Abstract
Minocycline has been implicated in the treatment for multiple diseases in the nervous system for its neuroprotective properties. However, the mechanism by which minocycline benefits postoperative anesthesia-induced cognitive dysfunction is still unclear. In this study, we introduced minocycline to a rat model of anesthetic-induced learning and memory impairment, to investigate the effects of minocycline on neuroinflammation, beta amyloid (Aβ) deposition, and activation of nuclear factor κB (NF-κB) signaling pathway in the hippocampus. Aged rats were treated with sevoflurane to induce cognitive impairment with and without pre-administration of minocycline. The rats were then subjected to Morris water maze tests to evaluate their learning and memory performance. Subsequently, apoptosis in the hippocampal tissue was assessed with TUNEL assays. Furthermore, the levels of apoptosis-related proteins and pro-inflammatory cytokines, Aβ responses, and activation of the NF-κB signaling pathway in the hippocampus were examined by Western blot analysis. Our results revealed that minocycline effectively alleviated sevoflurane-induced cognitive impairment in aged rats. Minocycline reduced sevoflurane-induced neuronal apoptosis and inflammation, as well as suppressed sevoflurane-induced Aβ accumulation and activation of NF-κB signaling pathway in the hippocampus of aged rats. In conclusion, our findings indicate that minocycline is a potent agent to counteract sevoflurane-induced cognitive impairment and neurotoxicity in the nervous system of aged rats, which is likely to be mediated via NF-κB signaling pathway.
Keywords: Minocycline, Sevoflurane, Anesthesia-induced cognitive impairment, Neurotoxicity, NF-κB signaling
Introduction
Postoperative cognitive dysfunction (POCD) is a clinical phenomenon characterized by cognitive impairment in patients after anesthesia and surgery, especially in the elderly surgical patients. It has been reported that about 25 % of elderly (60 years old and above) patients exhibit POCD one week after surgery, and about 10 % of elderly patients exhibit persistent POCD for three months or longer (Moller et al. 1998). POCD can be self-limiting in most patients, but it may be long-term or even permanent in some patients and associated with postoperative complications and mortality (Bekker and Weeks 2003, Rasmussen et al. 2003). For example, anesthetics-induced POCD has been reported to be a risk factor for Alzheimer’s disease in elderly surgery patients (Steinmetz et al. 2009; Bittner et al. 2011). In the past decade, extensive work has been done to elucidate the mechanisms for anesthetics-induced POCD, and it was found that anesthetics such as sevoflurane can induce neuronal apoptosis in experimental animal models and in elderly Alzheimer’s patients, suggesting that anesthetics neurotoxicity may play an important part in POCD (Silbert et al. 2011; Brosnan and Bickler 2013; Xiong et al. 2013). Hence, it is a practically and clinically important task to seek agents that can counteract anesthetics-induced neurotoxicity and cognitive dysfunction, especially for the well-being of the elderly surgery patients.
Minocycline is a semi-synthetic, second-generation tetracycline derivative capable of penetrating the blood–brain barrier (Yang et al. 2007a). Minocycline is commonly used to treat infectious diseases in the clinic for its anti-inflammation (Desjarlais et al. 2014), anti-apoptosis (Liu et al. 2014), and anti-oxidation (Karachitos et al. 2012) properties. Interestingly, experimental data have indicated that minocycline exhibits neuroprotective functions in many nervous system-associated diseases, such as cerebral ischemia (Yrjanheikki et al. 1999), spinal cord injury (Stirling et al. 2004), Parkinson’s disease (Wu et al. 2002), Huntington’s disease (Palazuelos et al. 2009), and Alzheimer’s disease (Choi et al. 2007). Recently, it was reported that minocycline could alleviate isoflurane anesthesia-induced cognitive impairment in aged rats (Kong et al. 2013), suggesting that minocycline might be a potential agent to antagonize the anesthetics-induced adverse effects in the brain, thereby protecting the nervous system of the elderly surgery patients. However, whether minocycline can mitigate the cognitive dysfunction induced by other anesthetics such as sevoflurane remains unknown, and it is worth addressing for the potential clinical implication in elderly surgery.
Herein, we employed an aged rat model of learning and memory impairment resulting from sevoflurane inhalation. We investigated the effects of minocycline administration on sevoflurane-induced cognitive impairment and the underlying molecular mechanisms. Our results revealed that minocycline effectively alleviated sevoflurane-induced learning and memory impairment in aged rats as evidenced by Morris water maze (MWM) tests. Moreover, minocycline exerted protection for the hippocampal neurons by suppressing sevoflurane-induced apoptosis, inflammation, beta amyloid (Aβ) accumulation, and activation of nuclear factor κB (NF-κB) signaling pathway in the hippocampus of aged rats. In summary, our findings demonstrate a neuroprotective property of minocycline in sevoflurane-induced neurotoxicity and cognitive impairment, which is likely to be mediated through NF-κB signaling pathway.
Materials and Methods
Establishment of a Rat Model of Cognitive Impairment and Minocycline Treatment
Male, 20-month-old SD rats were purchased from the Laboratory Animal Center of China Medical University. Prior to the experiments, the animals were maintained under specific pathogen free (SPF) conditions for a week for adaptation, during which period they were allowed to consume food and water ad libitum. The animal experiment protocol was approved by the Animal Ethics Committee of China Medical University.
Twenty-four rats were randomly divided into four treatment groups with six animals in each group: control group, minocycline group, sevoflurane group, and minocycline + sevoflurane group. The rats in minocycline group and minocycline + sevoflurane group received intraperitoneal injection of 45 mg/kg minocycline 12 h prior to anesthesia, whereas the animals in the other two groups received a same volume of saline. The animals in sevoflurane group and minocycline + sevoflurane group were anesthetized in the environment containing 2 % sevoflurane for 5 h. 24 h later, physiological data of the animals including heart rate (HR), mean arterial pressure (MAP), arterial carbon dioxide tension (PaCO2), and arterial oxygen tension (SPO2) were documented. After MWM tests, the animals were sacrificed. For each rat, half of the brain was fixed in 4 % paraformaldehyde, paraffin-embedded, and the hippocampal tissue in the other half was frozen at −70 °C and kept for subsequent experiments.
Morris Water Maze
MWM test consisting of acquisition test and special probe trial is a well-established method to evaluate learning and memory ability in small animals (Blokland et al. 2004). Acquisition test: this experiment lasted for four days with two training sessions on each day and a 15-min interval between the two sessions. During training, each animal was placed, facing the tank wall, at a designated starting point in different quadrants of the maze every day. All groups shared the same starting point in each training session. If a rat swam for an amount of time before climbing onto the platform and remained there for over 3 s, its swimming time was documented and considered to be the latency for seeking escape. If a rat failed to find the platform within 120 s, or it was ushered to the platform and remained there for 30 s, the latency of these animals was recorded as 120 s. The latency of finding the hidden platform to escape reflects an animal’s performance at acquiring experience, or learning ability.
Spatial probe trial: this experiment was only performed once, whereby the platform was removed. A rat was placed at any starting point not adjacent to the platform while facing the wall. The frequency of the rat swimming across the original platform location within 120 s was documented. This experiment reveals an animal’s performance at recalling experience, or memory ability.
TUNEL Assay
Apoptosis was examined using In Situ Cell Death Detection Kit (Roche Diagnostics, Mannheim, Germany) following the manufacturer’s instructions. Specifically, 5-μm sections of the paraffin-embedded brain tissues were dewaxed, permeabilized, and blocked. The sections were treated with 50 μl TUNEL reaction solution and incubated in a humidified, dark box at 37 °C for 60 min. Subsequently, 50 μl Converter-POD was added to the sections for another incubation for 30 min in a dark box, and 50 μl DAB substrate solution was then added for color development. Hematoxylin was used to stain the nuclei, followed by routine dehydration, decolorization, and mounting. The sections were microscopically examined and images were captured. For each captured image, the numbers of total nuclei and TUNEL-positive nuclei were counted and the apoptosis ratio was calculated as follows: apoptosis ratio = (number of TUNEL-positive nuclei/number of total nuclei) × 100 %.
Western Blot Analysis
Hippocampal tissues were lysed with radio-immunoprecipitation assay (RIPA) lysis buffer (Beyotime Institute of Biotechnology, Haimen, China) to extract total proteins and nucleoproteins as previously described (Maguire et al. 2011). Protein concentration was determined using a bicinchoninic acid (BCA) protein assay kit (Beyotime). 40 μg total proteins from each sample were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) and transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, Bedford, MA, USA), which were probed with primary antibodies against the proteins of interest at 4 °C overnight. The primary antibodies against cleaved caspase-3, Bax, Bcl-2, tumor necrosis factor (TNF)-α, interleukin (IL)-6, IL-1β, nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor α (IκBα), and NF-κB were from Santa Cruz (Dallas, TX, USA). Anti-BACE, anti-Aβ (1–42), and anti-p-IκBα antibodies were purchased from Bioss (Beijing, China). The membranes were then incubated with corresponding HRP-conjugated secondary antibodies (1:5,000; Beyotime) at room temperature for 1 h. The signals of the proteins of interest were visualized with the ECL system (7SeaPharmTech, Shanghai, China) followed by exposure to films in a darkroom. The films were scanned and analyzed with Gel-Pro-Analyzer to reveal the densitometry values of the target bands. β-actin and lamin A were used as the internal controls for cytoplasmic proteins and nuclear proteins, respectively, in the experiment for detecting NF-κB nuclear translocation, otherwise, β-actin was the internal control for total cellular proteins.
Statistical Analysis
The data are expressed as the mean ± standard deviation. One-way analysis of variance was used for comparisons between groups. Bonferroni post hoc analysis was performed for multiple comparisons. GraphPad Prism 5.0 was employed for data processing and graph plotting. The differences were considered statistically significant when P < 0.05.
Results
Minocycline Alleviated Sevoflurane-Induced Learning and Memory Impairment in Aged Rats
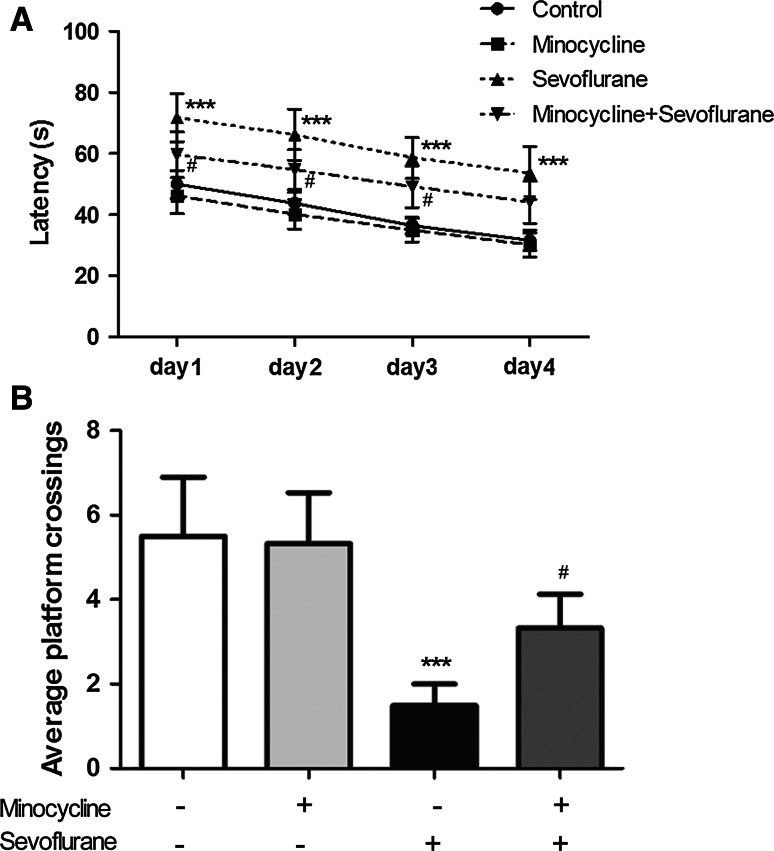
The cognitive impairment model of aged rats was induced by sevoflurane inhalation. At 24 h post-treatment, the physiological conditions of the rats were examined before MWM tests, and the indicators of arterial blood gas were not affected by sevoflurane or minocycline (Table 1). MWM tests were performed to assess the effects of minocycline on learning and memory ability of the sevoflurane-treated rats. Compared with control group, sevoflurane-treated rats displayed an extended latency and a reduced number of swimming passes above the platform in MWM tests, and the differences were statistically significant (Fig. 1a, b, P < 0.001 and P < 0.001, respectively), indicating an impaired learning and memory ability due to sevoflurane anesthesia. Compared with the rats treated with sevoflurane only, those treated with both minocycline and sevoflurane exhibited a remarkably shortened latency and an increased number of passes above the platform (Fig. 1a, b, P < 0.05). The MWM results indicated that minocycline alleviated sevoflurane-induced learning and memory impairment in aged rats.
Table 1.
Arterial blood gas index
| Groups (n = 6) | HR (beats/min) | MAP (mmHg) | PaCO2 (mmHg) | SPO2 (mmHg) |
|---|---|---|---|---|
| Control | 382.7 ± 13.5 | 105.78 ± 5.92 | 37.95 ± 1.31 | 97.78 ± 1.13 |
| Minocycline | 384.3 ± 19.4 | 107.68 ± 5.58 | 37.28 ± 1.18 | 97.57 ± 1.24 |
| Sevoflurane | 390.0 ± 17.1 | 103.05 ± 6.13 | 38.43 ± 1.29 | 97.78 ± 1.10 |
| Minocycline + sevoflurane | 389.3 ± 17.5 | 104.43 ± 6.39 | 37.53 ± 1.32 | 97.70 ± 1.10 |
Fig. 1.
Minocycline alleviated sevoflurane-induced cognitive impairment in aged rats. Learning and memory ability of the sevoflurane-treated rats with and without pre-administration of minocycline was examined with Morris water maze (MWM) tests (n = 6 for each group). a Acquisition tests of MWM were performed to examine rats’ learning ability as indicated by the latency to find the platform; b MWM navigation tests were performed to examine rats’ memory ability of recalling the original position of the platform. All experimental data are expressed as the mean ± SD. Compared with the untreated control group, ***P < 0.001; compared with sevoflurane treatment group, # P < 0.05
Minocycline Mitigated Sevoflurane-Induced Neuronal Apoptosis in Rat Hippocampus
After MWM tests, the rats were sacrificed and the brains were harvested. To investigate the effect of minocycline on neuronal apoptosis in sevoflurane-treated rats, TUNEL assay was performed to detect the apoptotic neurons in the hippocampus. The results revealed that sevoflurane induced degeneration of rat hippocampal neurons, as evidenced by the sparse, irregular arrangement of cells that manifested a pronounced tail-like appearance, whereas minocycline ameliorated sevoflurane-induced neuronal degeneration to a significant degree (Fig. 2a). Statistical analysis on the TUNEL results showed that sevoflurane anesthesia resulted in elevated numbers of TUNEL-positive nuclei in the hippocampal region, which was significantly reduced by minocycline co-treatment (Fig. 2b, P < 0.05), implying that minocycline could attenuate sevoflurane-induced cell apoptosis in the hippocampal region of aged rats. Furthermore, the levels of several apoptosis-related proteins including cleaved caspase-3, Bax, and Bcl-2 (Wang et al. 2004) in the rat hippocampus were examined by Western blot analysis. Sevoflurane inhalation resulted in increased levels of cleaved caspase-3 and Bax in rat hippocampus, which was significantly inhibited by minocycline (Fig. 2c, d, P < 0.01). Meanwhile, minocycline administration reversed sevoflurane-induced downregulation of Bcl-2 in rat hippocampus (Fig. 2d, P < 0.01). In summary, these results indicated that minocycline suppressed sevoflurane-induced apoptosis in the hippocampal neurons of aged rats.
Fig. 2.
Minocycline inhibited sevoflurane-induced hippocampal neuronal apoptosis in aged rats. After MWM tests, the rats were sacrificed and the rat brains were harvested. a The brains were fixed, paraffin-embedded, and sectioned, followed by TUNEL assay to detect chromosomal fragmentation in the apoptotic cells. Cell nuclei were stained blue with hematoxylin, whereas fragmented DNA was labeled and turned brownish under an optical microscope; b statistical analysis of the TUNEL results (n = 6 for each group). The apoptosis ratio is calculated as (number of TUNEL-positive nuclei/number of total nuclei) × 100 %; c–e the hippocampi of the experimental rats were isolated and lysed with RIPA to extract total proteins. The levels of cleaved caspase-3, Bax, and Bcl-2 in rat hippocampus were determined by Western blots followed by densitometry analysis using β-actin as the internal control. This figure shows the representative images of all the animals examined, and the data are expressed as the mean ± SD (n = 6 for each group). Compared with control group, **P < 0.01, ***P < 0.01; compared with sevoflurane group, # P < 0.05, ## P < 0.01
Minocycline Inhibited Sevoflurane-Induced Aβ Accumulation
Amyloid precursor protein is present in neurons and can be cleaved to generate Aβ peptide by β-site APP-converting enzyme (BACE) (Lammich et al. 2004; Dong et al. 2011). Aβ deposition is frequently observed in the neurons of Alzheimer’s patients and it is assumed to play a critical role in the neuropathogenesis of the diseases (O’Brien and Wong 2011). Western blot analysis was performed to examine the levels of Aβ and BACE in the hippocampus of sevoflurane-treated rats with and without minocycline pre-administration (Fig. 3). The results revealed that the levels of both of Aβ and BACE were significantly elevated in the hippocampus of sevoflurane-treated rats compared with the untreated rats (P < 0.01 for Aβ, P < 0.001 for BACE), indicating an activated Aβ response to sevoflurane anesthesia. Notably, minocycline reduced sevoflurane-induced elevation of Aβ and BACE in rat hippocampus, compared with the rats treated with sevoflurane alone (P < 0.05 and P < 0.01, respectively). Thus, these results indicated that minocycline suppressed sevoflurane-induced upregulation of BACE and resultant Aβ accumulation in rat hippocampus.
Fig. 3.
Minocycline inhibited sevoflurane-induced Aβ accumulation in rat hippocampus. The levels of Aβ and BACE in rat hippocampus were revealed by Western blots. Densitometry analysis was performed using β-actin as the internal control. Figure shows the representative images of all the experimental rats, and the values are expressed as the mean ± SD (n = 6 for each group). Compared with control group, **P < 0.01, ***P < 0.001; compared with sevoflurane group, # P < 0.05, ## P < 0.01
Minocycline Suppressed Sevoflurane-Induced Inflammation in the Hippocampus of Aged Rats
To investigate the effects of minocycline on sevoflurane-induced neuroinflammation, Western blot analysis was performed to detect the levels of inflammatory cytokines including TNF-α, IL-1β, and IL-6 in rat hippocampus (Fig. 4). Compared with the untreated rats, the levels of TNF-α, IL-1β, and IL-6 in the hippocampus of sevoflurane-treated rats were all markedly elevated (P < 0.01, P < 0.01 and P < 0.01, respectively) and such elevation was significantly suppressed by minocycline (P < 0.05, P < 0.01 and P < 0.01, respectively). Minocycline inhibited sevoflurane-induced upregulation of inflammatory cytokines in the rat hippocampus, suggesting that sevoflurane-induced neuroinflammation could be attenuated by minocycline.
Fig. 4.
Minocycline suppressed sevoflurane-induced elevation of inflammatory cytokines in the hippocampus of aged rats. The levels of TNF-α, IL-1β, and IL-6 in rat hippocampus were examined by Western blot analysis. Densitometry analysis was performed using β-actin as the internal control. This figure shows the representative images of all the experimental rats, and the values are expressed as the mean ± SD (n = 6 for each group). Compared with control group, **P < 0.01; compared with sevoflurane group, # P < 0.05, ## P < 0.01
Minocycline Inhibited Sevoflurane-Induced Activation of the NF-κB Signaling Pathway in the Hippocampus of Aged Rats
NF-κB signaling pathway is one of the key pathways mediating inflammation and stress responses in the cells (Abdullah et al. 2013). Proteolysis and phosphorylation of IκBα as well as NF-κB nuclear translocation are the major events for the activation of the NF-κB signaling (Huang et al. 2013; Nguyen et al. 2014). To elucidate whether minocycline attenuated sevoflurane-induced neuronal apoptosis and inflammation via NF-κB signaling pathway, the levels of full-length IκBα and phosphorylated form p-IκBα, as well as nuclear and cytoplasmic levels of NF-κB in rat hippocampus were determined by Western blot analysis. The levels of the active form p-IκBα and nuclear NF-κB were significantly increased in the hippocampus of sevoflurane-treated rats (Fig. 5a, c, P < 0.01), resulting in reduced levels of full-length IκBα and cytoplasmic NF-κB, compared with the untreated control (Fig. 5b, d, P < 0.01, P < 0.05), indicating that NF-κB signaling pathway was activated by sevoflurane anesthesia. However, sevoflurane-induced IκBα phosphorylation and NF-κB nuclear translocation were inhibited by minocycline to a significant extent. Hence, these results suggest that the minocycline inhibited sevoflurane-induced activation of the NF-κB signaling pathway in the hippocampus of aged rats.
Fig. 5.
Minocycline inhibited sevoflurane-induced activation of the NF-κB signaling pathway in the hippocampus of aged rats. The levels of p-IκBα, full-length IκBα, nuclear NF-κB, and cytoplasmic NF-κB in rat hippocampus were examined by Western blot analysis. Densitometry analysis was performed using β-actin as the internal control for total proteins in a and b, whereas β-actin and lamin A were used as the internal controls for cytoplasmic proteins and nuclear proteins, respectively, in c and d. This figure shows the representative images of all the experimental rats, and the values are expressed as the mean ± SD (n = 6 for each group). Compared with control group, *P < 0.05, **P < 0.01; compared with sevoflurane group, # P < 0.05, ## P < 0.01
Discussion
Preclinical studies have shown that sevoflurane, one of the most commonly used inhalational anesthetics, could induce widespread cerebral neuronal apoptosis and cognitive impairment (Jevtovic-Todorovic et al. 2003). Moreover, it has been reported that sevoflurane could induce neuroinflammation and deposition of Aβ in the hippocampus (Liu et al. 2013). In the present study, aged rats that were exposed to 2 % sevoflurane for 5 h displayed cognitive impairment and neurodegenerative symptoms including apoptosis, inflammation, and Aβ accumulation in the hippocampus. Minocycline administration significantly alleviated sevoflurane-induced cognitive impairment, hippocampal neurodegeneration, inflammation, and Aβ deposition, suggesting that minocycline could be a potent agent to counteract cognitive impairment and neurotoxicity induced by sevoflurane anesthesia. It has been reported that long-term anesthesia may cause hypoxia in the brain, therefore damaging the neurons and resulting in cognitive impairment (Kong et al. 2013). However, in our study, the indicators of arterial blood gas were not affected by sevoflurane or minocycline, implying that sevoflurane-induced neurotoxicity was not due to cerebral hypoxia. In fact, we found that sevoflurane anesthesia led to activation of NF-κB signaling, which was suppressed by minocycline, suggesting that NF-κB signaling pathway may be an important mechanism accounting for sevoflurane-induced neurotoxicity as well as the neuroprotective effects of minocycline.
MWM, which is a well-established test to evaluate learning and memory performance in animals (Blokland et al. 2004), was employed to study the effects of minocycline on sevoflurane-induced cognitive impairment in aged rats. The differences in the escape latency and number of passes over the original platform location revealed that sevoflurane compromised the animals’ learning and memory ability, which was effectively improved by the administration of minocycline. We further investigated the molecular mechanism for minocycline-mediated neuroprotection. Western blot analysis showed that minocycline counteracted sevoflurane-induced elevation of the anti-apoptotic protein Bcl-2, and reduction of the pro-apoptotic proteins Bax and cleaved caspase-3 in rat hippocampus, implying that minocycline significantly mitigated sevoflurane-induced apoptosis in the hippocampal neurons, which was consistent with results of TUNEL assays. Minocycline also inhibited sevoflurane-induced upregulation of BACE, resulting in suppression of β-secretory pathway of APP and ultimate generation of Aβ, indicating that minocycline inhibited sevoflurane-induced Aβ accumulation in the hippocampus of aged rats. In addition, minocycline suppressed sevoflurane-induced elevation of pro-inflammatory cytokines including TNF-α, IL-1β, and IL-6 in rat hippocampus. It is generally believed that pro-inflammatory cytokines in the hippocampus are secreted by activated microglials, which are key mediators for neuroinflammation (King et al. 2001; Streit 2004). Thus, these results suggest that minocycline may attenuate sevoflurane-induced microglial activation and the consequent neuroinflammation in the hippocampus of aged rats. Overall, the above results demonstrate the neuroprotective effects of minocycline in sevoflurane-induced neurotoxicity by suppressing sevoflurane-induced neuronal apoptosis, Aβ accumulation, and inflammation in the hippocampus. These data are consistent with the work by Kong et al. on the neuroprotective effects of minocycline on isoflurane-induced neural injury in aged rats (Kong et al. 2013) and also confirms the neuroprotective mechanisms of minocycline including promoting neuronal survival (Huang et al. 2010), inhibiting caspase cascades and microglial-mediated neuroinflammation (Chen et al. 2000), and diminishing Aβ deposition in hippocampal neurons (Seabrook et al. 2006). In addition, inhalational anesthetics have been reported to induce cell injury by disrupting intracellular calcium homeostasis (Yang et al. 2008), and minocycline is able to target mitochondria and maintain intracellular calcium homeostasis (Fernandez-Gomez et al. 2005; Garcia-Martinez et al. 2010), thereby exerting neuroprotective effects. Hence, the involvement of minocycline in intracellular calcium homeostasis of hippocampal neurons may be also involved in the neuroprotective actions of minocycline against sevoflurane anesthesia.
In addition to the aforementioned roles of minocycline in neuroprotection, in the present study, we bring new insights into NF-κB signaling pathway as a critical molecular mechanism in anesthesia-induced cognitive impairment and in minocycline-mediated neuroprotection. NF-κB is a homologous or heterologous dimer consisting of polypeptide chain p50 and p65. The activity of NF-κB is inhibited by IκB, which binds to NF-κB dimer to form a trimer that is stably present in the cytoplasm. In response to an external stimulus, IκB is phosphorylated, resulting in its dissociation from the trimer. Subsequently, NF-κB dimmers translocate from cytoplasm into nucleus to activate transcription of downstream genes that play critical roles in inflammation and apoptosis (Yang et al. 2007b; Gilmore and Wolenski 2012). NF-κB signaling has been implicated to play a part in cognitive deficits in animal models (Mattson and Camandola 2001; Camandola and Mattson 2007), thus we further investigated whether minocycline counteracted sevoflurane-induced cognitive impairment and neurotoxicity via NF-κB signaling pathway. Our results revealed that sevoflurane induced phosphorylation of IκB, resulting in NF-κB nuclear translocation and activation of transcription of downstream genes, whereas minocycline significantly inhibited sevoflurane-induced activation of NF-κB signaling pathway in rat hippocampus. These data suggest that NF-κB signaling pathway may play a critical role in sevoflurane-induced cognitive impairment and neural injury, and it might serve as an important mechanism accounting for the neuroprotective effects of minocycline.
Alzheimer’s disease is characterized by increased Aβ deposition, extracellular senile plaques, intracellular neurofibrillary tangles, and massive neuronal loss in the brain (Blennow et al. 2006). Cognitive impairment is commonly a transition stage between normal aging and pathogenesis of Alzheimer’s disease, and POCD has been reported to be a risk factor for Alzheimer’s disease in elderly surgery patients (Steinmetz et al. 2009; Bittner et al. 2011). In the present study, we found that minocycline could mitigate sevoflurane-induced cognitive impairment accompanying with downregulation of BACE and reduction of Aβ in rat hippocampus, providing new evidence for the potential beneficial effects of minocycline on Alzheimer’s disease (Choi et al. 2007).
Minocycline has long been proposed to treat POCD and neurodegenerative diseases (Blum et al. 2004; Fan et al. 2011), and our study reinforces the clinical potential of minocycline in POCD prevention/treatment. In humans, long-term treatment with minocycline at doses of up to 200 mg/day is generally safe and well tolerated as demonstrated by tolerability tests and clinical trials in rheumatoid arthritis, acne vulgaris, and Huntington’s disease (Blum et al. 2004). In some rare cases, minocycline treatment can cause some minor side effects such as digestive disorders, staining of teeth, or benign intracranial hypertension, but the side effects are mostly reversible upon termination of use (Donnet et al. 1992; Shapiro et al. 1997; Good and Hussey 2003). Hence, minocycline is a safe compound and can be further evaluated as a promising agent to alleviate POCD in clinical studies.
In summary, our study reveals that minocycline displays neuroprotective properties against sevoflurane-induced learning and memory impairment in aged rats and that this effect correlates with minocycline-mediated inhibition of sevoflurane-induced apoptosis, inflammation, Aβ accumulation, and activation of NF-κB signaling pathway in rat hippocampus. Our data suggest that minocycline may counteract sevoflurane-induced cognitive impairment and neurotoxicity through NF-κB signaling pathway, and that minocycline has potential clinical values as a neuroprotective agent to prevent or treat POCD.
Acknowledgments
This study was supported by grants from the National Natural Science Foundation of China (Nos. 81473285, 81101402, and 81171782).
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were approved by the Animal Ethics Committee of China Medical University and in accordance with the ethical standards of the institution.
References
- Abdullah M, Rani AA, Sudoyo AW, Makmun D, Handjari DR, Hernowo BS (2013) Expression of NF-kB and COX2 in colorectal cancer among native Indonesians: the role of inflammation in colorectal carcinogenesis. Acta Med Indones 45:187–192 [PubMed] [Google Scholar]
- Bekker AY, Weeks EJ (2003) Cognitive function after anaesthesia in the elderly. Best Pract Res Clin Anaesthesiol 17:259–272 [DOI] [PubMed] [Google Scholar]
- Bittner EA, Yue Y, Xie Z (2011) Brief review: anesthetic neurotoxicity in the elderly, cognitive dysfunction and Alzheimer’s disease. Can J Anaesth 58:216–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blennow K, de Leon MJ, Zetterberg H (2006) Alzheimer’s disease. Lancet 368:387–403 [DOI] [PubMed] [Google Scholar]
- Blokland A, Geraerts E, Been M (2004) A detailed analysis of rats’ spatial memory in a probe trial of a Morris task. Behav Brain Res 154:71–75 [DOI] [PubMed] [Google Scholar]
- Blum D, Chtarto A, Tenenbaum L, Brotchi J, Levivier M (2004) Clinical potential of minocycline for neurodegenerative disorders. Neurobiol Dis 17:359–366 [DOI] [PubMed] [Google Scholar]
- Brosnan H, Bickler PE (2013) Xenon neurotoxicity in rat hippocampal slice cultures is similar to isoflurane and sevoflurane. Anesthesiology 119:335–344 [DOI] [PubMed] [Google Scholar]
- Camandola S, Mattson MP (2007) NF-kappa B as a therapeutic target in neurodegenerative diseases. Expert Opin Ther Targets 11:123–132 [DOI] [PubMed] [Google Scholar]
- Chen M, Ona VO, Li M, Ferrante RJ, Fink KB, Zhu S, Bian J, Guo L, Farrell LA, Hersch SM, Hobbs W, Vonsattel JP, Cha JH, Friedlander RM (2000) Minocycline inhibits caspase-1 and caspase-3 expression and delays mortality in a transgenic mouse model of Huntington disease. Nat Med 6:797–801 [DOI] [PubMed] [Google Scholar]
- Choi Y, Kim HS, Shin KY, Kim EM, Kim M, Park CH, Jeong YH, Yoo J, Lee JP, Chang KA, Kim S, Suh YH (2007) Minocycline attenuates neuronal cell death and improves cognitive impairment in Alzheimer’s disease models. Neuropsychopharmacology 32:2393–2404 [DOI] [PubMed] [Google Scholar]
- Desjarlais M, Pratt J, Lounis A, Mounier C, Haidara K, Annabi B (2014) Tetracycline derivative minocycline inhibits autophagy and inflammation in concanavalin-a-activated human hepatoma cells. Gene Regul Syst Bio 8:63–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Xu Z, Zhang Y, McAuliffe S, Wang H, Shen X, Yue Y, Xie Z (2011) RNA interference-mediated silencing of BACE and APP attenuates the isoflurane-induced caspase activation. Med Gas Res 1:5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnet A, Dufour H, Graziani N, Grisoli F (1992) Minocycline and benign intracranial hypertension. Biomed Pharmacother 46:171–172 [DOI] [PubMed] [Google Scholar]
- Fan L, Wang TL, Xu YC, Ma YH, Ye WG (2011) Minocycline may be useful to prevent/treat postoperative cognitive decline in elderly patients. Med Hypotheses 76:733–736 [DOI] [PubMed] [Google Scholar]
- Fernandez-Gomez FJ, Galindo MF, Gomez-Lazaro M, Gonzalez-Garcia C, Cena V, Aguirre N, Jordan J (2005) Involvement of mitochondrial potential and calcium buffering capacity in minocycline cytoprotective actions. Neuroscience 133:959–967 [DOI] [PubMed] [Google Scholar]
- Garcia-Martinez EM, Sanz-Blasco S, Karachitos A, Bandez MJ, Fernandez-Gomez FJ, Perez-Alvarez S, de Mera RM, Jordan MJ, Aguirre N, Galindo MF, Villalobos C, Navarro A, Kmita H, Jordan J (2010) Mitochondria and calcium flux as targets of neuroprotection caused by minocycline in cerebellar granule cells. Biochem Pharmacol 79:239–250 [DOI] [PubMed] [Google Scholar]
- Gilmore TD, Wolenski FS (2012) NF-kappaB: where did it come from and why? Immunol Rev 246:14–35 [DOI] [PubMed] [Google Scholar]
- Good ML, Hussey DL (2003) Minocycline: stain devil? Br J Dermatol 149:237–239 [DOI] [PubMed] [Google Scholar]
- Huang WC, Qiao Y, Xu L, Kacimi R, Sun X, Giffard RG, Yenari MA (2010) Direct protection of cultured neurons from ischemia-like injury by minocycline. Anat Cell Biol 43:325–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Xu L, Zhou X, Gao C, Yang M, Chen G, Zhu J, Jiang L, Gan H, Gou F, Feng H, Peng J, Xu Y (2013) High glucose induces activation of NF-kappaB inflammatory signaling through IkappaBalpha sumoylation in rat mesangial cells. Biochem Biophys Res Commun 438:568–574 [DOI] [PubMed] [Google Scholar]
- Jevtovic-Todorovic V, Hartman RE, Izumi Y, Benshoff ND, Dikranian K, Zorumski CF, Olney JW, Wozniak DF (2003) Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci 23:876–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karachitos A, Jordan J, Kmita H (2012) Cytoprotective activity of minocycline includes improvement of mitochondrial coupling: the importance of minocycline concentration and the presence of VDAC. J Bioenerg Biomembr 44:297–307 [DOI] [PubMed] [Google Scholar]
- King BR, Smith R, Nicholson RC (2001) The regulation of human corticotrophin-releasing hormone gene expression in the placenta. Peptides 22:1941–1947 [DOI] [PubMed] [Google Scholar]
- Kong F, Chen S, Cheng Y, Ma L, Lu H, Zhang H, Hu W (2013) Minocycline attenuates cognitive impairment induced by isoflurane anesthesia in aged rats. PLoS One 8:e61385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammich S, Schobel S, Zimmer AK, Lichtenthaler SF, Haass C (2004) Expression of the Alzheimer protease BACE1 is suppressed via its 5′-untranslated region. EMBO Rep 5:620–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Pan N, Ma Y, Zhang S, Guo W, Li H, Zhou J, Liu G, Gao M (2013) Inhaled sevoflurane may promote progression of amnestic mild cognitive impairment: a prospective, randomized parallel-group study. Am J Med Sci 345:355–360 [DOI] [PubMed] [Google Scholar]
- Liu FY, Wu YH, Zhou SJ, Deng YL, Zhang ZY, Zhang EL, Huang ZY (2014) Minocycline and cisplatin exert synergistic growth suppression on hepatocellular carcinoma by inducing S phase arrest and apoptosis. Oncol Rep 32:835–844 [DOI] [PubMed] [Google Scholar]
- Maguire O, Collins C, O’Loughlin K, Miecznikowski J, Minderman H (2011) Quantifying nuclear p65 as a parameter for NF-kappaB activation: correlation between ImageStream cytometry, microscopy, and western blot. Cytometry A 79:461–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Camandola S (2001) NF-kappaB in neuronal plasticity and neurodegenerative disorders. J Clin Investig 107:247–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller JT, Cluitmans P, Rasmussen LS, Houx P, Rasmussen H, Canet J, Rabbitt P, Jolles J, Larsen K, Hanning CD, Langeron O, Johnson T, Lauven PM, Kristensen PA, Biedler A, van Beem H, Fraidakis O, Silverstein JH, Beneken JE, Gravenstein JS (1998) Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. international study of post-operative cognitive dysfunction. Lancet 351:857–861 [DOI] [PubMed] [Google Scholar]
- Nguyen DP, Li J, Yadav SS, Tewari AK (2014) Recent insights into NF-kappaB signalling pathways and the link between inflammation and prostate cancer. BJU Int 114:168–176 [DOI] [PubMed] [Google Scholar]
- O’Brien RJ, Wong PC (2011) Amyloid precursor protein processing and Alzheimer’s disease. Annu Rev Neurosci 34:185–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palazuelos J, Aguado T, Pazos MR, Julien B, Carrasco C, Resel E, Sagredo O, Benito C, Romero J, Azcoitia I, Fernandez-Ruiz J, Guzman M, Galve-Roperh I (2009) Microglial CB2 cannabinoid receptors are neuroprotective in Huntington’s disease excitotoxicity. Brain 132:3152–3164 [DOI] [PubMed] [Google Scholar]
- Rasmussen LS, Johnson T, Kuipers HM, Kristensen D, Siersma VD, Vila P, Jolles J, Papaioannou A, Abildstrom H, Silverstein JH, Bonal JA, Raeder J, Nielsen IK, Korttila K, Munoz L, Dodds C, Hanning CD, Moller JT (2003) Does anaesthesia cause postoperative cognitive dysfunction? A randomised study of regional versus general anaesthesia in 438 elderly patients. Acta Anaesthesiol Scand 47:260–266 [DOI] [PubMed] [Google Scholar]
- Seabrook TJ, Jiang L, Maier M, Lemere CA (2006) Minocycline affects microglia activation, Abeta deposition, and behavior in APP-tg mice. Glia 53:776–782 [DOI] [PubMed] [Google Scholar]
- Shapiro LE, Knowles SR, Shear NH (1997) Comparative safety of tetracycline, minocycline, and doxycycline. Arch Dermatol 133:1224–1230 [PubMed] [Google Scholar]
- Silbert B, Evered L, Scott DA, Maruff P (2011) Anesthesiology must play a greater role in patients with Alzheimer’s disease. Anesth Analg 112:1242–1245 [DOI] [PubMed] [Google Scholar]
- Steinmetz J, Christensen KB, Lund T, Lohse N, Rasmussen LS (2009) Long-term consequences of postoperative cognitive dysfunction. Anesthesiology 110:548–555 [DOI] [PubMed] [Google Scholar]
- Stirling DP, Khodarahmi K, Liu J, McPhail LT, McBride CB, Steeves JD, Ramer MS, Tetzlaff W (2004) Minocycline treatment reduces delayed oligodendrocyte death, attenuates axonal dieback, and improves functional outcome after spinal cord injury. J Neurosci 24:2182–2190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit WJ (2004) Microglia and Alzheimer’s disease pathogenesis. J Neurosci Res 77:1–8 [DOI] [PubMed] [Google Scholar]
- Wang J, Wei Q, Wang CY, Hill WD, Hess DC, Dong Z (2004) Minocycline up-regulates Bcl-2 and protects against cell death in mitochondria. J Biol Chem 279:19948–19954 [DOI] [PubMed] [Google Scholar]
- Wu DC, Jackson-Lewis V, Vila M, Tieu K, Teismann P, Vadseth C, Choi DK, Ischiropoulos H, Przedborski S (2002) Blockade of microglial activation is neuroprotective in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson disease. J Neurosci 22:1763–1771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong WX, Zhou GX, Wang B, Xue ZG, Wang L, Sun HC, Ge SJ (2013) Impaired spatial learning and memory after sevoflurane-nitrous oxide anesthesia in aged rats is associated with down-regulated cAMP/CREB signaling. PLoS One 8:e79408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang LP, Zhu XA, Tso MO (2007a) Minocycline and sulforaphane inhibited lipopolysaccharide-mediated retinal microglial activation. Mol Vis 13:1083–1093 [PMC free article] [PubMed] [Google Scholar]
- Yang LP, Zhu XA, Tso MO (2007b) Role of NF-kappaB and MAPKs in light-induced photoreceptor apoptosis. Invest Ophthalmol Vis Sci 48:4766–4776 [DOI] [PubMed] [Google Scholar]
- Yang H, Liang G, Hawkins BJ, Madesh M, Pierwola A, Wei H (2008) Inhalational anesthetics induce cell damage by disruption of intracellular calcium homeostasis with different potencies. Anesthesiology 109:243–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yrjanheikki J, Tikka T, Keinanen R, Goldsteins G, Chan PH, Koistinaho J (1999) A tetracycline derivative, minocycline, reduces inflammation and protects against focal cerebral ischemia with a wide therapeutic window. Proc Natl Acad Sci USA 96:13496–13500 [DOI] [PMC free article] [PubMed] [Google Scholar]