Abstract
Gene therapy that targets the ROCK2 gene has yielded promising results in the treatment of AD. Our previous study indicated that PEG–PEI/siROCK2 could effectively suppress ROCK2 mRNA expression and showed a promising prospect for the treatment of Alzheimer’s disease. However, the ability of PEG–PEI/siROCK2 to reduce Aβ-induced cytotoxicity is unknown. To investigate the effect of PEG–PEI/siROCK2 against Aβ42-induced neurotoxicity, primary cultured cortical neurons were pretreated with PEG–PEI/siROCK2 for 24 h and then treated with 5 μM Aβ42 for 24 h. We found that PEG–PEI/siROCK2 increased the cell viability and reduced the number of apoptotic cells induced by Aβ42, as measured using an MTT assay and Annexin V/PI staining. A further study revealed that PEG–PEI/siROCK2 can activate p-Akt, and treatment with the PI3K inhibitor LY294002 attenuated the neuroprotective effects. These results suggest that PEG–PEI/siROCK2 prevents Aβ42-induced neurotoxicity and that the activation of PI3K/Akt pathway is involved in neuroprotection. Taken together, these findings shed light on the role of PEG–PEI/siROCK2 as a potential therapeutic agent for AD.
Keywords: Alzheimer disease, Rho-associated kinase 2, Gene therapy, Polyethylene glycol–polyethyleneimine, β-Amyloid, Neurotoxicity
Introduction
Alzheimer’s disease (AD) is the most common neurodegenerative disorder, and it results in the progressive impairment of cognitive function that eventually leads to dementia. Although some drugs have been shown to slow the progression of the disease, treatments that can stop or reverse the progression of the pathology are currently lacking (Glat and Offen 2013). The prospects appear hopeful that gene therapy has the potential to be a disease-modifying treatment for AD (Nilsson et al. 2010). RNA interference (RNAi) is regarded as a promising technique for gene silencing because the small interfering RNA (siRNA) can recognize its complementary mRNA with high specificity and degrade it in a short time (Liang et al. 2012). The efficient delivery of genetic material to the required cells within a patient without significant toxicity and side effects is a key problem in gene therapy that has been extensively studied for two decades (Wei et al. 2013). In recent years, the superior physical and biological properties of nanoparticles can be utilized to overcome challenges in gene and drug delivery (Kou et al. 2014). Our previous study indicated that the high pH-buffering capacity, reduced cytotoxicity, and high transfection efficiency of polyethylene glycol–polyethyleneimine (PEG–PEI) co-polymer make this material a strong potential gene carrier for gene therapy (Liu et al. 2013).
Rho-associated kinase (ROCK) is a major downstream effector of the small GTPase RhoA, which mediates cell survival and axonal regeneration in AD. The inhibition of ROCK reportedly protects rat retinal ganglion cells from apoptosis in vitro and in vivo (Lingor et al. 2008). However, few studies have investigated the effects of ROCK on the neuronal apoptosis induced by Amyloid-β (Aβ). Aβ deposition-induced neuronal apoptosis plays a crucial role in the pathogenesis of AD (Fu et al. 2014). The accumulation of toxic Aβ, which consists of extracellular plaques, has been suggested to lead to neuronal loss in AD (Hardy and Selkoe 2002). Therefore, the investigation of novel therapies to reduce the loss of neurons induced by Aβ will shed new light on AD treatments. Many studies have attempted to confirm ROCK2 as a therapeutic target for AD by showing that the inhibition of ROCK2 suppresses Aβ production (Chen et al. 2010; Herskowitz et al. 2013). However, the influence of ROCK2 on Aβ-induced neuronal death has seldom been reported.
Therefore, we synthesized a PEG–PEI to deliver ROCK-2-siRNA (siROCK2) into cultured mouse cortical neurons treated with Aβ1–42(Aβ42). The subsequent changes in the cell viability and apoptosis were then evaluated to confirm the neuroprotective effect of PEG–PEI/siROCK2. Furthermore, we investigated the involvement of the PI3K/Akt pathway in effect of PEG–PEI/siROCK2 against Aβ42-induced neurotoxicity.
Materials and Methods
Materials
PEG–PEI was synthesized in-house using previously reported techniques (Liu et al. 2013). The siROCK2 that targeted ROCK2 mRNA in mice and the negative control siRNA (siNC) were purchased from GenePharma (Shanghai, China). A 3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) kit was purchased from Sigma Aldrich (St. Louis, MO, USA). Aβ42 peptide, kinase inhibitor LY294002, and ROCK inhibitor Y-27632 were obtained from Sigma Aldrich (St. Louis, MO, USA). Antibodies to ROCK2, Akt, and phospho-Akt (Ser473) were purchased from Cell Signaling (Beverly, MA, USA).
Cell Culture and Treatment
Primary cortical neurons were prepared from E16 mouse embryonic brains. The cortexes were plated at 2 × 105 cells/ml on poly-d-lysine-coated plates and maintained in neurobasal medium supplemented with B27 (Invitrogen, Carlsbad, California, USA) and 25 nM glutamine at 37 °C in a humidified 5 % CO2 incubator. The purity of neurons exceeded 90 %. Aβ42 oligomers were prepared as previously described by Manterola et al. (2013). The cultured primary neurons at day 7 were incubated with PEG–PEI/siROCK2, PEG–PEI/siNC or saline treatment for 24 h, followed by treatment with Aβ42 oligomers for 24 h.
The cells in primary culture were first treated with several concentrations of Aβ42 oligomers (0, 1, 2, 5, and 10 μM) for 24 h to explore the appropriate toxic concentration. To observe the protective effect of PEG–PEI/siROCK2, the neurons were pretreated with several concentrations of PEG–PEI/siROCK2 (50, 100, and 200 nM) or ROCK inhibitor Y-27632 (final concentration 10 μM) for 24 h, followed by co-treatment with 5 μmol/l Aβ42 oligomers for 24 h. The cells were then carefully washed several times with phosphate-buffered saline (PBS). We evaluated the protective mechanism of PEG–PEI/siROCK2 by co-administering a phosphoinositide 3-kinase (PI3K) inhibitor, LY294002 (final concentration 10 μM), with PEG–PEI/siROCK2 to the primary culture.
Preparation of PEG–PEI/siROCK2 Complexes and In Vitro Transfection
PEG–PEI/siROCK2 complexes were synthesized and characterized as described previously (Wen et al. 2014). The specific N/P ratio (molar ratio of PEG–PEI amino groups/siRNA phosphate groups) was 50. The solution was stirred for 30 min at room temperature prior to use. The particle size and size distribution of PEG–PEI/siROCK2 complexes were characterized. The mean hydrodynamic diameter and polydispersity index were assessed by dynamic light scattering using a 90 Plus particle size analyzer (Brookhaven Instruments Corporation, Holtsville, NY, USA). The primary cortical neurons were seeded and transfected with PEG–PEI/siROCK2, PEG–PEI/siNC, siROCK2, or the same volumes of PBS for 24 h, and the transfection was conducted according to our previously described method.
Cell Viability Assays
The cell viability was determined with a conventional MTT assay. After treatment, the primary cortical neurons were incubated with 0.5 mg/ml MTT for 4 h at 37 °C. The MTT solution was removed, and the resulting formazan crystals were dissolved in 100 μl of dimethyl sulfoxide (DMSO). The absorption was measured at 570 nm with a reference wavelength of 630 nm. The cell survival rates are expressed as percentages of the value of normal cells.
Apoptotic Assay by Flow Cytometry
The primary cortical neurons were centrifuged to remove the medium and then washed with PBS and stained with Annexin V-FITC and propidium iodide (PI) using the Annexin V-FITC Apoptosis Detection Kit (BD Biosciences, San Jose, CA, USA) according to the manufacturer’s instructions. Briefly, the primary cortical neurons were harvested and washed with PBS after treatment. The neurons were then re-suspended in 400 µL of binding buffer. Next, 5 µl of Annexin V-FITC and 1 µl of PI were added. The flow cytometric analysis was performed immediately after supravital staining. The data were acquired and analyzed on a flow cytometer (FACSCalibur, BD Biosciences) using the CellQuest software. The apoptotic rate (%) was quantified with flow cytometry, and viable cells were negative for both Annexin V-PE and PI.
Western Blot Analysis
The total amount of protein was extracted using Proteo JET™ mammalian cell lysis reagent (Formentas Life Science) with phenylmethanesulfonyl fluoride (PMSF). The concentrations of these reagents were determined using a BCA-100 Protein Quantitative Analysis Kit (Biocolors, Shanghai, China). The protein samples were separated by SDS-PAGE and transferred onto polyvinylidene difluoride (PVDF) membranes. The membranes were blocked using 5 % skim milk in 1× Tris-buffered saline and then incubated at 4 °C overnight with respective primary antibodies to anti-ROCK2 (1:1000), Akt (1:1000), p-Akt (1:1000), or β-actin (internal control, 1:2000). After being washed three times with TBST, the membranes were incubated with horseradish peroxidase (HRP)-conjugated goat anti-rabbit antibody (1:3000) for 2 h at room temperature. The antibody-reactive bands were visualized using enhanced chemiluminescence detection reagents and imaged using a UV imaging system.
Statistical Analysis
All experiments were repeated at least three times, and the data were statistically analyzed using the SPSS 16.0 software. Significant differences between groups were assessed with one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison tests. The results are presented as the mean ± standard deviation (SD), and a P value of <0.05 was considered statistically significant.
Results
Aβ42-Induced Neurotoxicity in Primary Neuron Cells
We first investigated the dose–response of primary cortical neurons to Aβ42 oligomers. As shown in Fig. 1a, exposing neurons to Aβ42 oligomers for 24 h dose-dependently reduced the neuronal viability; 2, 5, and 10 µM Aβ42 oligomers significantly reduced the viability. We selected 5 µM as the effective concentration of Aβ42 oligomers in the subsequent experiments unless otherwise indicated.
Fig. 1.
The neurotoxicity of Aβ42 and the characterization of PEG–PEI. a Aβ42-induced neurotoxicity in primary neuron cells. Neuronal cells were exposed to different concentrations of Aβ42 for 24 h, and the cell viability was estimated with an MTT assay. b Size distribution of PEG–PEI/siSNCA complexes at N/P = 50 ratio measured with a Zeta-Plus instrument. The data are presented as the mean ± SD, **P < 0.01, compared with the control
Synthesis and Characterization of PEG–PEI/siROCK2
The size distribution of PEG–PEI/siROCK2 complexes at an N/P ratio of 50 is shown in Fig. 1b. The mean diameter of the complexes was approximately 100 nm, and the extreme values of the distribution may represent particles that did not complex well.
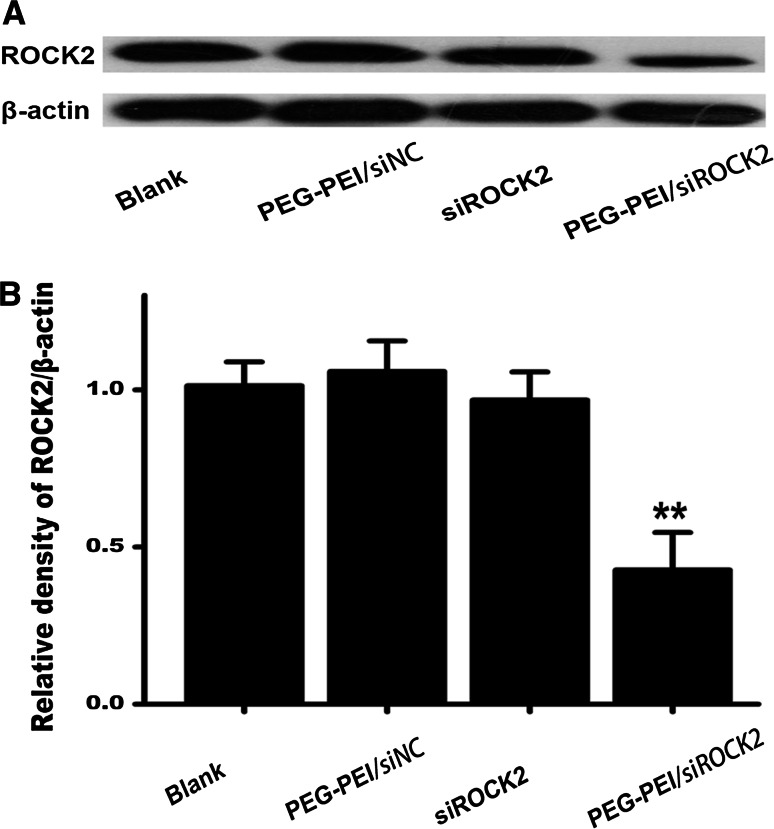
Gene-Silencing Effect of PEG–PEI/siROCK2 in Primary Cortical Neurons
A western blotting analysis was conducted to verify the gene-silencing effect of PEG–PEI/siROCK2 (100 nM) in primary cortical neurons. As shown in Fig. 2a, b, PEG–PEI/siROCK2 significantly decreased the ROCK2 protein expression in primary cortical neurons (P < 0.01).
Fig. 2.
Gene-silencing effect of PEG–PEI/siROCK2 in primary cortical neurons. a The gene-silencing effect of PEG–PEI/siROCK2 (100 nM) in primary cortical neurons was detected by western blotting. b The relative density of the corresponding western blotting bands was determined using the Image-J software. The blank control was treated with PBS, and siNC was a negative nonsense sequence of siROCK2. Values are mean ± SD, **P < 0.01, compared with the blank group
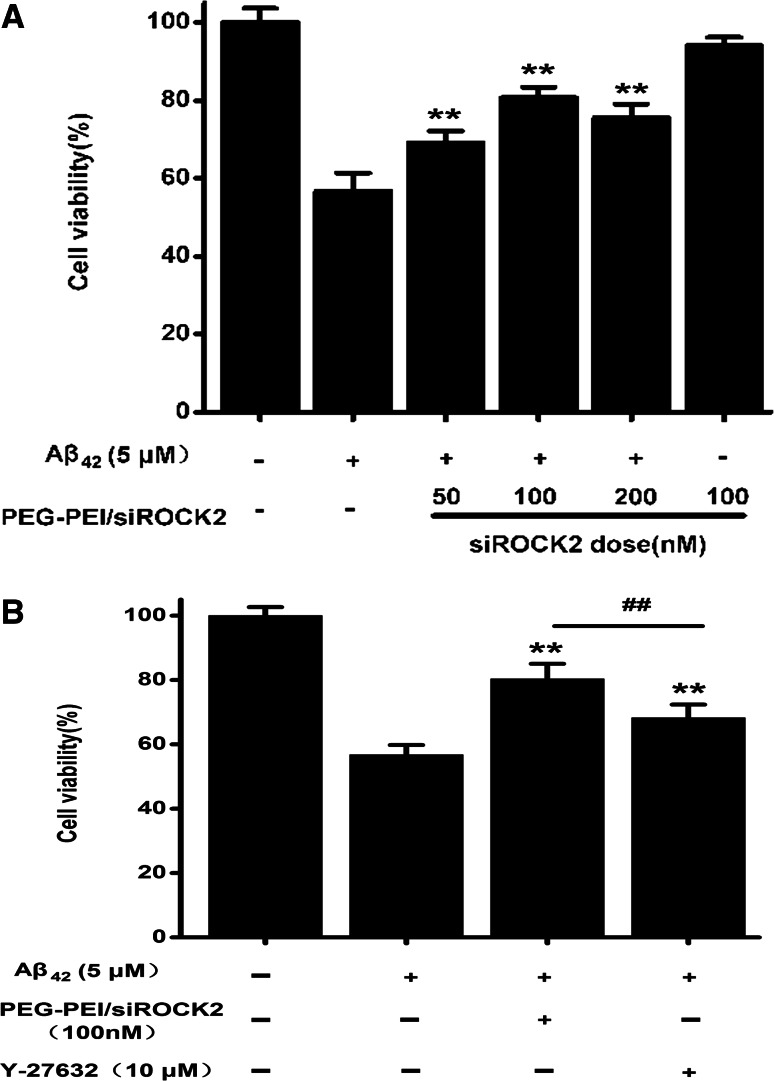
Effect of PEG–PEI/siROCK2 on Aβ42-Induced Neurotoxicity
To determine the neuroprotective effect of PEG–PEI/siROCK2 in response to Aβ42-induced neurotoxicity, primary cortical neurons were pretreated with PEG–PEI/siROCK2 for 24 h and then exposed to 5 µM Aβ42 oligomers for 24 h. The cell viability decreased to 56.7 % of the control after treatment with Aβ42, while the viability of neurons pretreated with PEG–PEI/siROCK2 (100 nM) for 24 h prior to Aβ42 oligomer exposure reached 80.7 % (Fig. 3a). To compare these results with the neuroprotective effect of the ROCK inhibitor Y-27632 on Aβ42-induced neurotoxicity, primary cortical neurons were pretreated with Y-27632 (10 μM) or PEG–PEI/siROCK2 (100 nM). The addition of the ROCK inhibitor Y-27632 (10 μM) to the culture medium significantly increased the number of MTT-positive cells (68.3 %). However, the neuroprotective effect of the Y-27632 treatment was less pronounced than that of PEG–PEI/siROCK2. These results imply that PEG–PEI/siROCK2 markedly protected neurons from Aβ42-induced neurotoxicity.
Fig. 3.
In vitro transfection and protective effects of PEG–PEI/siROCK2 on Aβ42-induced neurotoxicity in primary cortical neurons. a PEG–PEI/siROCK2 protected primary cortical neurons from Aβ42-induced cytotoxicity. b The comparison of the ability of ROCK inhibitor Y-27632 (10 μM) and PEG–PEI/siROCK2 (100 nM) to protect cells from Aβ42-induced neurotoxicity. The cell viability was measured with an MTT assay. All data shown represent the mean ± SD. **P < 0.01, compared with Aβ42-treated group. ## P < 0.01, compared with Aβ42 + PEG/PEI/siROCK2-treated group
PEG–PEI/siROCK2 Protects Neurons Against Aβ42-Induced Apoptosis
To further confirm the ability of PEG–PEI/siROCK2 to protect cells from Aβ42 oligomer-mediated neurotoxicity, neuronal apoptosis was detected by Annexin V and PI staining. As shown in Fig. 4, cell apoptosis was demonstrated to be 24.06 ± 1.67 % in Aβ42-treated neurons, and this rate was significantly higher than that of the control. Treatment with PEG–PEI/siROCK2 significantly attenuated this rate (9.38 ± 3.06 %).
Fig. 4.
Effects of PEG–PEI/siROCK2 on cell apoptosis induced by Aβ42 assessed by flow cytometry. a Percentage of apoptotic cells in the control group and Aβ42 group. b Quantification of the percentage of apoptotic cells after exposure to Aβ42 in the absence or presence of PEG–PEI/siROCK2. The data are presented as the mean ± SD, **P < 0.01 versus the control group, ## P < 0.01 versus Aβ42 + control group and Aβ42 + siNC group
PEG–PEI/siROCK2 Protects Against Aβ42-Induced Neurotoxicity by Upregulating The Activity of the PI3K/Akt Pathway
To identify the signal transduction pathways that may be involved in the protective effect of PEG–PEI/siROCK2 against Aβ42-induced neurotoxicity, we examined the activation of Akt, which is a major signaling enzyme involved in the protection of cells from apoptosis. As shown in Fig. 5a, exposure to Aβ42 oligomers decreased the phosphorylation of Akt (p-Akt), while pre-treatment with PEG–PEI/siROCK2 significantly increased the level of p-Akt (P < 0.01). We also examined the effects on the PI3K/Akt pathway by administering the specific PI3K inhibitor LY294002 in conjunction with PEG–PEI/siROCK2 to the primary culture. We found that LY294002 partly blocked p-Akt expression, as shown in Fig. 5b. Furthermore, we performed MTT and annexin V analyses on cortical neurons treated with PEG–PEI/siROCK2 and Aβ42 in the presence or absence of the PI3K inhibitor. As shown in Fig. 6a, LY294002 significantly reduced the viability (P < 0.01). The annexin V analysis revealed that LY294002 significantly increased apoptosis. These findings suggest that LY294002 could attenuate the neuroprotective effects of PEG–PEI/siROCK2 against Aβ42-induced neurotoxicity in primary cortical neurons.
Fig. 5.
PEG–PEI/siROCK2 protects against Aβ42-induced neurotoxicity by upregulating p-Akt/Akt. a Pre-treatment with PEG–PEI/siROCK2 significantly increased the expression of p-Akt. b Pre-treatment with both PEG–PEI/siROCK2 and LY294002 reduced the ratio of p-Akt/Akt. The data are presented as the mean ± SD. **P < 0.01, compared with the Aβ42 group. ## P < 0.01, compared with the Aβ42 + siROCK2 group
Fig. 6.
LY294002 attenuated the neuroprotective effects of PEG–PEI/siROCK2. a MTT analysis of cortical neurons treated with PEG–PEI/siROCK2 in the presence or absence of LY294002. b Annexin V analysis of cortical neurons treated with PEG–PEI/siROCK2 in the presence or absence of LY294002. Data are reported as the mean ± SD. **P < 0.01, compared with the siROCK2 group
Discussion
Aβ is a short peptide that originates from the sequential proteolytic cleavage of Aβ precursor protein, and its most common isoforms are Aβ40 and Aβ42 (Zhou et al. 2014). The accumulation of Aβ42 peptides in plaques has been strongly associated with the progression of AD (Lambracht-Washington et al. 2011). The neurotoxicity of Aβ in AD is associated with apoptosis (Napolitano et al. 2014). Our study demonstrated that the Aβ42 is toxic to primary cortical neurons, and this cytotoxicity was dose dependent. Considering the important role of Aβ oligomers in AD-related neuronal cell death, exploring neuroprotective therapies that target Aβ-induced neurotoxicity may offer an effective treatment strategy for AD (Santos et al. 2012).
A previous study showed that fasudil reduced hippocampal neuronal damage and improved cognitive impairment in an Aβ-induced AD rat model, which suggested that ROCK might regulate Aβ-induced neurotoxicity in AD (Song et al. 2013). ROCK is believed to play a critical role in the regulation of cell migration, cell proliferation, stress fiber formation, and smooth muscle contraction (Amano et al. 2000). The present findings reveal that Aβ42-induced cell death was remarkably reduced in the presence of PEG–PEI/siROCK2, and this neuroprotective effect was better than that of Y27632. This difference may be because PEG–PEI/siROCK2 is a more efficient and specific gene therapy strategy than Y27632. These results suggest that siRNA target ROCK2 might have the potential to be used in AD therapeutic.
The most significant challenge to the implementation RNAi therapy is a lack of an efficient gene delivery vector that maintains efficient delivery without inducing significant toxicity and other adverse effects. The use of viral vectors in human therapy raises several concerns, such as an increased immune response, the possible recombination of oncogenes, and the difficulty of scale-up (Fornaguera et al. 2014). However, the reduced immune response, low toxicity, and high safety of non-viral vectors have garnered considerable interest (Puras et al. 2014). PEI is one of the most widely used cationic polymers that can directly coat a gene-expressing plasmid and is often considered the gold standard of gene transfection (Yao et al. 2014). Various coatings, such as polyethylene glycol, allow the functional properties of the nanoparticles to be customized to a number of clinical applications (Murray et al. 2013). A previous study showed that siROCK2 delivered with a non-viral vector consisting of PEG engrafted onto PEI exhibited a remarkable potential for the treatment of AD (Liu et al. 2013). Prior to conducting biological experiments, the characteristics of the PEG–PEI/siROCK2 nanoparticles need to be evaluated. Our results revealed that PEG–PEI fully condensed siROCK2 at an N/P ratio of 50:1, and the particle size was approximately 100 nm. The optimal particle size for a targeting nanocarrier has been suggested to range from 20 to 200 nm, which is sufficiently large to avoid filtration but small enough to penetrate through the cell membrane (Zhao et al. 2014). The results of the western blotting analyses demonstrated that PEG–PEI/siROCK2 complexes effectively silenced the ROCK2 gene to suppress protein expression in primary cortical neurons, and this gene silencing was more effective than that promoted by naked siROCK2 or PEG–PEI/siNC. PEG–PEI nanoparticles that deliver siRNA-targeting ROCK2 are efficient for transfection and may be used as an AD therapeutic.
The mechanism underlying the role of ROCK2 in the reduction of Aβ42-induced neurotoxicity in primary cortical neurons remains unknown. A previous study demonstrated that the Akt kinase signaling pathway contributes to the effect of ROCK inhibition on neurite elongation and RGCs survival (Lingor et al. 2008). PI3K/Akt signaling pathways play an important role in the protection of ROCK inhibition to reduce ischemia–reperfusion injury, and a PI3K/Akt inhibitor partly abolished this protection (Li et al. 2012). The regulation of PI3K/Akt activity by Rho GTPases is complex, and RhoA signaling via ROCK can either activate or downregulate PI3K/Akt signaling depending on the cell type and cellular context (Bourguignon et al. 2003; Li et al. 2005). The PI3K/Akt signaling pathway plays a crucial role in cell survival and apoptosis (Schorey and Cooper 2003). Akt is a main effector in the PI3K signaling pathway, and the activity of Akt blocks the mitochondrial apoptotic pathway (Datta et al. 1997). The PI3K/Akt signaling pathway plays a crucial role in neuronal survival, and the activation of PI3K/Akt has previously been shown to prevent Aβ-induced neuronal death (Lou et al. 2011). Our results showed that PEG–PEI/siROCK2 could increase the level of p-Akt to prevent Aβ42-induced cell death, and treatment with the specific PI3K inhibitor LY294002 attenuated the upregulation of p-AKT by PEG–PEI/siROCK2. In addition, LY294002 significantly but not completely reversed the neuroprotective effect of PEG–PEI/siROCK2, which implies that other mechanisms in addition to PI3K/Akt may be involved in the ROCK2-mediated reduction of Aβ42-induced neurotoxicity in primary cortical neurons. Further work is needed to clarify the mechanisms of ROCK2 in reducing Aβ42-induced neurotoxicity in detail.
In summary, this study demonstrates that PEG–PEI/siROCK2 is effective in reducing the neurotoxicity induced by Aβ42 in primary cortical neurons. We further concluded that the activation of PI3K/Akt is involved in this protection. However, our studies did not identify the specific mechanism by which PEG–PEI/siROCK2 activates the PI3K/Akt pathway. A recent study suggested that PTEN may be involved in the interaction between ROCK and the PI3K/AKT survival pathway (Fusella et al. 2014). Overall, the mechanisms remain poorly understood and require further study.
Acknowledgments
This work was supported by the Medical Scientific Research Foundation of Guangdong Province, China (B2014169), and Natural Science Foundation of Guangdong Province, China (S2013010014550 and 2014A030310352). We thank the Center of Biomedical Engineering of Sun Yat-sen University for assisting in the preparation of nanoparticles.
Conflict of interest
The authors have no conflict of interest.
Footnotes
Yunyun Liu and Xingyi Yang have contributed equally to this work.
Contributor Information
Zhong Li, Email: zslylizhong@163.com.
Zhonglin Liu, Email: zhonglinliu@126.com.
References
- Amano M, Fukata Y, Kaibuchi K (2000) Regulation and functions of Rho-associated kinase. Exp Cell Res 261(1):44–51 [DOI] [PubMed] [Google Scholar]
- Bourguignon LY, Singleton PA, Zhu H, Diedrich F (2003) Hyaluronan-mediated CD44 interaction with RhoGEF and Rho kinase promotes Grb2-associated binder-1 phosphorylation and phosphatidylinositol 3-kinase signaling leading to cytokine (macrophage-colony stimulating factor) production and breast tumor progression. J Biol Chem 278(32):29420–29434 [DOI] [PubMed] [Google Scholar]
- Chen TJ, Hung HS, Wang DC, Chen SS (2010) The protective effect of Rho-associated kinase inhibitor on aluminum-induced neurotoxicity in rat cortical neurons. Toxicol Sci 116(1):264–272 [DOI] [PubMed] [Google Scholar]
- Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME (1997) Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 91(2):231–241 [DOI] [PubMed] [Google Scholar]
- Fornaguera C, Grijalvo S, Galan M, Fuentes-Paniagua E, de la Mata FJ, Gomez R, Eritja R, Caldero G, Solans C (2014) Novel non-viral gene delivery systems composed of carbosilane dendron functionalized nanoparticles prepared from nano-emulsions as non-viral carriers for antisense oligonucleotides. Int J Pharm 478(1):113–123 [DOI] [PubMed] [Google Scholar]
- Fu Q, Gao N, Yu J, Ma G, Du Y, Wang F, Su Q, Che F (2014) Diazoxide pretreatment prevents Abeta1-42 induced oxidative stress in cholinergic neurons via alleviating NOX2 expression. Neurochem Res 39(7):1313–1321 [DOI] [PubMed] [Google Scholar]
- Fusella F, Ferretti R, Recupero D, Rocca S, Di Savino A, Tornillo G, Silengo L, Turco E, Cabodi S, Provero P et al (2014) Morgana acts as a proto-oncogene through inhibition of a ROCK-PTEN pathway. J Pathol 234(2):152–163 [DOI] [PubMed] [Google Scholar]
- Glat MJ, Offen D (2013) Cell and gene therapy in Alzheimer’s disease. Stem Cells Dev 22(10):1490–1496 [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ (2002) The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297(5580):353–356 [DOI] [PubMed] [Google Scholar]
- Herskowitz JH, Feng Y, Mattheyses AL, Hales CM, Higginbotham LA, Duong DM, Montine TJ, Troncoso JC, Thambisetty M, Seyfried NT et al (2013) Pharmacologic inhibition of ROCK2 suppresses amyloid-beta production in an Alzheimer’s disease mouse model. J Neurosci 33(49):19086–19098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kou Z, Wang X, Yuan R, Chen H, Zhi Q, Gao L, Wang B, Guo Z, Xue X, Cao W et al (2014) A promising gene delivery system developed from PEGylated MoS2 nanosheets for gene therapy. Nanoscale Res Lett 9(1):587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambracht-Washington D, Qu BX, Fu M, Anderson LJ, Stuve O, Eagar TN, Rosenberg RN (2011) DNA immunization against amyloid beta 42 has high potential as safe therapy for Alzheimer’s disease as it diminishes antigen-specific Th1 and Th17 cell proliferation. Cell Mol Neurobiol 31(6):867–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Dong X, Wang Z, Liu W, Deng N, Ding Y, Tang L, Hla T, Zeng R, Li L et al (2005) Regulation of PTEN by Rho small GTPases. Nat Cell Biol 7(4):399–404 [DOI] [PubMed] [Google Scholar]
- Li Y, Zhu W, Tao J, Xin P, Liu M, Li J, Wei M (2012) Fasudil protects the heart against ischemia-reperfusion injury by attenuating endoplasmic reticulum stress and modulating SERCA activity: the differential role for PI3K/Akt and JAK2/STAT3 signaling pathways. PLoS One 7(10):e48115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y, Liu Z, Shuai X, Wang W, Liu J, Bi W, Wang C, Jing X, Liu Y, Tao E (2012) Delivery of cationic polymer-siRNA nanoparticles for gene therapies in neural regeneration. Biochem Biophys Res Commun 421(4):690–695 [DOI] [PubMed] [Google Scholar]
- Lingor P, Tonges L, Pieper N, Bermel C, Barski E, Planchamp V, Bahr M (2008) ROCK inhibition and CNTF interact on intrinsic signalling pathways and differentially regulate survival and regeneration in retinal ganglion cells. Brain 131(Pt 1):250–263 [DOI] [PubMed] [Google Scholar]
- Liu Y, Liu Z, Wang Y, Liang YR, Wen X, Hu J, Yang X, Liu J, Xiao S, Cheng D (2013) Investigation of the performance of PEG-PEI/ROCK-II-siRNA complexes for Alzheimer’s disease in vitro. Brain Res 1490:43–51 [DOI] [PubMed] [Google Scholar]
- Lou H, Fan P, Perez RG, Lou H (2011) Neuroprotective effects of linarin through activation of the PI3K/Akt pathway in amyloid-beta-induced neuronal cell death. Bioorg Med Chem 19(13):4021–4027 [DOI] [PubMed] [Google Scholar]
- Manterola L, Hernando-Rodriguez M, Ruiz A, Apraiz A, Arrizabalaga O, Vellon L, Alberdi E, Cavaliere F, Lacerda HM, Jimenez S et al (2013) 1-42 beta-amyloid peptide requires PDK1/nPKC/Rac 1 pathway to induce neuronal death. Transl Psychiatry 3:e219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray AR, Kisin E, Inman A, Young SH, Muhammed M, Burks T, Uheida A, Tkach A, Waltz M, Castranova V et al (2013) Oxidative stress and dermal toxicity of iron oxide nanoparticles in vitro. Cell Biochem Biophys 67(2):461–476 [DOI] [PubMed] [Google Scholar]
- Napolitano M, Costa L, Piacentini R, Grassi C, Lanzone A, Gulino A (2014) 17beta-Estradiol protects cerebellar granule cells against beta-amyloid-induced toxicity via the apoptotic mitochondrial pathway. Neurosci Lett 561:134–139 [DOI] [PubMed] [Google Scholar]
- Nilsson P, Iwata N, Muramatsu S, Tjernberg LO, Winblad B, Saido TC (2010) Gene therapy in Alzheimer’s disease—potential for disease modification. J Cell Mol Med 14(4):741–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puras G, Mashal M, Zarate J, Agirre M, Ojeda E, Grijalvo S, Eritja R, Diaz-Tahoces A, Martinez NG, Aviles-Trigueros M et al (2014) A novel cationic niosome formulation for gene delivery to the retina. J Control Release 174:27–36 [DOI] [PubMed] [Google Scholar]
- Santos AN, Ewers M, Minthon L, Simm A, Silber RE, Blennow K, Prvulovic D, Hansson O, Hampel H (2012) Amyloid-beta oligomers in cerebrospinal fluid are associated with cognitive decline in patients with Alzheimer’s disease. J Alzheimers Dis 29(1):171–176 [DOI] [PubMed] [Google Scholar]
- Schorey JS, Cooper AM (2003) Macrophage signalling upon mycobacterial infection: the MAP kinases lead the way. Cell Microbiol 5(3):133–142 [DOI] [PubMed] [Google Scholar]
- Song Y, Chen X, Wang LY, Gao W, Zhu MJ (2013) Rho kinase inhibitor fasudil protects against beta-amyloid-induced hippocampal neurodegeneration in rats. CNS Neurosci Ther 19(8):603–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Cheang T, Tang B, Xia H, Xing Z, Chen Z, Fang Y, Chen W, Xu A, Wang S et al (2013) The inhibition of human bladder cancer growth by calcium carbonate/CaIP6 nanocomposite particles delivering AIB1 siRNA. Biomaterials 34(4):1246–1254 [DOI] [PubMed] [Google Scholar]
- Wen X, Wang L, Liu Z, Liu Y, Hu J (2014) Intracranial injection of PEG-PEI/ROCK II-siRNA improves cognitive impairment in a mouse model of Alzheimer’s disease. Int J Neurosci 124(9):697–703 [DOI] [PubMed] [Google Scholar]
- Yao X, Zhou N, Wan L, Su X, Sun Z, Mizuguchi H, Yoshioka Y, Nakagawa S, Zhao RC, Gao JQ (2014) Polyethyleneimine-coating enhances adenoviral transduction of mesenchymal stem cells. Biochem Biophys Res Commun 447(3):383–387 [DOI] [PubMed] [Google Scholar]
- Zhao X, Cui H, Chen W, Wang Y, Cui B, Sun C, Meng Z, Liu G (2014) Morphology, structure and function characterization of PEI modified magnetic nanoparticles gene delivery system. PLoS One 9(6):e98919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F, Xu Y, Hou XY (2014) MLK3-MKK3/6-P38MAPK cascades following N-methyl-D-aspartate receptor activation contributes to amyloid-beta peptide-induced apoptosis in SH-SY5Y cells. J Neurosci Res 92(6):808–817 [DOI] [PubMed] [Google Scholar]