 μl of saline solution, following a 1:10 ratio. Afterward, 30 μl of this suspension were seeded on PDA plates (potato dextrose agar) and incubated for 96 h at 30°C. All experiments were conducted in triplicate.
1.3.4. Cell viability assays
The cytotoxic activity was assessed using the MTT (3-(4,5-dimethylthiazol-2-yl)-diphenyltetrazolium bromide) colorimetric assay, as proposed by Mosmann in 1989 [35]. Briefly, L929-fibroblasts (ATCC® CCL-1™) and Hepa1c1c7-hepatocytes (ATTC® CRL-2026™) were added to 96-well microplates at a final concentration of 7 × 104 cells ml-1. After incubation for 18 h, the cells were exposed to varying concentrations of EUG (1–200 μg ml-1), determined based on the MIC results obtained from the broth microdilution assay and then incubated for 24 h. Following incubation, 100 μl of MTT (1 mg ml-1) was added to each well and incubated for an additional 2 h. Subsequently, 100 μl of DMSO was added to dissolve the formazan crystals. ITZ and EUG were used as positive controls. The absorbance of purple formazan, which is proportional to the number of viable cells, was measured at 595 nm using a microplate reader (Molecular Devices, Spectra Max 190, CA, USA). The experiments were performed in triplicate.
1.4. Computational studies
Docking studies were carried out using Gold Suite 5.1 software (Genetic Optimization for Ligand Docking 5.1, CCDC Software Ltd). A genetic algorithm was applied to the crystallographic structures of lanosterol 14α-demethylase wild-type, with PDB code 4WMZ, at a resolution of 2.05 Å and the G73E mutant, with PDB code 5ESG at a resolution of 1.98 Å, obtained from Saccharomyces cerevisiae. The binding mode of compounds 2a and 2i, reveals that these EUG triazole derivatives exhibit complementarity within the heme cavity, similar to ITZ, with a score value of 45.15 (PDB code: 5ESG) and FCZ, with a score of 49.73 (PDB code: 4WMZ). GOLD was utilized with a Goldscore function for 4WMZ and ChemScore for the 5ESG fitness function. Goldscore considers factors such as H-bonding energy, van der Waals energy, metal interaction and ligand torsion strain, while ChemScore is an empirical scoring function that estimates the binding affinity of a complex by summing up important energetic factors for protein-ligand binding, including hydrogen bonds, hydrophobic effects and steric clashes. Docking simulations were performed within a 5 Å radius sphere for 4WMZ and a 10 Å radius sphere for 5ESG, centered at the ligand in chain A, using the available pattern parameters. These parameters included a population of 100 conformers, 100,000 operations, 95 mutations and 95 crossovers. Water molecules W743 and W790 were retained in docking simulations for 4WMZ. The docked lowest-energy structure exhibited a root mean square deviation of 1.583 Å, based on the ligand in chain A in the ITZ/lanosterol 14α-demethylase G73E mutant complex and 1.609 Å, for the complex between the ligand in chain A in the FCZ/lanosterol 14α-demethylase wild-type (Figure 1).
Figure 1.

The overlap between the docked lowest-energy structure for (A) FCZ (blue) and (B) ITZ (yellow) and ligand in chain (A) for the lanosterol/14α-demethylase wild-type and G73E mutant complexes, respectively.
FCZ: Fluconazole; ITZ: Itraconazole.
1.5. Statistical analysis
The results were analyzed by analysis of variance, followed by the Tukey test to determine a significant difference between the means (p < 0.05), using the Biostat 5.0 software.
2. Results
2.1. Synthesis of EUG derivatives with 1,2,3-triazole fragments
The derivatives of EUG containing the 1,2,3-triazole fragments were prepared in two steps according to the synthetic route presented in Figure 2. The terminal alkyne (1) was obtained in 81% yield from EUG, while the triazole derivatives 2a–2i were obtained within 62%–83% yield.
Figure 2.
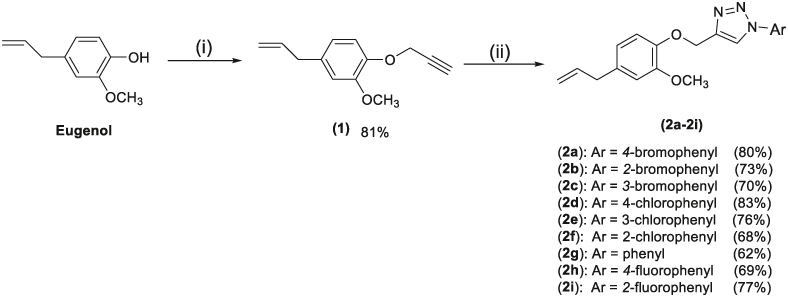
Reactions involved in the preparation of triazolic compounds derived from EUG. Reagents and conditions: (i) acetonitrile, NaOH, CH3OH, 3-bromoprop-1-yne, 40°C, r.t.; (ii) ArN3, sodium ascorbate (40 mol%), CuSO4·5H2O (20 mol%), EtOH/H2O (1:1 v/v).
EUG: Eugenol.
The compounds 2a–2i were fully characterized by spectroscopic methods including NMR (1H and 13C), IR and MS. 1H and 13C NMR, IR and LC-MS/MS spectra (Supplementary Figures S1–S48) for compounds 2a–2i can be found in the Supplementary Material.
2.2. Antifungal activity
Once synthesized, the antifungal activity of EUG-containing 1,2,3-triazol derivatives 2a–2i was assessed against strains of C. albicans, A. fumigatus, A. flavus and T. rubrum, with the results presented in Table 1. The compounds presented different degrees of efficiency on the strains. Notably, EUG derivatives 2a and 2i demonstrated significant activity against the dermatophyte T. rubrum, with MICs of 16 and 32 μg ml-1, respectively. The MIC values were higher than those determined for the positive controls ITZ and FCZ. The efficiency of the compounds was also compared with EUG.
Table 1.
Antifungal activity of triazoles derived from EUG (2a–2i) against A. fumigatus, A. flavus, T. rubrum and C. albicans.
| Compound | A. fumigatus | A. flavus | T. rubrum | C. albicans | ||||
|---|---|---|---|---|---|---|---|---|
| MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | |
| EUG | 256 | – | 512 | – | 64 | 64 | 128 | 256 |
| 2a | 128 | – | 128 | – | 16 | 32 | 128 | – |
| 2b | 128 | – | 128 | – | 128 | – | 100 | – |
| 2c | 128 | – | 128 | – | 128 | – | 100 | – |
| 2d | 128 | – | 128 | – | 128 | – | 64 | – |
| 2e | 128 | – | 128 | – | 64 | 128 | 64 | – |
| 2f | 128 | – | 128 | – | 64 | 128 | 64 | – |
| 2g | 128 | – | 128 | – | 64 | 128 | 64 | – |
| 2h | 128 | – | 128 | – | 64 | 128 | 64 | – |
| 2i | 128 | – | 128 | – | 32 | 128 | 64 | – |
| ITZ | 0.5 | 1 | 0.5 | 0.5 | 0.03 | 0.03 | 0.25 | 0.5 |
| FCZ | >128 | – | >128 | – | 4 | 8 | 0.5 | 4 |
Results expressed in μg ml-1; (-) no activity.
EUG: Eugenol; FCZ: Fluconazole; ITZ: Itraconazole; MFC: Minimum fungicidal concentration; MIC: Minimum inhibitory concentration.
Figure 3 illustrates the time-kill study curves, showcasing the fungicidal effect of compounds 2a and 2i from the 1 × MIC concentration, thereby confirming the results presented in Table 1. The time-kill curves of compounds 2a, 2i, ITZ, FCZ and EUG express this time-dose-dependent effect, as depicted in Figure 3.
Figure 3.

Time-kill curve studies of compounds 2a and 2i, EUG, ITZ and FCZ against T. rubrum.
EUG: Eugenol; FCZ: Fluconazole; ITZ: Itraconazole.
2.3. Cell viability evaluation
The compounds 2a–2i had also their effects evaluated against hepatocytes (H1C1C7) and fibroblasts (L929) in order to assess the selectivity of EUG derivatives and the results are depicted in Table 2. In vitro cell viability assays serve as a screening method to assess the selectivity of a compound, providing insights into the safety profile of new drugs [51]. Given that compounds bearing triazole moieties can exhibit cytotoxicity, particularly in liver cells and considering the potential topical application of these compounds for controlling dermatophytosis caused by T. rubrum [52], the in vitro cell viability of compounds 2a and 2i was evaluated using the cytotoxicity test (MTT) on hepatocyte (H1C1C7) and fibroblast (L929) cells, as detailed in Table 2.
Table 2.
Cell viability test of hepatocytes (H1C1C7) and fibroblasts (L929) of compounds 2a and 2i.
| Cell viability IC50 (μg ml-1) | ||
|---|---|---|
| Compound | Hepatocyte (IC50 μg ml-1) | Fibroblast (IC50 μg ml-1) |
| 2a | 125 ± 1.0a | 55.5 ± 4.4a,b |
| 2i | 42.8 ± 3.8d | 48.9 ± 3.8a |
| EUG | 109 ± 1.7b | 160 ± 6.1 |
| ITZ | 95.9 ± 1.0c | 83.7 ± 1.9b |
Different letters between columns represent significant differences (p < 0.05). Cell viability was determined by the MTT assay after 96 h of treatment for hepatocyte (h1c1c7) and fibroblast (L929) cell lines. Values are expressed as mean ± SD of each compound from three independent experiments.
EUG: Eugenol; ITZ: Itraconazole.
The IC50 value of compound 2a was significantly higher than that of the controls EUG and ITZ on hepatocytes, indicating lower cellular toxicity. Regarding fibroblasts, no difference was found between compound 2a and ITZ (Table 2). On the other hand, compound 2i significantly presented the lowest IC50 values among the compounds evaluated for both the hepatocytes and fibroblasts.
2.4. Docking simulations
The binding mode results for EUG-triazole derivatives 2a and 2i with lanosterol 14α-demethylase wild-type are depicted, respectively, in Figures 4 & 5 and indicate that both compounds are located within the heme cavity of this protein.
Figure 4.

Binding mode prediction for compound 2a (pink) with lanosterol 14α-demethylase wild-type.
Figure 5.
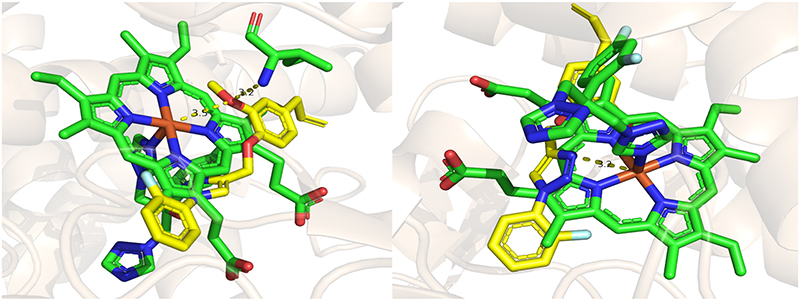
Binding mode prediction for compound 2i (yellow) with lanosterol 14α-demethylase wild-type.
Figure 6 shows the results of docking studies for the triazole derivatives 2a and 2i against lanosterol 14α-demethylase G73E mutant. A complementarity with the heme group similar to ITZ is observed (Figure 6).
Figure 6.

(A) Overlap between ITZ (green) and triazole EUG derivatives 2a (pink) and 2i (yellow). (B) Binding mode prediction for 2a against lanosterol 14α-demethylase G73E mutant. (C) Binding mode prediction for 2i against the same enzyme.
EUG: Eugenol; ITZ: Itraconazole.
2.5. Pharmacokinetic prediction
The prediction of pharmacokinetic parameters of the EUG derivatives 2a and 2i (Supplementary Figures S49 & S50 in the Supplementary material) revealed that these derivatives demonstrate good gastrointestinal absorption.
3. Discussion
Over the past three decades, the incidence of fungal infections has risen sharply, primarily due to the increasing number of immunocompromised individuals. Despite the existence of over 6 million fungal species worldwide, only a small percentage can cause severe diseases in humans. The limited therapeutic options for treating fungal infections, especially in comparison to bacterial infections, contribute to high mortality rates. Therefore, it is crucial to research and develop new antifungal agents to more effectively manage these infections and improve patient outcomes.
Herein, we investigated the antifungal activity of a series of EUG-containing 1,2,3-triazole derivatives against four human pathogenic fungal species. Our investigation began with the preparation of the 1,2,3-triazole derivatives, which was achieved in just two steps (Figure 2). The first step involved the alkylation reaction between EUG and propargyl bromide in the presence of sodium hydroxide, resulting in compound 1. In the second step, nine derivatives (2a–2i) were prepared with synthetically useful yields through the copper-catalyzed azide-alkyne cycloaddition reaction, also known as the ‘click’ reaction [36–38].
Regarding the characterization of the compounds, the IR spectra revealed a band assigned to the stretching of the N=N bond, characteristic of structures containing the 1,2,3-triazole fragment, observed between 1588 and 1493 cm-1. In the 1H NMR spectra, the signal attributed to the hydrogen present in the triazole moiety was observed as a singlet in the range of 8.07–8.99 ppm. The signal corresponding to the methylene hydrogens linked to oxygen and the triazole moiety was also observed as a singlet in the range of 5.14–5.38 ppm. In the 13C NMR spectra, the chemical shift values and the number of signals were consistent with the structures of the substances, with signals corresponding to the carbons of the triazole ring observed between 120.6 and 146.8 ppm. The molecular formulas of all triazoles were confirmed by their mass spectra. Notably, the synthesis of the compounds was planned to evaluate the effect of different aromatic groups linked to the triazole portion (Figure 2).
For antifungal therapy, the process of new drug discovery involves in silico and in vitro investigations to provide a robust experimental direction when screening promising compounds [39]. The sensitivity test is the gold standard for evaluating the antifungal potency of a new drug candidate [40,41]. Considering these factors, the compounds were evaluated against strains of the fungi species A. fumigatus, A. flavus, T. rubrum and C. albicans. The more active compounds corresponded to 2a (MIC = 16 μg ml-1) and 2i (MIC = 32 μg ml-1) against the dermatophyte T. rubrum. These data are promising compared with the antifungal effect of EUG against the same strain (Table 1), suggesting that the compounds herein investigated may have pharmacological applications or serve as a basis for future studies. Similar findings were reported by Pinto et al. [42], demonstrating up to 2× optimization of novel EUG derivatives against Trychophyton spp. However, it is important to highlight that based on the MIC values found in this study, compound 2a is four-times more potent in comparison with EUG.
Although the MIC values of derivatives 2a and 2i are higher than those of the antifungals ITZ and FCZ (Table 1), it is important to note that the maximum MIC range of these antifungals can reach up to 64 μg ml-1 [43,44], compared with different isolates of T. rubrum. Therefore, 2a and 2i may hold promise for antifungal therapy against dermatophytosis [45]. Furthermore, it is essential to highlight that 2a has a bromine atom in the para position and derivative 2i has a fluorine atom in the ortho position of the phenyl ring (Figure 1). It was also observed that the other brominated derivatives (2b ortho and 2c meta) and fluorinated (2h para), along with the three chlorinated derivatives (2d, 2e and 2f), did not exhibit significant fungal inhibition against any of the fungi tested. According to Jeschke [46], the use of compounds bearing fluorine atoms in their structures in biological assays, particularly in the para position in benzene rings, has become a common practice aiming to increase the stability of the substance, leading to improved activity.
The findings from the time-kill investigation (Figure 3) indicate that compounds 2a and 2i exhibit a dose-dependent fungicidal effect akin to ITZ, FCZ and EUG. This observation aligns with the studies conducted by Pannu et al. [47] and Saracino et al. [48]. The time-kill study provides a robust assessment of the interaction between antifungals and fungi poststatic MIC determinations. This assay holds clinical significance in guiding the management of fungal infections during treatment, as a fungicidal effect is highly desirable for effective antifungal therapy [49]. Generally, azoles demonstrate a time-dose-dependent fungicidal effect, due to their ability to block the ergosterol biosynthetic pathway, causing distortions in the fungal cell over time [50].
In vitro cell viability assays serve as a screening method to assess the selectivity of a compound, providing insights into the safety profile of new drugs [51]. Given that compounds bearing triazole moieties can exhibit cytotoxicity, particularly in liver cells and considering the potential topical application of these compounds for controlling dermatophytosis caused by T. rubrum [52], the in vitro cell viability of compounds 2a and 2i was evaluated using the cytotoxicity test (MTT) on hepatocyte (H1C1C7) and fibroblast (L929) cells (Table 2). It is essential to note that the cell viability test serves as a preliminary cytotoxicity assessment and is not conclusive. Additional in vivo tests are necessary to fully understand the structure-activity relationship in this class of compounds. However, it is important to emphasize that the MIC values found for T. rubrum for compounds 2a and 2i were, respectively, 16 μg ml-1 and 32 μg ml-1, which are below the IC50 found in cell viability tests, both for hepatocytes and fibroblasts (Table 2). This suggests that the compounds can be administered both orally or topically, as MIC values are commonly found in cells or tissues where they are metabolized [53]. As previously highlighted, the use of halogen elements, mainly in the para position in the benzene ring, has become a common practice in synthesizing new compounds to enhance substance stability and improved activity, as seen in the case of compound 2a, which features a Br in the para position of the phenyl ring [46].
Azole antifungal agents, such as ITZ, have been extensively employed in the treatment of dermatophytosis. However, the rising resistance of dermatophytes to antifungal drugs has become a concern [45]. Beyond resistance, these drugs may induce side effects, including damage to liver function and damage to epidermal tissues, as illustrated by examples such as ketoconazole [54]. Therefore, it is crucial to underscore the need for further investigations into the in vivo cytotoxicity of compounds 2a and 2i, along with the assessments of pharmacokinetic and pharmacodynamic interactions.
Molecular docking investigations with lanosterol 14α-demethylase wild-type and compounds 2a and 2i revealed that the binding mode prediction for triazole EUG derivative 2a (Figure 4) suggests a distinctive bond to the heme iron through the oxygen atom in the methoxyl group (2.9 Å). Additionally, there is a possibility of performing a hydrogen-bonding interaction with the residue Thr318 (3.9 Å) within the hydrogen bond network, as highlighted in Figure 4. The derivative 2i demonstrated the capability to engage in a highly polarized interaction with the heme iron through the oxygen atom of the methoxyl group (3.5 Å) and the free nitrogen atom (3.2 Å) located on the azole ring (Figure 5), similar to FCZ. This binding is crucial for the inhibitory activity of this protein as it prevents oxygen activation, thereby inhibiting the demethylation of lanosterol and consequently disrupting the process of ergosterol biosynthesis. Additionally, the oxygen atom in the methoxyl group (3.2 Å) carried out a hydrogen bond interaction with the amino acid residue Ile471 and the free nitrogen atom (3.2 Å) on the triazole ring may potentially participate in an interaction with the residue Leu380 (3.9 Å) within the hydrogen bond network, as illustrated in Figure 5.
In terms of the docking studies for the triazole derivatives 2a and 2i against G73E mutant, both compounds can establish a crucial hydrogen bond interaction with the amino acid residue Tyr140, which is intrinsically associated with inhibitory activity. This interaction is facilitated through the oxygen atom of the methoxyl group, with distances of 3.3 Å for 2a and 2.6 Å for 2i.
By utilizing the SwissADME software, a free web tool designed to predict absorption, distribution, metabolism and excretion parameters, pharmacokinetic properties and drug-like nature, a pharmacokinetic prediction study involving compounds 2a and 2b was carried out. Absorption, distribution, metabolism and excretion are pivotal properties that determine drug potency and are intrinsically linked to its physicochemical profile [55]. Subsequently, utilizing topological polar surface area and Water Partition Coefficient parameters, along with physicochemical data, the BOILED-Egg statistical graph was generated to predict passive tract gastrointestinal absorption of derivatives 2a and 2i (Supplementary Figure S49 in Supplementary material). These parameters are represented as ellipses. Consequently, these derivatives demonstrate good gastrointestinal absorption (white region) and are capable of crossing the blood-brain barrier (indicated by the yellow ellipse). These findings position 2a and 2i as bioavailable and promising candidates for oral administration [56]. These observations align with the IC50 values obtained from the cell viability test, which indicate the non-toxicity of compound 2a in hepatocytes, where drug metabolism typically occurs following oral administration.
Furthermore, bioavailability radar provides valuable insights into the drug properties of novel compounds. Here, we consider six pharmacokinetic parameters: lipophilicity (Xlogp3 between –0.7 and 5.00), molar mass (ranging 150–500 g mol-1), polarity (topological polar surface area between 20 and 130 Å2), insolubility (log S between 0 and 6), unsaturation (sp3 portion ranging from 0.25 to 1) and flexibility (number of flexible bonds between 0 and 9). Oral bioavailability is depicted in the colored region (Supplementary Figure S50 in Supplementary material). It is noteworthy that EUG derivatives 2a and 2i exhibit a good pharmacokinetic profile, with only a slightly higher level of unsaturation, as anticipated by the presence of aromatic rings. Finally, Lipinski's rule of five establishes that small molecules are considered drug candidates if they have a molar mass of less than 500 g mol-1, lipophilicity expressed as cLogP less than 5, hydrogen acceptor groups less than 10 and hydrogen donor groups less than 5 [57]. The EUG derivatives 2a and 2i comply with Lipinski’s rule of five.
4. Conclusion
A set of nine EUG-containing triazole derivatives has been synthesized and assessed for their antifungal activity. Among these derivatives, compound 2a demonstrated promising antifungal activity against T. rubrum, commonly associated with dermatophytosis. Additionally, it exhibited antifungal activity four-times greater than EUG itself. Importantly, it showed no toxicity to hepatocyte and fibroblast cells at concentrations effective against fungi. Moreover, computational studies indicate a robust interaction between the new compound and the target enzyme lanosterol 14α-demethylase. Furthermore, the findings described here suggest that the compounds possess favorable gastrointestinal absorption characteristics, making them potentially suitable for oral administration. Further studies are required to develop such compounds into viable drugs.
Supplementary Material
Funding Statement
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brazil - Finance Code 001), Fundação de Amparo à Pesquisa e Inovação do Espírito Santo (FAPES, Brazil – Edital 11/2019, Term of grant 532/2020; Edital 003/2021, Term of grant 472/2021; Edital 021/2022, Term of grant 1055/2022), Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG, Brazil, Grant Number APQ02957-17) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil, Grant Number 306873/2021-4).
Supplemental material
Supplemental data for this article can be accessed at https://doi.org/10.1080/17568919.2024.2385292
Financial disclosure
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brazil - Finance Code 001), Fundação de Amparo à Pesquisa e Inovação do Espírito Santo (FAPES, Brazil – Edital 11/2019, Term of grant 532/2020; Edital 003/2021, Term of grant 472/2021; Edital 021/2022, Term of grant 1055/2022), Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG, Brazil, Grant Number APQ02957-17) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil, Grant Number 306873/2021-4). The authors are supported by Research Fellowships from FAPES (VTQ) and CNPq (AVC and LCAB). The authors thank the researchers from graduate Programs in Agrochemistry (UFES) and Natural Products and Organic Synthesis Research Group (GEAPS-CNPq) for their learning support. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Competing interests disclosure
The authors have no competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Writing disclosure
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest
- 1.Wiederhold NP. Emerging fungal infections: new Species, new names and antifungal resistance. Clin Chem. 2022;68(1):83–90. doi: 10.1093/clinchem/hvab217 [DOI] [PMC free article] [PubMed] [Google Scholar]; • This review highlights emerging fungal infections, including newly described species.
- 2.Strickland AB, Shi M. Mechanisms of fungal dissemination. Cell Mol Life Sci. 2021;78(7):3219–3238. doi: 10.1007/s00018-020-03736-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scorzoni L, Silva ACAPE, Marcos CM, et al. Antifungal therapy: new advances in the understanding and treatment of mycosis. Front Microbiol. 2017;8:36. doi: 10.3389/fmicb.2017.00036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Monk BC, Sagatova AA, Hosseini P, Ruma YN, et al. Fungal lanosterol 14α-demethylase: a target for next-generation antifungal design. Biochim Biophys Acta Proteins Proteom. 2020;1868(3):140206. doi: 10.1016/j.bbapap.2019.02.008 [DOI] [PubMed] [Google Scholar]; • This article highlights the importance of the enzyme 14α-desmethylase for the design of new antifungal agents.
- 5.Allen D, Wilson D, Drew R, et al. Azole antifungals: 35 years of invasive fungal infection management. Expert Rev Anti Infect Ther. 2015;13(6):787–798. doi: 10.1586/14787210.2015.1032939 [DOI] [PubMed] [Google Scholar]
- 6.Shafiei M, Peyton L, Hashemzadeh M, et al. History of the development of antifungal azoles: a review on structures, SAR and mechanism of action. Bioorg Chem. 2020;104:104240. doi: 10.1016/j.bioorg.2020.104240 [DOI] [PubMed] [Google Scholar]; • In this review article, it is summarized the advances concerning the azole fungicides, one of the most important classes of fungicide agents.
- 7.Shaveta, Mishra S, Singh P. Hybrid molecules: the privileged scaffolds for various pharmaceuticals. Eur J Med Chem. 2016;124:500–536. doi: 10.1016/j.ejmech.2016.08.039 [DOI] [PubMed] [Google Scholar]
- 8.Caramalho R, Tyndall JDA, Monk BC, et al. Intrinsic short-tailed azole resistance in mucormycetes is due to an evolutionary conserved aminoacid substitution of the lanosterol 14α-demethylase. Sci Rep. 2017;7(1):15898. doi: 10.1038/s41598-017-16123-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sagatova AA, Keniya MV, Wilson RK, et al. Structural insights into binding of the antifungal drug fluconazole to Saccharomyces cerevisiae lanosterol 14α-demethylase. Antimicrob Agents Chemother. 2015;59(8):4982–4989. doi: 10.1128/AAC.00925-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Monk BC, Tomasiak TM, Keniya MV, et al. Architecture of a single membrane spanning cytochrome P450 suggests constraints that orient the catalytic domain relative to a bilayer. Proc Natl Acad Sci USA. 2014;111(10):3865–3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khurana A, Sardana K, Chowdhary A. Antifungal resistance in dermatophytes: recent trends and therapeutic implications. Fungal Genet Biol. 2019;132:103255. doi: 10.1016/j.fgb.2019.103255 [DOI] [PubMed] [Google Scholar]; • This review provides an account of the in vitro and in vivo resistance to clinically used antifungals among dermatophytes.
- 12.Sardana K, Kaur R, Arora P, et al. Is antifungal resistance a cause for treatment failure in dermatophytosis: a study focused on tinea corporis and cruris from a tertiary centre? Indian Dermatol Online J. 2018;9(2):90–95. doi: 10.4103/idoj.IDOJ_137_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinez-Rossi NM, Bitencourt TA, Peres NTA, et al. Dermathophyte resistance to antifungal drugs: mechanism and prospectus. Front Microbiol. 2018;9:1108. doi: 10.3389/2Ffmicb.2018.01108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwans JP, Sunden F, Lassila JK, et al. Use of anion-aromatic interactions to position the general base in the ketosteroid isomerase active site. Proc Natl Acad Sci USA. 2013;110(28):11308–11313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tyndall JDA, Sabherwal M, Sagatova AA, et al. Structural and functional elucidation of yeast lanosterol 14α-demethylase in complex with agrochemical antifungals. PLOS ONE. 2016;11(12):e0167485. doi: 10.1371/journal.pone.0167485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sagatova AA, Keniya MV, Wilson RK, Sabherwal M, Tyndall JDA, Monk BC. Triazole resistance mediated by mutations of a conserved active site tyrosine in fungal lanosterol 14α-demethylase. Sci Rep. 2016;6:26213. doi: 10.1038/srep26213 [DOI] [PMC free article] [PubMed] [Google Scholar]; • This research paper highlights how commonly occurring single-site mutations in pathogenic fungi affect triazole binding.
- 17.Barbosa LCA, Costa AV, Piló-Veloso D, et al. Phytogrowth-inhibitory lactones derivatives of glaucolide B. Z Naturforsch C J Biosci. 2004;59(11–12):803–810. doi: 10.1515/znc-2004-11-1207 [DOI] [PubMed] [Google Scholar]
- 18.Lima AMA, de Paula WT, Leite ICHL, et al. Synthesis of eugenol-fluorinated triazole derivatives and evaluation of their fungicidal activity. J Braz Chem Soc. 2022;33(10):1200–1210. doi: 10.21577/0103-5053.20220040 [DOI] [Google Scholar]
- 19.Sampaio JG, Pressete CG, Costa AV, et al. Methoxylated cinnamic esters with antiproliferative and antimetastatic effects on human lung adenocarcinoma cells. Life. 2023;13(7):1428. doi: 10.3390/life13071428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barbosa LCA, Demuner AJ, Alvarenga ES, et al. Phytogrowth- and photosynthesis-inhibiting properties of nostoclide analogues. Pest Manag Sci. 2006;62(3):214–222. doi: 10.1002/ps.1147 [DOI] [PubMed] [Google Scholar]
- 21.Heard SC, Wu G, Winter JM. Antifungal natural products. Curr Opin Biotech. 2021;69:232–241. doi: 10.1016/j.copbio.2021.02.001 [DOI] [PubMed] [Google Scholar]
- 22.Khwaza V, Aderibigbe BA. Antifungal activities of natural products and their hybrid molecules. Pharmaceuticals. 2023;15(12):2673. doi: 10.3390/pharmaceutics15122673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang CW, Zhong X-J, Zhao Y-S, et al. Antifungal natural products and their derivatives: a review of their activity and mechanism of actions. Pharmacol Res – Mod Chin Med. 2023;7(40);100262. doi: 10.1016/j.prmcm.2023.100262 [DOI] [Google Scholar]
- 24.Ulanowska M, Olas B. Biological properties and prospects for the application of eugenol – A review. Int J Mol Sci. 2021;22(7):3671. doi: 10.3390/ijms22073671 [DOI] [PMC free article] [PubMed] [Google Scholar]; • This review covers the biological properties of eugenol, including fungicide activity.
- 25.Pereira FO, Mendes JM, Lima EO. Investigation on mechanism of antifungal activity of eugenol against Trichophyton rubrum. Med Mycol. 2013;51(5):507–513. doi: 10.3109/13693786.2012.742966 [DOI] [PubMed] [Google Scholar]
- 26.Marchese A, Barbieri R, Coppo E, et al. Antimicrobial activity of eugenol and essential oils containing eugenol: a mechanistic viewpoint. Crit Rev Microbiol. 2017;43(6):668–689. doi: 10.1080/1040841X.2017.1295225 [DOI] [PubMed] [Google Scholar]
- 27.de Souza TB, Brito KMO, Silva NC, et al. New eugenol glucoside-based derivative shows fungistatic and fungicidal activity against opportunistic Candida glabrata. Chem Biol Drug Des. 2016;87(1):83–90. doi: 10.1111/cbdd.12625 [DOI] [PubMed] [Google Scholar]
- 28.Abrão PHO, Pizi RB, de Souza TB, et al. Synthesis and biological evaluation of new eugenol Mannich bases as promising antifungal agents. Chem Biol Drug Des. 2015;86(4):459–465. doi: 10.1111/cbdd.12504 [DOI] [PubMed] [Google Scholar]
- 29.Hipólito TMM, Bastos GTL, Barbosa TWL, et al. Synthesis, activity and docking studies of eugenol-based glucosides as new agents against Candida sp. Chem Biol Drug Des. 2018;92(2):1514–1524. doi: 10.1111/cbdd.13318 [DOI] [PubMed] [Google Scholar]
- 30.CLSI . Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard — third edition. 2010. [Google Scholar]
- 31.CLSI . Reference method for broth dilution antifungal susceptibility testing of filamentous fungi approved standard — second edition. 2008. [Google Scholar]
- 32.Espinel-Ingroff A, Fothergil A, Peter J, et al. Testing conditions for determination of minimum fungicidal concentrations of new and established antifungal agents for Aspergillus spp.: NCCLS collaborative study. J Clin Microbiol. 2002;40(9):3204–3208. doi: 10.1128/JCM.40.9.3204-3208.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghannoum M, Isham N, Verma A, et al. In vitro antifungal activity of naftifine hydrochloride against dermatophytes. Antimicrob Agents Chemother. 2013;57(9):4369–4372. doi: 10.1128/AAC.01084-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klepser ME, Ernst EJ, Lewis RE, et al. Influence of test conditions on antifungal time-kill curve results: proposal for standardized methods. Antimicrob Agents Chemother. 1998;42(5):1207–1212. doi: 10.1128/AAC.42.5.1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1):55–63. doi: 10.1016/0022-1759(83)90303-4 [DOI] [PubMed] [Google Scholar]
- 36.Kolb HC, Finn MG, Sharpless KB. Click chemistry: diverse chemical function from a few good reations. Angew Chem Int Ed. 2001;40(11):2004–2021. doi: [DOI] [PubMed] [Google Scholar]
- 37.Rostovtsev VV, Green LG, Forkin VV, et al. A stepwise Huisgen cycloaddition process: copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew Chem Int Ed. 2002;41(14):2596–2599. doi: [DOI] [PubMed] [Google Scholar]
- 38.Tornoe CW, Christensen C, Meldal M. Peptidotriazoles on solid phase:[1,2,3]-Triazoles by regiospecific copper(I)-catalyzed 1,3-dipolar cycloaddition of terminal alkynes to azides. J Org Chem. 2002;67(9):3057–3064. doi: 10.1021/jo011148j [DOI] [PubMed] [Google Scholar]
- 39.Maksimov AY, Balandina SY, Topanov PA, et al. Organic antifungal drugs and targets of their action. Curr Top Med Chem. 2021;21(8):705–736. doi: 10.2174/1568026621666210108122622 [DOI] [PubMed] [Google Scholar]
- 40.Balouiri M, Sadiki M, Ibnsouda SK. Methods for in vitro evaluating antimicrobial activity: a review. J Pharm Anal. 2016;6(2):71–79. doi: 10.1016/j.jpha.2015.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wiederhold NP. Antifungal susceptibility testing: a primer for clinicians. Open Forum Infect Dis. 2021;8(11):1–13. doi: 10.1093/ofid/ofab444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pinto SML, Rivera Y, Sandoval LVH, et al. Semisynthetic eugenol derivatives as antifungal agents against dermatophytes of the genus Trichophyton. J Med Microbiol. 2019;68(7):1109–1117. doi: 10.1099/jmm.0.001019 [DOI] [PubMed] [Google Scholar]
- 43.Deng S, Zhang C, Seyedmousavi S, et al. Comparison of the in vitro activities of newer triazoles and established antifungal agents against Trichophyton rubrum. Antimicrob Agents Chemother. 2015;59(7):4312–4314. doi: 10.1128/aac.00244-15 [DOI] [PMC free article] [PubMed] [Google Scholar]; • A comparison of the effects of different triazoles on T. rubrum is outlined.
- 44.Rezaei-Matehkolaei A, Khodavaisy S, Alshahni MM, et al. In vitro antifungal activity of novel triazole efinaconazole and five comparators against dermatophyte isolates. Antimicrob Agents Chemother. 2018;62(5):e02423–17. doi: 10.1128/aac.02423-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sardana K, Khurana A, Gupta A. Parameters that determine dissolution and efficacy of itraconazole and its relevance to recalcitrant dermatophytoses. Expert Rev Clin Pharmacol. 2019;12(5):443–452. doi: 10.1080/17512433.2019.1604218 [DOI] [PubMed] [Google Scholar]
- 46.Jeschke P. The unique role of halogen substituents in the design of modern agrochemicals. Pest Manag Sci. 2010;66(1):10–27. doi: 10.1002/ps.1829 [DOI] [PubMed] [Google Scholar]
- 47.Pannu J, McCarthy A, Martin A, et al. NB-002, a novel nanoemulsion with broad antifungal activity against dermatophytes, other filamentous fungi and Candida albicans. Antimicrob Agents Chemother. 2009;53(8):3273–3279. doi: 10.1128/aac.00218-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saracino IM, Foschi C, Pavoni M, et al. Antifungal activity of natural compounds vs. Candida spp.: a mixture of cinnamaldehyde and eugenol shows promising in vitro results. Antibiotics. 2022;11(1):73. doi: 10.3390/antibiotics11010073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Öz Y, Özdemir HG, Gökbolat E, et al. Time-kill kinetics and in vitro antifungal susceptibility of non-fumigatus Aspergillus Species isolated from patients with ocular mycoses. Mycopathologia. 2016;181(3):225–233. doi: 10.1007/s11046-015-9969-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nivoix Y, Ledoux M-P, Herbrecht R. Antifungal therapy: new and evolving therapies. Semin Respir Crit Care Med. 2020;41(1):158–174. doi: 10.1055/s-0039-3400291 [DOI] [PubMed] [Google Scholar]
- 51.Adan A, Kiraz Y, Baran Y. Cell proliferation and cytotoxicity assays. Curr Pharm Biotechnol. 2016;17(14):1213–1221. doi: 10.2174/1389201017666160808160513 [DOI] [PubMed] [Google Scholar]
- 52.Kumar D, Jain SK. A comprehensive review of N-heterocycles as cytotoxic agents. Curr Med Chem. 2016;23(38):4338–4394. doi: 10.2174/0929867323666160809093930 [DOI] [PubMed] [Google Scholar]
- 53.Giuffrida R, Caradonna E, Borgia F, et al. Pulse itraconazole in the treatment of lymphocutaneous sporotrichosis: a case report from Southern Italy and review of the literature. Dermatol Ther. 2020;33(4):e13716. doi: 10.1111/dth.13716 [DOI] [PubMed] [Google Scholar]
- 54.Gupta AK, Lyons DCA. The rise and fall of oral ketoconazole. J Cutan Med Surg. 2015;19(4):352–357. doi: 10.1177/1203475415574970 [DOI] [PubMed] [Google Scholar]
- 55.Daina A, Michielin O, Zoete V. SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep. 2017;7:42717. doi: 10.1038/srep42717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eldar-Finkelman H, Martinez A. GSK-3 inhibitors: preclinical and clinical focus on CNS. Front Mol Neurosci. 2011;4:32. doi: 10.3389/fnmol.2011.00032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lipinski CA, Lombardo F, Dominy BW, et al. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 2001;46(1):3–26. doi: 10.1016/s0169-409x(00)00129-0 [DOI] [PubMed] [Google Scholar]; • This paper describes experimental and computational approaches to estimate solubility and permeability in discovery and development settings. It also highlights the ‘rule of five’, which predicts the likelihood of drug absorption or permeation.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


