Abstract
Venezuelan equine encephalitis virus (VEE) is an important equine and human pathogen of the Americas. In the adult mouse model, cDNA-derived, virulent V3000 inoculated subcutaneously (s.c.) causes high-titer peripheral replication followed by neuroinvasion and lethal encephalitis. A single change (G to A) at nucleotide 3 (nt 3) of the 5′ untranslated region (UTR) of the V3000 genome resulted in a virus (V3043) that was avirulent in mice. The mechanism of attenuation by the V3043 mutation was studied in vivo and in vitro. Kinetic studies of virus spread in adult mice following s.c. inoculation showed that V3043 replication was reduced in peripheral organs compared to that of V3000, titers in serum also were lower, and V3043 was cleared more rapidly from the periphery than V3000. Because clearance of V3043 from serum began 1 to 2 days prior to clearance of V3000, we examined the involvement of alpha/beta interferon (IFN-α/β) activity in VEE pathogenesis. In IFN-α/βR−/− mice, the course of the wild-type disease was extremely rapid, with all animals dying within 48 h (average survival time of 30 h compared to 7.7 days in the wild-type mice). The mutant V3043 was as virulent as the wild type (100% mortality, average survival time of 30 h). Virus titers in serum, peripheral organs, and the brain were similar in V3000- and V3043-infected IFN-α/βR−/− mice at all time points up until the death of the animals. Consistent with the in vivo data, the mutant virus exhibited reduced growth in vitro in several cell types except in cells that lacked a functional IFN-α/β pathway. In cells derived from IFN-α/βR−/− mice, the mutant virus showed no growth disadvantage compared to the wild-type virus, suggesting that IFN-α/β plays a major role in the attenuation of V3043 compared to V3000. There were no differences in the induction of IFN-α/β between V3000 and V3043, but the mutant virus was more sensitive than V3000 to the antiviral actions of IFN-α/β in two separate in vitro assays, suggesting that the increased sensitivity to IFN-α/β plays a major role in the in vivo attenuation of V3043.
Venezuelan equine encephalitis virus (VEE) is a member of the Alphavirus genus in the Togaviridae family. The genome of this enveloped virus is a single-stranded, messenger-sense RNA molecule of approximately 11.5 kb (24), capped at the 5′ end and polyadenylated at the 3′ end. The genomic RNA encodes four nonstructural proteins (nsP1 through -4) and three structural proteins (capsid and two envelope glycoproteins, E1 and E2). The 5′ untranslated region (UTR) in VEE is 45 nucleotides (nt) long, and although its sequence is not conserved among alphaviruses, the sequence predicts a stem-loop structure that is conserved across the Alphavirus genus (55). It has been proposed that the complementary sequence at the 3′ end of the minus strand also folds into a conserved secondary structure that may play a role as a promoter for the initiation of genome RNA synthesis from the minus-strand template (10, 38, 55). The nonstructural proteins are translated directly from the genomic RNA as a polyprotein that is cleaved by a viral protease to produce the enzyme complex necessary for RNA replication. Thus, the 5′ UTR is likely involved both in translation of genomic mRNA and in initiation of plus-strand RNA synthesis through interactions with host and/or viral proteins. The structural proteins are expressed from an abundant 26S subgenomic mRNA as a polyprotein. The precursor is cleaved into the capsid protein that assembles to form the T=4 nucleocapsid, as well as into two transmembrane glycoproteins, E1 and E2, that are arranged within the viral envelope in a T=4 icosahedral lattice (39, 50).
VEE is an arthropod-borne virus that has been associated with periodic epidemics and equine epizootics in the Western Hemisphere since the 1920s. These epidemics are associated with high mortality (19 to 83%) and severe morbidity in equines and with up to tens of thousands of human cases, with a case mortality rate of around 1% (21, 46, 61). Recent studies indicate that epizootic viruses can evolve from enzootic equine-avirulent strains that circulate continuously among wild rodents and Culex melanoconio mosquitoes in lowland tropical forests (43, 60). The mechanism of emergence and the molecular determinants of natural equine virulence have been examined recently but are not well understood (34, 42).
Experimental infection of the adult mouse with VEE closely parallels the biphasic disease seen in horses (12). In the mouse, subcutaneous (s.c.) inoculation with VEE is followed by initial viral replication in the draining lymph nodes (DLN), viral spread to all of the major lymphoid organs, and a significant viremia. This peripheral infection is cleared by 3 to 4 days postinfection (p.i.). In the second phase, VEE invades the central nervous system first through the olfactory and trigeminal nerves, replicating predominantly in neurons and causing lethal encephalitis (6, 13).
Molecular determinants of VEE virulence in the mouse model have been studied using cDNA clones of wild-type virulent VEE and tissue culture-adapted, attenuated strains, including the investigational live-attenuated vaccine strain TC-83 (2, 9, 13, 23, 40, 51). TC-83 was derived by serial passage of the virulent epizootic VEE Trinidad donkey strain (VEE-TRD) in guinea pig heart cells (3). Comparison of the nucleotide sequences of TRD and TC-83 revealed 12 nucleotide changes. Mouse virulence studies of chimeric TRD–TC-83 viruses suggested that two changes were associated with the attenuated phenotype, a G-to-A substitution at nt 3 in the 5′ UTR and an E2-120 Thr-to-Arg mutation (23). It was suggested that the 5′ UTR and the E2-120 mutations represent two independent loci that act synergistically in the attenuation of TC-83. The contributions of several individual mutations in E1 and E2 glycoproteins to VEE virulence in mice have been well characterized (4, 9, 13). However, the role of the 5′ UTR in the attenuation of VEE has not been addressed separately.
The involvement of alpha/beta interferon (IFN-α/β) in the virulence of VEE for hamsters and mice was first proposed more than 25 years ago, when pairs of virulent and benign isolates were compared with respect to their sensitivity to IFN-α/β in cultures of hamster cells or in vivo in hamsters (20, 22). These early studies showed a correlation between virulence and relative resistance to IFN-α/β. More recently, the IFN-α/β sensitivity of TC-83 and 24 enzootic and epizootic isolates of VEE were compared in a tissue culture cytopathic effect (CPE) reduction assay (54). The IFN-α/β resistance or sensitivity phenotype correlated with the epizootic or enzootic potential, respectively, supporting the idea that one of the adaptations leading to the emergence of epizootic strains is the evolution of resistance to IFN-α/β (54). The IFN-α/β sensitivity or resistance phenotype of chimeric viruses containing the 5′ UTR and nonstructural genes from either enzootic (sensitive) or epizootic (resistant) strains of VEE seems to segregate with the 5′ UTR and nonstructural genes of the parental strain, even though it does not segregate with genes conferring guinea pig virulence (42).
Viral double-stranded RNA (dsRNA) replication intermediates are major inducers of the IFN-α and IFN-β genes and cofactors in the activation of IFN-induced antiviral enzymes (49, 56, 62). Since cis-acting sequences involved in the regulation of alphavirus RNA replication are in the 5′ UTR (55), it is possible that virulence determinants in the 5′ UTR of the VEE genome might act through either a direct effect on viral replication and yield and/or an indirect effect on early host responses, such as IFN-α/β.
Noncoding regions of many viral genomes carry virulence determinants, including picornaviruses, influenza virus, retroviruses, and other alphaviruses (1, 10, 36, 41, 44). For the prototype alphavirus, Sindbis virus, 5′ UTR mutants typically show defects in RNA accumulation and altered growth rates in cell culture (37). A single nucleotide change in the 5′ UTR of Sindbis virus confers neurovirulence in rats (26). However, the pathogenic mechanism at the molecular level has not been elucidated for these alphavirus noncoding viral determinants.
In this study, the mutation in the 5′ UTR of the TC-83 genome was evaluated as to its effect on mouse virulence. A single change (G to A) in nt 3 of the 5′ UTR of the virulent V3000 genome resulted in a virus, V3043, that was avirulent in mice and had reduced growth in cell culture. In delineating the mechanism of attenuation in vivo and in vitro, two possibilities were considered: an intrinsic defect in viral replication and an altered interaction with the innate immune response. Full-length cDNA clones of the virulent parent (V3000) and single-site mutant (V3043) genomes were used as genetically stable sources of homogeneous virus for infections of mice with a targeted disruption of the IFN-α/β receptor gene and infections of cells derived from these mice (35). In the absence of a functional IFN-α/β pathway, V3043 replicated like V3000 in vivo and in vitro. Our results demonstrated that IFN-α/β plays a major role in the pathogenesis of V3000 and in the attenuated phenotype of the 5′ UTR mutant V3043. One mechanism is an increased sensitivity of V3043 to the antiviral actions of IFN-α/β.
MATERIALS AND METHODS
Viruses.
The construction of the full-length cDNA clone pV3000, derived from the natural VEE isolate, TRD, has been described previously (9). Nucleotide 3 of the 5′ UTR was changed from G to A by site-directed mutagenesis. The 500-bp XbaI-RsrII fragment of pV3000 was replaced with the mutant fragment to produce pV3043, and the sequence of the replaced fragment was confirmed. To produce virus stocks, pV3000 and pV3043 were transcribed in vitro and the infectious RNA was electroporated into baby hamster kidney cells (BHK-21; ATCC CCL-10) as described previously (8). Virus particles were harvested from the supernatant at 24 h p.i. (hpi) when significant CPE was produced and were clarified by centrifugation (10,000 × g, 30 min, 4°C) and stored as single-use aliquots at −70°C. Virus titers were determined by a standard plaque assay on BHK-21 cells. For some experiments, virus stocks were concentrated by pelleting the clarified virus preparations through 20% (wt/vol) sucrose in low-endotoxin phosphate-buffered saline (PBS) at 71,934 × g for 5 h at 4°C.
VEE replicon particles (VRP) expressing green fluorescent protein (GFP) were packaged using a split helper system as described previously (45). The wild-type replicon plasmid pV5005 contained the gene for GFP mutant 2 (7) directly downstream of the 26S promoter, in place of the VEE structural protein genes (31). A mutant replicon genome containing the nt 3 G-to-A (nt3A) mutation, pV5505, was constructed by substituting the 546-bp XbaI-RsrII fragment from V3043 for the equivalent fragment in the pV5005 plasmid and confirmed by sequencing. Wild-type and mutant VRP were produced by coelectroporation into BHK-21 cells of RNA transcripts from pV5005 or pV5505, respectively, with two VEE helper transcripts expressing either the capsid or glycoprotein genes, as described previously (31). The capsid and glycoprotein helpers were of wild-type origin (V3000); therefore, wild-type and nt3A mutant VRP were denoted V5005-3000 and V5505-3000, respectively. Due to the lack of structural protein genes, infectious VRP undergo only one round of infection. The titers of VRP stocks were determined by the infection of BHK-21 cells with serial dilutions followed by counting the number of GFP-expressing BHK-21 cells by using a fluorescent microscope (Nikon) with a fluorescein isothiocyanate filter set. Titers were expressed as infectious units (IU) per milliliter.
Mouse studies.
Specific-pathogen-free female CD-1 mice were obtained from Charles River Breeding laboratories (Raleigh, N.C.). Breeding pairs of IFN-α/βR+/+ 129Sv/Ev and IFN-α/βR−/− mice were kindly provided by Herbert Virgin (Washington University, St. Louis, Mo.) and Barbara Sherry (North Carolina State University, Raleigh, N.C.), respectively. Mice were bred under specific-pathogen-free conditions in the Department of Laboratory Animal Medicine breeding colony facilities at the University of North Carolina, Chapel Hill.
Mouse studies were performed in an environmentally controlled room in a biosafety level 3 facility. CD-1 mice obtained commercially were acclimatized for 7 days. 129Sv/Ev and IFN-α/βR−/− mice, which were bred in the same building, were acclimatized for 2 to 5 days in the biosafety level 3 laboratory before experimental manipulation. Standard mouse chow and water were provided ad libitum. Mice were between 6 and 8 weeks old when inoculated with virus.
For the morbidity and mortality studies, mice (8 to 11 per experimental group) were anesthetized in a Metofane-saturated chamber and inoculated s.c. in the left rear footpad (LRFP) or intracranially (i.c.) with 103 PFU of virus diluted in endotoxin-free PBS containing 1% donor calf serum (DCS) in a 10-μl volume by using a 27-gauge needle and a 100-μl Hamilton syringe. Mock-infected animals received diluent alone. All mice except for IFN-α/βR−/− mice were observed and weighed every 24 h for 14 days. IFN-α/βR−/− mice were observed every 12 h. The clinical signs of disease included ruffled fur, paresis, ataxia, and/or paralysis. Morbidity was defined as greater than 10% weight loss and/or clinical signs for two or more consecutive days. To confirm a productive infection in the surviving mice, they were challenged with 104 PFU of V3000 inoculated intraperitoneally in a 100-μl volume and were scored for morbidity and mortality for 14 days. All previously inoculated mice survived this lethal challenge without morbidity.
To determine in vivo virus growth kinetics, mice were inoculated s.c. in the LRFP with 103 PFU of virus. At various times postinoculation, three mice per experimental group were anesthetized with Metofane, the thoracic cavity was opened, and blood was collected by cardiac puncture. The serum, separated in microtainer tubes, was aliquoted and stored at −70°C. Each mouse was then perfused with PBS–1% DCS to minimize the presence of blood-associated virus in the organs to be collected. DLN, spleen, thymus, and brain were collected, and PBS–1% DCS supplemented with Ca+ and Mg+ was added to make 20% (brain) or 10% (other organs) (wt/vol) suspensions in Kontes microfuge tubes. Samples were homogenized (Kontes pestles) after one freeze-thaw and clarified by centrifugation at 10,000 × g. Samples were stored at −70°C prior to processing. Virus titers were determined by a standard BHK-21 plaque assay.
Cells and in vitro infections.
BHK-21 cells, murine L929 fibroblasts (ATCC CCL-1), and murine Swiss 3T3 fibroblasts (ATCC CCL-92) were maintained in alpha-minimum essential media (Gibco) supplemented with 10% DCS, 10% tryptose phosphate broth, 0.29 mg of l-glutamine/ml, 100 U of penicillin/ml, and 0.05 mg of streptomycin/ml (37°C, 5% CO2). Primary bone marrow macrophages (BMMΦ) from 129Sv/Ev and IFN-α/βR−/− mice were generated as previously described (17). Briefly, the bone marrow cells were collected by flushing the bone marrow cavity of the femur and tibia bones of cervically dislocated mice with cold growth media. The cells were pelleted by centrifugation (60 × g, 4°C, 8 min) and resuspended in low-endotoxin Dulbecco's modified Eagle's medium (Gibco) supplemented with 10% fetal bovine serum (Gibco), 2 mM l-glutamine, 100 U of penicillin/ml, 0.05 mg of streptomycin/ml, and 20% of L-cell-conditioned media. The cells were seeded onto low-adherent bacterial dishes and allowed to differentiate for 7 to 10 days.
For the in vitro growth curves, the BMMΦ were removed from the low-adhesion dishes by using enzyme-free cell dissociation buffer (Sigma) and then were seeded into 24-well plates at a density of 5 × 105 cells/well. The BHK, L929, and 3T3 cells were seeded into 60-mm-diameter dishes and incubated at 37°C with 5% CO2. When the monolayers were 80 to 90% confluent, the media were removed and the cells were infected at a multiplicity of infection (MOI) of 5 to 12 PFU/cell. After 1 h of adsorption at 37°C, the monolayers were washed three times with PBS–1% DCS at room temperature, and complete growth medium was added to each dish. At different times p.i., 70- or 20-μl aliquots of media were collected and an equal volume of fresh media was added to keep the volume constant. Samples were frozen at −70°C until analysis by plaque assay.
IFN-α/β assay.
The levels of murine IFN-α/β present in serum from infected mouse and in supernatants from infected cell cultures were measured by a standard biological assay on L929 murine fibroblasts as previously described (58). Briefly, L929 cultures in 96-well plates were treated with standard murine IFN-α/β (Lee Biomolecular) (twofold dilutions ranging from 1,000 to 0.49 IU/ml) or twofold dilutions of the experimental samples, previously acid treated and neutralized. After 24 h of IFN-α/β treatment, the cells were infected with 2 × 105 PFU of encephalomyocarditis virus (EMCV)/well in a 50-μl volume and incubated at 37°C for 24 h. The remaining cells were then stained with 1% crystal violet. The percent CPE in each well was scored by direct observation. The IFN-α/β titers (IU/ml) were calculated based on the standard curves generated with commercial IFN-α/β; the end-point titer was calculated from the dilution of IFN-α/β required to protect 50% of the cell monolayer from EMCV-induced CPE.
IFN-α/β sensitivity assay.
The relative sensitivity of V3000 and V3043 to murine IFN-α/β was measured in murine fibroblast L929 cells and Swiss 3T3 cells in a cell viability assay. The cells were maintained in alpha-minimum essential media as described above and used within five passages post-thaw. Cells seeded in 96-well plates were treated with twofold dilutions of standard murine IFN-α/β (Lee Biomolecular) in triplicate in doses ranging from 505 to 0 IU/ml. After 24 h at 37°C, the cells were infected with 2 × 106 PFU of virus (V3000 or V3043)/well or virus diluent in a volume of 50 μl in the presence of 20,000 IU of rabbit anti-murine IFN-α/β antibodies/ml (55,000 NIH neutralizing units per ml) (Lee Biomolecular) to minimize the effect of virus-induced IFN-α/β. At 72 hpi, cell viability was determined by colorimetric quantification using the MTT assay (mitochondrial dehydrogenase substrate) (Sigma M5655) as described previously (52). Internal controls on each plate consisted of unprimed infected cells, IFN-α/β-primed uninfected cells, and unprimed uninfected cells. The percentage of viable cells in each well was calculated relative to that of the uninfected controls. These values were used to calculate the percentage of CPE [(1 − fraction of viable cells) × 100].
RNA isolation and analysis by RPA.
L929 cells grown in 60-mm-diameter dishes were treated with 0, 21, or 500 IU of murine IFN-α/β/ml for 24 h at 37°C. The media were removed and the cells were infected for 1 h at 37°C with V3000 or V3043 at an MOI of 4 PFU per cell. The inoculum was removed and replaced with prewarmed media. At 4 hpi, cells were placed on ice and harvested for total cytoplasmic RNA by using the Ultraspec II RNA isolation system (Biotecx). The levels of viral RNA were detected by an RNase protection assay (RPA) using the RPAII system (Ambion). A negative-sense VEE-specific riboprobe for the detection of plus-strand genomic and subgenomic VEE RNA was constructed by inserting the 375-bp BsrGI-BsgI fragment (nt 1925 to nt 2290) of V3000 into the SmaI-HindIII-digested pGEM-3 plasmid. The pG3V VEE plus-sense specific probe was made by using the resulting plasmids, which were linearized at the unique BsrGI site. In vitro T7 polymerase transcription reaction mixtures (Maxiscript; Ambion) containing [α-32P]UTP generated a 586-nt radiolabeled probe that would produce 405- and 163-nt protected fragments corresponding to genomic and subgenomic RNA, respectively. As an internal control for this assay, a pGEM-GAPDH plasmid, kindly provided by Charles Rice (School of Medicine, Washington University, St. Louis, Mo.), was used to generate a specific probe for the detection of cellular control GAPDH (glyceraldehyde-3-phosphate dehydrogenase) mRNA. The undigested probe was 130 bp and the protected fragment was 116 bp. Ten micrograms of total RNA was hybridized overnight at 45°C in 30 μl of hybridization buffer to a molar excess of each radiolabeled probe (routinely 1 × 106 to 2 × 106 cpm). Unhybridized RNA was digested with RNase T1/RNase A mix, and the remaining RNA was precipitated. The protected radiolabeled RNA was analyzed in 6% acrylamide–8 M urea–Tris-borate-EDTA gels and visualized with a phosphorimager.
RESULTS
A single change in the 5′ UTR of the VEE genome attenuates the virus in mice after s.c. and i.c inoculations.
To assess the individual contribution of the 5′ UTR nt3A mutation to the attenuation of VEE, we introduced that single change into the virulent, TRD-derived, full-length cDNA clone (pV3000) and completely sequenced the mutagenized region to ensure that no additional changes had been introduced. The virulence of the resulting clone-derived virus, V3043, was determined by comparing the morbidity and mortality rates of V3000 and V3043 in adult 129Sv/Ev mice after s.c. and i.c. inoculation (Table 1). As has been shown previously for CD-1 mice (4, 13), V3000 at a dose of 103 PFU was virulent in 129Sv/Ev mice (100% morbidity and 100% mortality) following s.c. or i.c. inoculation, with similar average survival times (AST) to those in CD-1 mice. In contrast, no mortality was observed in mice inoculated with V3043 by the s.c. route, nor did they show clinical signs of disease. By the i.c. route, all the mice inoculated with V3043 showed signs of disease (ruffled fur, paresis, and/or more than 10% weight loss) but nevertheless survived the infection (100% morbidity, 0% mortality). Similar morbidity and mortality scores were obtained with outbred CD-1 mice inoculated s.c and i.c. with V3043 (data not shown). All the mice infected with V3043 were protected against a lethal challenge with 104 PFU of V3000, confirming that they had undergone a productive V3043 infection.
TABLE 1.
Virulence of V3000 and V3043 in adult 129Sv/Ev mice inoculated with 103 PFU of virus by s.c. and i.c. routes
| Virus | s.c. inoculation
|
i.c. inoculation
|
||||
|---|---|---|---|---|---|---|
| % Morbidity (sick/total) | % Mortality (dead/total) | AST ± SE (days) | % Morbidity (sick/total) | % Mortality (dead/total) | AST ± SE (days) | |
| V3000 | 100 (11/11) | 100 (11/11) | 7.7 ± 0.9 | 100 (8/8) | 100 (8/8) | 6.1 ± 1.2 |
| V3043 | 0 (0/11) | 0 (0/11) | NAa | 100 (8/8) | 0 (0/8) | NA |
NA, not applicable.
Replication and spread of V3043 mutant virus in the mouse.
To determine the point at which the replication of V3043 was restricted in the mouse, time course studies were performed and the kinetics of virus replication in various tissues (DLN, spleen, serum, and brain) were determined after inoculation with 103 PFU of either V3000 or V3043 in the LRFP (Fig. 1A to D). At 12 hpi, replication of both viruses in the DLN and spleen resulted in similar titers. After 24 hpi, the titers of V3043 in the spleen and serum started to decline. By 48 hpi, V3043 titers had dropped 2 to 2.5 logs from the peak titer, and by 72 hpi, V3043 was below the limit of detection in the serum, while V3000 titers were still 3 to 4 orders of magnitude higher. In the DLN, the drop in titer was slower than that in the spleen and serum, and the difference in titers between V3000 and V3043 was evident only by 96 hpi (data not shown). In the brains of mice inoculated s.c., there were detectable levels of V3000, but not V3043, at 24 hpi. The V3000 titers increased thereafter until death, while the V3043 titers peaked at 48 hpi, with only one out of three mice showing titers above the limit of detection. V3043 titers declined to below the limit of detection by 96 hpi (data not shown). Therefore, V3043 was able to replicate in the same tissues as V3000 and reached comparable titers early in the infection. However, V3043 titers were lower at 48 hpi in spleen, serum, and brain. V3043 viremia was lower and of shorter duration than that of V3000. Serum titers of both viruses were above the threshold required for neuroinvasion in the mouse (K. A. Bernard et al., personal communication). When V3043 was injected directly into the brain, the kinetics of replication in brain were similar to those of V3000 at early times p.i.; however, the mutant virus was cleared from the brain while the wild-type virus continued to replicate until the death of the animal (data not shown).
FIG. 1.
Virus titers of V3000 and V3043 in 129Sv/Ev mouse tissues. Six- to 8-week-old 129Sv/Ev mice were inoculated s.c. in the LRFP with 103 PFU of V3000 (solid bars) or V3043 (hatched bars). At the indicated times p.i., three mice per group were sacrificed and the following tissues were harvested: DLN (A), spleen (B), serum (C), and brain (D). Virus titers were determined by plaque assay of BHK-21 cells. The values are the geometric mean titers (log10 PFU/milliliter or gram) of three mice. Error bars represent standard deviations. The lower limit of detection is indicated by a broken line. ∗, all samples had virus titers below the limit of detection; ∗∗, one out of three samples had virus liters above the limit of detection.
V3043 is as virulent as V3000 and grows to similar titers in IFN-α/βR−/− mice.
The rapid, early clearance of V3043 from spleen and serum suggested two possible mechanisms of nt3A attenuation. The nt3A mutation may have a slightly reduced rate of replication in one or more cell types that is detectable only after amplification through several rounds of replication. Alternatively, the mutation may affect the interaction of the virus with some aspect of the host nonspecific innate immune response, such as induction of or sensitivity to IFN-α/β. To distinguish between these two possibilities, V3000 and V3043 infections were compared in IFN-α/βR−/− mice (35), the congenic background strain 129Sv/Ev, and cells derived from them. We hypothesized that in the absence of a functional IFN-α/β pathway, any effect of the nt3A mutation on the intrinsic growth of the virus independent of the IFN-α/β response would be detected. The virulence of V3000 and V3043 in the IFN-α/βR−/− mouse was compared to that in the control mice after inoculating the animals s.c. with 103 PFU of either virus (Fig. 2). V3000 infection of either the IFN-α/βR−/− or normal 129Sv/Ev mouse resulted in 100% mortality, but the AST was significantly shorter in the IFN-α/βR−/− mouse (30 h) than in the control 129Sv/Ev mouse (7.7 days). The results indicate that V3000 replication in control mice is significantly restricted by IFN-α/β but is not sufficiently restricted to prevent the virus from causing a fatal encephalitis, as reported earlier (15). In contrast to results obtained with the 129Sv/Ev control mouse, in the IFN-α/βR−/− mouse V3043 was as virulent as the wild-type virus, causing 100% mortality with an AST similar to that of V3000 (30 h). Even when mice were inoculated with lower doses (102, 10, and 1 PFU), V3043-infected IFN-α/βR−/− mice showed the same AST of V3000-infected mice (30 h).
FIG. 2.
Survival of 129Sv/Ev (closed symbols) and IFN-α/βR−/− (open symbols) mice infected with V3000 or V3043. Six- to 8-week-old mice, 11 mice per group, were inoculated in the LRFP with 103 PFU of V3000 or V3043. The mice were observed every 6 h for the first 48 h and every 24 h for 12 days. The survival curves of V3000 and V3043 in the IFN-α/βR−/− mice are superimposable.
To compare the replication and spread of V3000 and V3043 virus in the IFN-α/βR−/− mouse, 6- to 8-week-old mice were inoculated in the LRFP with 103 PFU of either virus. At different times p.i., serum and selected organs were harvested in triplicate as described for Fig. 1. The levels of virus in the DLN, spleen, and serum of the IFN-α/βR−/− mice were equivalent for both V3000 and V3043 and increased continuously with no evidence of clearance until the death of the animal (Fig. 3). Under these conditions, V3043 did not show a growth restriction in the organs tested, except for the brain at 27 hpi, in which the titers of V3043 were consistently lower than those of V3000.
FIG. 3.
Virus titers of V3000 and V3043 in IFN-α/βR−/− mouse tissues. Six- to 8-week-old IFN-α/βR−/− mice were inoculated s.c. in the LRFP with 103 PFU of V3000 (solid bars) or V3043 (hatched bars). At the indicated times p.i., three mice per group were sacrificed, tissues were harvested, and virus titers were determined as indicated in Fig. 1. The following tissues were harvested: DLN (A), spleen (B), serum (C), and brain (D). The values are the geometric mean titers (log10 PFU/milliliter or gram) of three mice. Error bars represent the standard deviations. The lower limit of detection is indicated as a broken line. ∗, samples with virus titers below the limit of detection.
The experiments depicted in Fig. 1 and 3 were performed in parallel, allowing comparison of the virus titers in the IFN-α/βR−/− mouse (Fig. 3) with those in the 129Sv/Ev mouse (Fig. 1). At 12 hpi, the titers of both viruses in the DLN of the IFN-α/βR−/− mice were equivalent to those in the 129 Sv/Ev mice, suggesting that early replication in the DLN was not significantly affected by either the nt3A mutation or the IFN-induced antiviral state. However, in the next 24 h, IFN-α/β played a crucial role and determined the kinetics of replication and spread for both viruses and the outcome of the infection by V3043.
Growth of V3043 in cell culture is reduced in the presence, but not in the absence, of a functional IFN-α/β pathway compared to that of V3000.
To further examine the mechanism of attenuation of this mutation, the in vitro growth phenotypes of V3000 and V3043 were examined. Growth curves were performed in mouse fibroblast cell lines L929 and Swiss 3T3, in the BHK-21 cell line, and in primary BMMΦ derived from 129Sv/Ev or IFN-α/βR−/− mice (Fig. 4). Triplicate monolayers of cells were infected with either virus at an MOI of 5 to 12 PFU/cell (PFU determined on BHK cells). At these MOIs, virtually 100% of the BHK, L929, and 3T3 cells and IFN-α/βR−/− BMMΦ were infected, as determined by infecting cells at equivalent MOIs with replicon particles 5005-3000 (nt3G) or 5505-3000 (nt3A) and measuring the percentage of cells expressing GFP. We consider these conditions representative of single-step growth curves. At the same MOI, less than 3% of 129 BMMΦ expressed GFP, reflecting a reduced permissivity to VEE replication, determined mainly by the status of the IFN-α/β system (see below). The specific infectivity (particle per PFU ratio) of V3000 and V3043 in BHK-21 cells is equivalent (K. A. Bernard and R. E. Johnston, unpublished observation); therefore, infections at the same MOI (BHK PFU/cell) can be compared. The relative infectivity of both viruses in the other cell lines was similar when compared by infecting the cells with GFP-expressing replicon particles derived from the wild-type or the mutant sequence and measuring the percentage of cells expressing GFP.
FIG. 4.
In vitro growth of V3000 and V3043 in L929 cells (A), Swiss 3T3 cells (B), BHK-21 cells (C), and primary BMMΦ (D) from 129Sv/Ev (closed symbols) and IFN-α/βR−/− (open symbols) mice. Monolayers grown in 60-mm-diameter dishes were infected in triplicate with V3000 (solid line) or V3043 (broken line) at an MOI of 5 to 12. After 1 h of adsorption at 37°C, the monolayers were washed three times and complete media were added. The first aliquot was collected at this time. Aliquots of culture media were collected at various times p.i., and equal volumes of fresh media were replaced every time. Virus titers were determined by a plaque assay on BHK-21 cells.
Even though there were variations in permissivity and growth kinetics of V3000 infection among the cell types tested, V3043 virus grew slower and to lower titers than V3000 in BHK-21, L929, and Swiss 3T3 cells and BMMΦ derived from 129Sv/Ev mice (Fig. 4A to D). Similar results were seen in Neuro2A cells and primary mouse embryo fibroblasts from CD-1 mice (data not shown). Although the virus titers in the BMMΦ derived from 129Sv/Ev were low, V3043 replication was even more restricted than that of V3000 (Fig. 4D), as shown for the other more permissive cell lines.
These experiments were designed to infect all permissive cells at time zero to limit the factors affecting the growth of V3043 to either an intrinsic growth defect or an autocrine or endogenous IFN-α/β response. We hypothesized that in cells derived from IFN-α/βR−/− mice, in which virus growth cannot be affected by an autocrine or paracrine IFN-α/β response, an attenuated growth phenotype would be the result of an intrinsic growth defect. In BMMΦ cells derived from the IFN-α/βR−/− mice, both viruses grew to titers several orders of magnitude higher than those in cells from normal controls. Interestingly, in contrast to all other cell types, V3043 grew to titers similar to or even higher than those of V3000 in BMMΦ from IFN-α/βR−/− mice, suggesting that the nt3A mutation does not cause a major intrinsic growth defect and that when the IFN-induced antiviral response was functional, it was more efficient in controlling V3043 infection than V3000 infection. The growth characteristics of V3000 and V3043 in the BMMΦ from IFN-α/βR−/− mice were consistent with the in vivo results presented in the previous section. These results supported the hypothesis that IFN-α/β plays an important role in the attenuated phenotype of V3043 and suggested that any IFN-α/β-independent effect of nt3A mutation on virus growth would play only a minor role.
Induction of IFN-α/β by V3000 and V3043 infections in cell culture and in vivo.
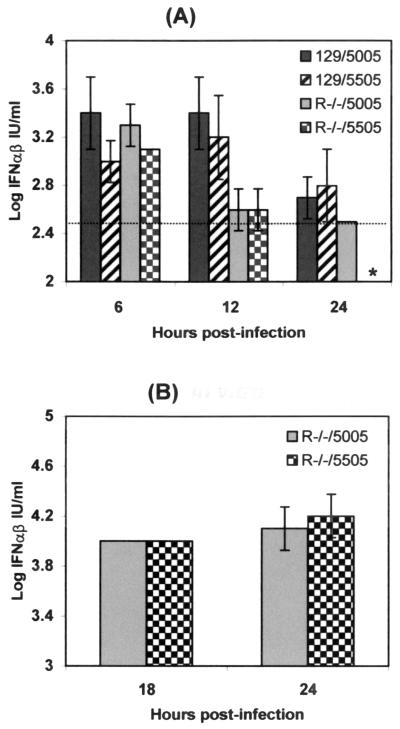
To further dissect the role of IFN-α/β in the attenuation of V3043, the nt3A mutation was examined for its effect on the ability of VEE to induce IFN-α/β. The production of murine IFN-α/β in vivo and in vitro was measured following infection with V3000 and V3043. The amount of IFN-α/β present in the sera of CD-1 mice inoculated s.c. with 103 PFU of V3000 or V3043 was proportional to the serum virus titers and followed similar kinetics. Peak titers of IFN-α/β were observed 18 to 24 hpi in both V3000- and V3043-infected mice, but higher titers were induced by the virulent V3000 (up to 80,000 IU/ml) than by V3043 (up to 20,000 IU/ml) (data not shown). Because serum IFN-α/β levels may reflect the levels of virus replication and spread in the peripheral tissues, we compared the wild-type and the mutant viruses in their ability to induce IFN-α/β after a single replication cycle. Wild-type R5005-3000 and mutant R5505-3000 VRPs, which can only undergo one round of replication, were used to infect 129Sv/Ev and IFN-α/βR−/− mice (Fig. 5A). Replicon particles contain a VEE replicon genome containing the GFP gene instead of the structural protein genes, with either nt3G (R5005) or nt3A (R5505). At 6 hpi, both replicons induced detectable levels of serum IFN-α/β in both strains of mice. No differences in induction were found between the wild-type and mutant VRP infections, nor between the two strains of mice at this time. At 12 hpi, the IFN-α/β levels in the serum of IFN-α/βR−/− mice had dropped significantly compared to the titers in the 129 Sv/Ev mice, but titers of the different VRPs were similar within each mouse strain. At 24 hpi, the levels of IFN-α/β in the serum had dropped in both mouse strains, being lower for the IFN-α/βR−/− mice (at or below the lower limit of detection of the assay) and again showing no differences between them for the different VRPs. By 48 hpi, IFN-α/β could not be detected in any sample (data not shown). Therefore, the IFN-α/β induction after a single round of replication in vivo was not different in mice infected with the wild type compared to those infected with nt3A mutant VRP. Interestingly, the kinetics of IFN-α/β induction were different depending on whether or not the mice expressed the receptor for IFN-α/β. The IFN-α/β induced early was not dependent on the presence of the IFN-α/β receptor, while later, in the absence of the IFN receptor, less IFN was induced.
FIG. 5.
In vivo (A) and in vitro (B) induction of IFN-α/β in 129Sv/Ev and IFN-α/βR−/− mice or cells derived from them. (A) Six- to 8-week-old 129Sv/Ev and IFN-α/βR−/− mice were inoculated s.c. in the LRFP with 105 IU of wild-type R5005-3000 or mutant R5505-3000 replicons expressing GFP. At 6, 12, and 24 hpi, three mice per experimental group were euthanatized for blood collection. Aliquots of serum were frozen at −70°C and were thawed only once for IFN-α/β detection by using a bioassay on L929 cells and EMCV as indicator virus. Values are the averages of data from three mice, and the error bars represent the standard deviations. The lower limit of detection is indicated as a broken line. ∗, samples with virus titers below the limit of detection (dotted line). The levels of IFN-α/β in the mock-infected mice were below the limit of detection. (B) Monolayers of BMMΦ from IFN-α/βR−/− mice prepared as described for Fig. 4 were infected with R5005-3000 or R5505-3000 GFP-expressing replicons at an MOI of 20. At 18 and 24 hpi, the media were collected and clarified by centrifugation and aliquots were frozen until used in IFN-α/β assays as described for panel A.
To compare IFN-α/β induction in vitro, BMMΦ cells from IFN-α/βR−/− mice were infected with either replicon particle preparation at an MOI of 20. At 18 and 24 hpi, the titers of IFN-α/β in the culture supernatants were determined by bioassay on L929 cells (Fig. 5B). The IFN-α/β induced in the absence of the IFN-α/β receptor and in further rounds of replication was equivalent at 18 and 24 hpi in the culture supernatants whether cells were infected with V5005-3000 or V5505-3000, confirming the in vivo results.
V3043 is more sensitive than V3000 to exogenous IFN-α/β in infected cell cultures.
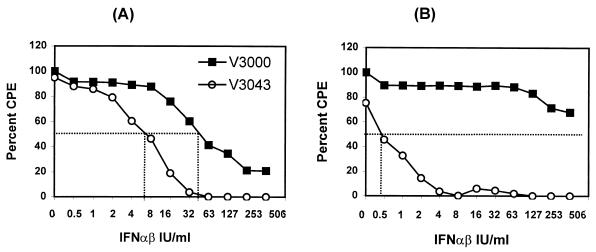
We next examined the effect of the nt3A mutation on the sensitivity of the virus to the antiviral effect induced by IFN-α/β. Two different assays were used to measure IFN-α/β sensitivity. First, mouse fibroblast L929 or Swiss 3T3 cells that had been pretreated with increasing concentrations (0 to 506 IU/ml) of murine IFN-α/β for 24 h were infected with V3000 or V3043 at an MOI of 10 to 20. The readout was virus-induced CPE at 72 hpi. To minimize the effect of the IFN-α/β produced by the infected cells on the readout of the assay, cells were infected in the presence of 20,000 IU of anti-IFN-α/β antibodies/ml, since in their absence, V3043 was not able to cause full CPE in the mock-IFN-treated controls. The dose of anti-IFN-α/β antibodies was estimated based on the maximum levels of IFN-α/β produced in such infected cultures (up to 10,000 IU/ml; data not shown). For both viruses, pretreatment with IFN-α/β decreased the percentage of cells exhibiting CPE compared to that of the untreated control, indicating that both were sensitive to the antiviral effects of IFN-α/β (Fig. 6). The sensitivity curve of V3000 in L929 cells was similar to that of the EMCV indicator virus used in this assay (data not shown), with concentrations of 32 to 63 IU of IFN/ml protecting 50% of the monolayer from virus-induced CPE. The sensitivity curve of V3043 was shifted to the left, reflecting a sensitivity to IFN-α/β that was 8- to 10-fold higher than that of V3000 (Fig. 6A). IFN-α/β at 8 IU/ml was able to protect 50% of the monolayer from V3043-induced CPE under infection conditions in which both viruses were able to cause close to 100% CPE in the untreated control. The increased sensitivity of V3043 to IFN-α/β was more dramatic in Swiss 3T3 cells, in which 0.5 IU of IFN-α/β/ml protected 50% in the V3043-infected culture, while more than 506 IU of IFN-α/β/ml was required to protect 50% in the V3000-infected culture (Fig. 6B).
FIG. 6.
IFN-α/β sensitivity assay of L929 (A) and 3T3 (B) cells. Monolayers of L929 cells in 96-well plates were treated with twofold dilutions of murine IFN-α/β (concentrations ranging from 506 to 0 IU/ml) for 24 h. Mice were infected with either V3000 or V3043 at an MOI of 10 to 20 PFU/cell in the presence of 20,000 IU of anti-IFN-α/β antibodies/ml to minimize any possible autocrine effects of IFN-α/β induction. The percentage of CPE was measured at 72 hpi by the MTT colorimetric assay.
The increased sensitivity of V3043 to IFN-α/β was also shown by analysis of viral RNA synthesis using an RPA. L929 cells were pretreated for 24 h with IFN-α/β at a high dose (500 IU/ml) or a low dose (21 IU/ml) or were mock treated with diluent. The cells were then infected with V3000 or V3043 at an MOI of 4. At 4 hpi, total cytoplasmic RNA was extracted for analysis of VEE genomic and subgenomic RNA by RPA. The levels of genomic VEE RNA in the untreated control cells were two- to threefold higher in the V3043-infected cells than in the V3000-infected cells (Fig. 7). This result is consistent with the one-step growth curves of the IFN-α/βR−/− cells and with the hypothesis that the attenuation of V3043 is not the result of a decreased ability of V3043 to replicate. When cells were pretreated with 500 IU of murine IFN-α/β/ml, viral RNA was not detected in either V3000- or V3043-infected cells, indicating that at high doses of IFN-α/β, the replication of both viruses was suppressed, consistent with the results in Fig. 6A. In contrast, when cells were treated with 21 IU of IFN-α/β/ml, the levels of V3043 RNA were reduced 78% in comparison to those of the untreated control, while the levels of V3000 RNA were reduced by only 9%, even though the RNA levels in the untreated control were higher for V3043. This result suggests that relative to the wild type, the mutation at nt 3 in the V3043 5′ UTR mediates a higher level of RNA replication, higher intracellular dsRNA levels, and greater sensitivity to IFN-α/β-induced antiviral effector pathways and, consequently, attenuation in vivo.
FIG. 7.
Mutant virus is more sensitive to IFN-α/β in L929 cells early in infection at the RNA synthesis level. L929 cells pretreated for 24 h with 0, 21, or 500 IU/ml of murine IFN-α/β were infected with V3000 or V3043 at an MOI of 4 or were mock infected. Total cytoplasmic RNAs were isolated at 4 hpi, and the levels of VEE plus-strand genomic and subgenomic RNA and of the mRNA for the housekeeping gene GAPDH were determined by RPA. Lane 1, undigested probes; lanes 2 to 4, 10, 100, and 1,000 ng, respectively, of plus-strand VEE RNA transcripts used as controls and subjected to the RPA. The presence (+) or absence (−) of IFN-α/β pretreatment is indicated. The concentrations of IFN-α/β (+) (in IU/milliliter) used are indicated.
DISCUSSION
The study of alphavirus virulence from a genetic perspective has yielded a number of interesting observations, perhaps none more interesting than the dramatic effects that a single nucleotide change can have on the course of disease in an animal. This is illustrated by the many examples of single-codon changes in alphavirus genomes, and indeed alternative amino acid substitutions at a given codon, that profoundly alter virulence and organ tropism (2, 4, 9, 13, 18, 25, 40, 51). The 5′ UTR of the genome of alphaviruses, which has been implicated in neurovirulence (10, 23, 26, 27, 33), is a case in point. In mice, a single nucleotide change in the 5′ UTR of VEE or Sindbis virus acts synergistically with determinants in the E2 glycoprotein to cause attenuation (10, 23, 33). In this study, we have examined the individual contribution of a single nucleotide change in the VEE 5′ UTR and have shown that it changes a virus which invariably causes 100% mortality to one which causes no deaths by either a peripheral or i.c. route of inoculation. In dissecting the in vivo and in vitro growth properties of the mutant virus, we have unveiled a major role for IFN-α/β in the attenuation of this single mutant virus. Our study contributes not only to a better understanding of alphavirus virulence but also of how the interplay between the virus genetics and the innate immune response affects the outcome of the infection.
Comparison of the pathogenesis of V3000 and V3043 revealed that the two viruses grew in the same tissues and replicated to similar titers through the first 12 hpi. After this time, however, a restriction in mutant replication was detected, peak titers in tissue and serum were reduced, clearance from the periphery was accelerated, and invasion of the central nervous system was delayed and less frequent. This profile suggested that the mutation in the 5′ UTR did not necessarily impede growth at the single-cell level but more likely caused V3043 to be more susceptible to an early host response.
The relative roles of the innate response to alphavirus infection, such as through the IFN-α/β system, and classical adaptive immune responses have been examined (5, 5a, 15, 16, 25, 28, 30, 35, 48, 57). In genetically modified animals lacking elements of the adaptive immune system, alphavirus infections appear less damaging than those in their immunologically intact counterparts. However, the virus typically is not cleared from the animal, which may ultimately die from continued virus replication (5a, 11, 29, 30). In contrast, defects in the IFN-α/β system render mice extremely susceptible to lethal infection (15, 19, 35), even with alphaviruses that do not cause any clinical illness at all in normal adult animals (48). Clearly, the IFN-α/β system provides an earlier and more protective benefit than adaptive immunity, the latter being demonstrably harmful in some cases.
In the work presented here, a significantly shorter AST and a 10,000-fold increase in virus titers in the IFN-α/βR−/− mice compared to those in normal mice were evidence that IFN-α/β does significantly inhibit VEE replication. These results were consistent with the mortality and AST of V3000 in IFN-α/βR−/− mice compared to those in the control mice reported by Grieder et al. (15). The comparable virulence of V3043 and V3000 in the IFN-α/βR−/− mice was consistent with the V3043 virus being more susceptible than V3000 to the early host response. Any mutation that reduces the replication competence of a virus could render it more susceptible to an early immune response. However, we predicted that an important growth defect would impair growth even in the absence of a functional IFN response, as has been shown for the Sindbis attenuating mutant TRSB-R114 (48). With the exception of brain titers, this was not the case for V3043. V3043 brain titers were consistently lower than V3000 titers at 24 hpi, suggesting that tissue-specific differences in growth between V3000 and V3043 also could contribute to the attenuated phenotype. Therefore, our in vivo data do not completely rule out a minor effect of the nt3A mutation on virus replication at the single-cell level, which could also indirectly result in a virus more susceptible to an early host response. A closer examination of the effect of nt3A on different steps in the replication cycle of the virus in cells lacking a functional IFN-α/β pathway is necessary to better understand any IFN-independent effect. There may be an altered interaction between the 5′ UTR and specific cell factors involved in translation or replication, higher endogenous levels of antiviral enzymes in different tissues, or an undefined IFN-independent mechanism in these tissues.
The attenuated phenotype of V3043 observed in vivo in the normal mouse was mimicked in cultured mouse and hamster cell lines and 129Sv/Ev mouse primary cells, in which V3043 replicated more slowly and to lower titers than V3000. The only cell culture in which V3043 did not show reduced growth was in the BMMΦ from IFN-α/βR−/− mice, consistent with the results from the in vivo infections that suggested a significant role for IFN-α/β in the reduced growth of V3043 in normal cells. We predicted that in one-step growth curves produced in the absence of a functional IFN-α/β system, any IFN-independent intrinsic growth defect in V3043, such as reduced viral RNA or protein synthesis, would be revealed. No growth reduction was observed; V3043 grew as well as, or better than, V3000 in cells from the IFN-α/βR−/− mice, suggesting that the mutant virus did not have an intrinsic growth restriction in these cells. Interestingly, the higher V3043 titers in the absence of a functional IFN system support the hypothesis that nt3A causes an early increase in genomic RNA replication (Fig. 7). This could increase the induction or the activation of IFN-induced antiviral proteins, which in the presence of a functional IFN-α/β response could result in increased sensitivity to IFN-α/β but in its absence could result in higher virus titers. The low permissivity of normal BMMΦ for VEE replication could be the result of an autocrine or paracrine IFN-α/β response mediated by IFN-α/β released by the infected cells that would induce an antiviral state in the infected and neighbor cells through the IFN-α/β receptor (48). Alternatively, a priming effect mediated by high endogenous levels of antiviral enzymes at the time of the infection could result in low permissivity.
Grieder and Nguyen (14) studied the growth kinetics of V3000 in mouse primary peritoneal macrophages, either quiescent or activated with lipopolysaccharide or IFN-γ prior to infection. Their results showed peak titers of about 104 PFU/ml at 18 hpi when infected at an MOI of 1. These titers are comparable to the ones reported here for the BMMΦ, even though our experiments were performed at higher MOIs (5 to 12) and used macrophages with a different phenotype and stage of differentiation.
To explain the in vivo and in vitro phenotypic differences observed in the presence and absence of a functional IFN pathway, we proposed two hypotheses: (ii) V3043 could be inducing higher levels of IFN-α/β than V3000 and/or (ii) V3043 could be more sensitive to the antiviral response induced by IFN-α/β.
To address IFN-α/β induction, we used identical virus particles carrying either V3043 or V3000 replicon genomes so that IFN-α/β induction could be measured without the confounding effects of multiple rounds of viral replication and subsequent alterations in the physiology of the host. We saw no differences in IFN-α/β induction between mutant and wild-type replicon particles in either normal or IFN-α/βR−/− mice. We would predict that higher levels of RNA synthesis by V3043 would induce higher levels of IFN-α/β, but our assays did not detect any difference. However, an interesting observation was made while addressing this question. Analysis of the kinetics of IFN-α/β induction after a single round of replication showed a prompt and transient response. However, early IFN-α/β levels (6 hpi) were not affected by the absence of the IFN-α/β receptor, while the faster drop of IFN-α/β titers in the sera of IFN-α/βR−/− mice compared to those of the normal mice suggested that feedback signaling through the IFN-α/β receptor was required for the continued production of IFN-α/β at 12 and 24 hpi. Recent studies have shown that in the absence of Stat1, only IFN-α4 is induced in response to Newcastle disease virus infection, while the induction of other species of IFN-α require signaling through the JAK-STAT pathway (involving new protein synthesis of IFN itself and IRF7) (32). This observation could explain our results of differential induction of IFN-α/β by both V3000 and V3043 in the IFN-α/βR−/− mice compared to induction in the normal mice.
When we compared the antiviral effects of murine IFN-α/β on V3000 and V3043 infections of murine fibroblasts, V3043 was 8- or >100-fold more sensitive to IFN-α/β in L929 or Swiss 3T3 cells, respectively. The mechanism of IFN-induced protection in the mouse fibroblasts involves binding of IFN-α and IFN-β to the common receptor on the cell surface, which triggers a cascade of events through the JAK-STAT pathway. This results in the transcriptional induction of a large group of IFN-stimulated genes, including RNA-dependent protein kinase, 2′-5′ oligoadenylate synthetase, RNase L, MX1, and adenosine deaminase. In the infected cell, viral dsRNA replication intermediates bind to the induced RNA-dependent protein kinase, 2′-5′ oligoadenylate synthetase, and/or adenosine deaminase as cofactors in the activation of these activities (62). Future studies will address the mechanism of increased sensitivity to IFN-α/β by determining what IFN effector mechanisms are active in the inhibition of VEE and what the relative sensitivity of the wild type and the nt3A mutant virus is to those antiviral pathways.
Attenuation determinants affecting sensitivity to IFN have been described for other viral systems. IFN-sensitive mutants of mengovirus (53) as well as a mutant of Sindbis virus resistant to mycophenolic acid and ribavirin with increased sensitivity to chicken IFN (47) have been described. However, the genetic loci in the virus responsible for the IFN-sensitive phenotype have not been defined, and the mechanisms that mediate their increased sensitivity to IFN are poorly understood.
Our results are consistent with previous reports showing an increased sensitivity to IFN-α/β of VEE strain TC-83 and horse-avirulent enzootic strains compared to those of their virulent counterparts (42, 54). These studies with chimeric viruses showed that IFN-α/β sensitivity segregates with the 5′ UTR and nonstructural genes (42). Our studies go further and associate the IFN-α/β sensitivity phenotype with a single locus in the 5′ UTR.
Alphaviruses are transmitted between vertebrate hosts by mosquito vectors which acquire the virus by taking a blood meal from an infected animal at a time of peak viremia (21). To insure a sufficiently high titer of virus for mosquito transmission, one would anticipate a selective pressure in favor of high-level virus replication and RNA synthesis. Decreased RNA synthesis would lead to a reduced viremia and concomitantly reduced fitness in nature. On the other hand, high levels of dsRNA could lead to increased induction of IFN-α/β as well as increased synthesis and/or activation of IFN-α/β-related antiviral effector mechanisms. Therefore, increased levels of RNA synthesis could also lead to the same negative fitness result by evoking elements of the IFN-α/β system. In terms of pathogenesis, either an increase or decrease in RNA synthesis would predict decreased virulence. In the case of V3043, early RNA synthesis is increased, the sensitivity of this virus to IFN-α/β is increased by 8- to >100-fold, replication in the animal is decreased, and the virus is avirulent.
Our working hypothesis to link the single nucleotide change at nt 3 of the 5′ UTR to the loss of virulence in mice is as follows. Although computer-generated RNA secondary structures only imperfectly predict authentic interactions, the Zucker programs do suggest that nt 3 is involved in a stable stem structure (59). Secondary structures of the 5′ UTR have been predicted for other alphaviruses (10, 38, 55). The change from G to A in V3043 is predicted to weaken this structure, which may create a more accessible 5′ terminus. This altered structure could affect RNA synthesis directly, as the 3′ end of the negative-strand complement serves as the promoter for the synthesis of progeny positive-strand genomes. Alternatively, the 5′ and 3′ ends of the genome may interact during RNA synthesis so that alterations in the genomic 5′ UTR could affect positive- and/or negative-strand synthesis. Finally, release of the extreme 5′ end of the genome from the stem could increase translation initiation for the nonstructural protein open reading frame, leading to increased levels of viral replicase components and hence increased RNA synthesis. (Preliminary evidence suggests that translation of nonstructural proteins is elevated in rabbit reticulocyte lysates primed with V3043 RNA and that higher levels of nonstructural proteins are synthesized in infected cells in culture.) We would predict that the increased IFN-α/β sensitivity of V3043 is a result of higher levels of RNA synthesis, leading to increased direct induction of IFN-α/β-related antiviral effectors, increased activation of such effectors, or both. Alternatively, increased IFN-α/β sensitivity could result from slower viral inhibition of host protein synthesis, allowing continued production of key antiviral activities in infected cells. This hypothesis is consistent with (i) the highly virulent infection of V3043 in IFN-α/βR−/− mice, (ii) the absence of a growth differential between the wild type and V3043 in cells lacking a functional IFN-α/β system, (iii) the lack of an early growth inhibition of V3043, followed by decreased titers in organ and more rapid clearance in normal animals, and (iv) reduced virulence.
While a number of individual elements of the hypothesis remain to be addressed, our characterization of this single nucleotide change in the 5′ UTR presents the opportunity to pursue a more comprehensive examination of the dynamic interaction of virus and host at the earliest times in the disease process.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grant NS26681 from the NIH.
We thank Kristen Bernard, Mark Heise, Brett Lidbury, William Klimstra, and Kate Ryman for critical reading of the manuscript. We thank Barbara Sherry and the entire Johnston laboratory for stimulating discussions. We also thank Cherice Conner, Michael Hawley, Jacque Bailey, and Dwayne Muhammad for excellent technical assistance with cell cultures.
REFERENCES
- 1.Almond J W. The attenuation of poliovirus neurovirulence. Annu Rev Microbiol. 1987;41:153–180. doi: 10.1146/annurev.mi.41.100187.001101. [DOI] [PubMed] [Google Scholar]
- 2.Aronson J F, Grieder F B, Davis N L, Charles P C, Knott T A, Brown K W, Johnston R E. A single-site mutant and revertants arising in vivo define early steps in the pathogenesis of Venezuelan equine encephalitis virus. Virology. 2000;270:111–113. doi: 10.1006/viro.2000.0241. [DOI] [PubMed] [Google Scholar]
- 3.Berge T O, Banks I S, Tigertt W D. Attenuation of Venezuelan equine encephalomyelitis virus by in vitro cultivation in guinea pig heart cells. Am J Hyg. 1961;73:209–218. [Google Scholar]
- 4.Bernard K A, Klimstra W B, Johnston R E. Mutations in the E2 glycoprotein of Venezuelan equine encephalitis virus confer heparan sulfate interaction, low morbidity and rapid clearance from blood of mice. Virology. 2000;276:93–103. doi: 10.1006/viro.2000.0546. [DOI] [PubMed] [Google Scholar]
- 5.Byrnes A P, Durbin J E, Griffin D E. Control of Sindbis virus infection by antibody in interferon-deficient mice. J Virol. 2000;74:3905–3908. doi: 10.1128/jvi.74.8.3905-3908.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5a.Charles, P. C., J. Trgovcich, N. L. Davis, and R. L. Johnston. Immunopathology and immune modulation of Venezuelan equine encephalitis virus-induced disease in the mouse. Virology, in press. [DOI] [PubMed]
- 6.Charles P C, Walters E, Margolis F, Johnston R E. Mechanism of neuroinvasion of Venezuelan equine encephalitis virus in the mouse. Virology. 1995;208:662–671. doi: 10.1006/viro.1995.1197. [DOI] [PubMed] [Google Scholar]
- 7.Cormack B P, Valdivia R H, Falkow S. FACS-optimized mutants of the green fluorescent protein (GFP) Gene. 1996;173:33–38. doi: 10.1016/0378-1119(95)00685-0. [DOI] [PubMed] [Google Scholar]
- 8.Davis N L, Brown K W, Johnston R E. A viral vaccine vector that expresses foreign genes in lymph nodes and protects against mucosal challenge. J Virol. 1996;70:3781–3787. doi: 10.1128/jvi.70.6.3781-3787.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis N L, Powell N, Greenwald G F, Willis L V, Johnson B J, Smith J F, Johnston R E. Attenuating mutations in the E2 glycoprotein gene of Venezuelan equine encephalitis virus: construction of single and multiple mutants in a full-length cDNA clone. Virology. 1991;183:20–31. doi: 10.1016/0042-6822(91)90114-q. [DOI] [PubMed] [Google Scholar]
- 10.Dubuisson J, Lustig S, Ruggli N, Akov Y, Rice C M. Genetic determinants of Sindbis virus neuroinvasiveness. J Virol. 1997;71:2636–2646. doi: 10.1128/jvi.71.4.2636-2646.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fazakerley J K, Khalili-Shirazi A, Webb H E. Semliki Forest virus (A7[74]) infection of adult mice induces an immune-mediated demyelinating encephalomyelitis. Ann N Y Acad Sci. 1988;540:672–673. doi: 10.1111/j.1749-6632.1988.tb27208.x. [DOI] [PubMed] [Google Scholar]
- 12.Gleiser C A, Gochenour W S, Jr, Berge T O, Tigertt W D. The comparative pathology of experimental Venezuelan equine encephalomyelitis virus infection in different animal hosts. J Infect Dis. 1961;110:80–97. doi: 10.1093/infdis/110.1.80. [DOI] [PubMed] [Google Scholar]
- 13.Grieder F B, Davis N L, Aronson J F, Charles P C, Sellon D C, Suzuki K, Johnston R E. Specific restrictions in the progression of Venezuelan equine encephalitis virus-induced disease resulting from single amino acid changes in the glycoproteins. Virology. 1995;206:994–1006. doi: 10.1006/viro.1995.1022. [DOI] [PubMed] [Google Scholar]
- 14.Grieder F B, Nguyen H T. Virulent and attenuated mutant Venezuelan equine encephalitis virus show marked differences in replication in infection in murine macrophages. Microb Pathog. 1996;21:85–95. doi: 10.1006/mpat.1996.0045. [DOI] [PubMed] [Google Scholar]
- 15.Grieder F B, Vogel S N. Role of interferon and interferon regulatory factors in early protection against Venezuelan equine encephalitis virus infection. Virology. 1999;257:106–118. doi: 10.1006/viro.1999.9662. [DOI] [PubMed] [Google Scholar]
- 16.Griffin D, Levine L, Tyor W, Ubol S, Despres P. The role of antibody in recovery from alphavirus encephalitis. Immunol Rev. 1997;159:155–161. doi: 10.1111/j.1600-065x.1997.tb01013.x. [DOI] [PubMed] [Google Scholar]
- 17.Heise M T, Connick M, Virgin H W. Murine cytomegalovirus inhibits interferon gamma-induced antigen presentation to CD4 T cells by macrophages via regulation of expression of major histocompatibility complex class II-associated genes. J Exp Med. 1998;187:1037–1046. doi: 10.1084/jem.187.7.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heise M T, Simpson D A, Johnston R E. A single amino acid change in nsP1 attenuates neurovirulence of the Sindbis-group alphavirus, S.A.AR86. J Virol. 2000;74:4207–4213. doi: 10.1128/jvi.74.9.4207-4213.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hwang S Y, Hertzog P J, Holland K A, Sumarsono S H, Tymms M J, Hamilton J A, Whitty G, Bertoncello I, Kola I. A null mutation in the gene encoding a type I interferon receptor component eliminates antiproliferative and antiviral responses to interferons alpha and beta and alters macrophage responses. Proc Natl Acad Sci USA. 1995;92:11284–11288. doi: 10.1073/pnas.92.24.11284. . (Erratum, 93:4519, 1996.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jahrling P B, Navarro E, Scherer W F. Interferon induction and sensitivity as correlates to virulence of Venezuelan encephalitis viruses for hamsters. Arch Virol. 1976;51:23–35. doi: 10.1007/BF01317831. [DOI] [PubMed] [Google Scholar]
- 21.Johnston R E, Peters C J. Alphaviruses. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 843–898. [Google Scholar]
- 22.Jordan G W. Interferon sensitivity of Venezuelan equine encephalomyelitis virus. Infect Immun. 1973;7:911–917. doi: 10.1128/iai.7.6.911-917.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kinney R M, Chang G J, Tsuchiya K R, Sneider J M, Roehrig J T, Woodward T M, Trent D W. Attenuation of Venezuelan equine encephalitis virus strain TC-83 is encoded by the 5′-noncoding region and the E2 envelope glycoprotein. J Virol. 1993;67:1269–1277. doi: 10.1128/jvi.67.3.1269-1277.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kinney R M, Johnson B J, Welch J B, Tsuchiya K R, Trent D W. The full-length nucleotide sequences of the virulent Trinidad donkey strain of Venezuelan equine encephalitis virus and its attenuated vaccine derivative, strain TC-83. Virology. 1989;170:19–30. doi: 10.1016/0042-6822(89)90347-4. [DOI] [PubMed] [Google Scholar]
- 25.Klimstra W B, Ryman K D, Nguyen K B, Biron C A, Johnston R E. Infection of neonatal mice with Sindbis virus results in a systemic inflammatory response syndrome. J Virol. 1999;73:10387–10398. doi: 10.1128/jvi.73.12.10387-10398.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kobiler D, Rice C M, Brodie C, Shahar A, Dubuisson J, Halevy M, Lustig S. A single nucleotide change in the 5′ noncoding region of Sindbis virus confers neurovirulence in rats. J Virol. 1999;73:10440–10446. doi: 10.1128/jvi.73.12.10440-10446.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuhn R J, Griffin D E, Zhang H, Niesters H G, Strauss J H. Attenuation of Sindbis virus neurovirulence by using defined mutations in nontranslated regions of the genome RNA. J Virol. 1992;66:7121–7127. doi: 10.1128/jvi.66.12.7121-7127.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.LeBlanc P A, Scherer W F, Sussdorf D H. Infections of congenitally athymic (nude) and normal mice with avirulent and virulent strains of Venezuelan encephalitis virus. Infect Immun. 1978;21:779–785. doi: 10.1128/iai.21.3.779-785.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levine B, Griffin D E. Molecular analysis of neurovirulent strains of Sindbis virus that evolve during persistent infection of scid mice. J Virol. 1993;67:6872–6875. doi: 10.1128/jvi.67.11.6872-6875.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levine B, Hardwick J M, Trapp B D, Crawford T O, Bollinger R C, Griffin D E. Antibody-mediated clearance of alphavirus infection from neurons. Science. 1991;254:856–860. doi: 10.1126/science.1658936. [DOI] [PubMed] [Google Scholar]
- 31.MacDonald G H, Johnston R E. Role of dendritic cell targeting in Venezuelan equine encephalitis virus pathogenesis. J Virol. 2000;74:914–922. doi: 10.1128/jvi.74.2.914-922.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marie I, Durbin J E, Levy D E. Differential viral induction of distinct interferon-alpha genes by positive feedback through interferon regulatory factor-7. EMBO J. 1998;17:6660–6669. doi: 10.1093/emboj/17.22.6660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McKnight K L, Simpson D A, Lin S-C, Knott T A, Polo J M, Pence D F, Johannsen D B, Heidner H W, Davis N L, Johnston R E. Deduced consensus sequence of Sindbis virus strain AR339: mutations contained in laboratory strains which affect cell culture and in vivo phenotypes. J Virol. 1996;70:1981–1989. doi: 10.1128/jvi.70.3.1981-1989.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meissner J D, Huang C Y, Pfeffer M, Kinney R M. Sequencing of prototype viruses in the Venezuelan equine encephalitis antigenic complex. Virus Res. 1999;64:43–59. doi: 10.1016/S0168-1702(99)00078-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muller U, Steinhoff U, Reis L F, Hemmi S, Pavlovic J, Zinkernagel R M, Aguet M. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 36.Muster T, Subbarao E K, Enami M, Murphy B R, Palese P. An influenza virus containing influenza B virus 5′- and 3′-noncoding regions on the neuraminidase gene is attenuated in mice. Proc Natl Acad Sci USA. 1991;88:5177–5181. doi: 10.1073/pnas.88.12.5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Niesters H G, Strauss J H. Defined mutations in the 5′ nontranslated sequence of Sindbis virus RNA. J Virol. 1990;64:4162–4168. doi: 10.1128/jvi.64.9.4162-4168.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ou J-H, Strauss E G, Strauss J H. The 5′-terminal sequences of the genomic of RNAs of several alphaviruses. J Mol Biol. 1983;168:1–15. doi: 10.1016/s0022-2836(83)80319-2. [DOI] [PubMed] [Google Scholar]
- 39.Paredes A M, Brown D T, Rothnagel R, Chiu W, Schoepp R J, Johnston R E, Prasad B V. Three-dimensional structure of a membrane-containing virus. Proc Natl Acad Sci USA. 1993;90:9095–9099. doi: 10.1073/pnas.90.19.9095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Polo J M, Johnston R E. Mutational analysis of a virulence locus in the E2 glycoprotein gene of Sindbis virus. J Virol. 1991;65:6358–6361. doi: 10.1128/jvi.65.11.6358-6361.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Portis J L, Perryman S, McAtee F J. The R-U5–5′ leader sequence of neurovirulent wild mouse retrovirus contains an element controlling the incubation period of neurodegenerative disease. J Virol. 1991;65:1877–1883. doi: 10.1128/jvi.65.4.1877-1883.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Powers A M, Brault A C, Kinney R M, Weaver S C. The use of chimeric Venezuelan equine encephalitis viruses as an approach for the molecular identification of natural virulence determinants. J Virol. 2000;74:4258–4263. doi: 10.1128/jvi.74.9.4258-4263.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Powers A M, Oberste M S, Brault A C, Rico-Hesse R, Schmura S M, Smith J F, Kang W, Sweeney W P, Weaver S C. Repeated emergence of epidemic/epizootic Venezuelan equine encephalitis from a single genotype of enzootic subtype ID virus. J Virol. 1997;71:6697–6705. doi: 10.1128/jvi.71.9.6697-6705.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pritchard A E, Calenoff M A, Simpson S, Jensen K, Lipton H L. A single base deletion in the 5′ noncoding region of Theiler's virus attenuates neurovirulence. J Virol. 1992;66:1951–1958. doi: 10.1128/jvi.66.4.1951-1958.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pushko P, Parker M, Ludwig G V, Davis N L, Johnston R E, Smith J F. Replicon-helper systems from attenuated Venezuelan equine encephalitis virus: expression of heterologous genes in vitro and immunization against heterologous pathogens in vivo. Virology. 1997;239:389–401. doi: 10.1006/viro.1997.8878. [DOI] [PubMed] [Google Scholar]
- 46.Rico-Hesse R, Weaver S C, de Siger J, Medina G, Salas R A. Emergence of a new epidemic/epizootic Venezuelan equine encephalitis virus in South America. Proc Natl Acad Sci USA. 1995;92:5278–5281. doi: 10.1073/pnas.92.12.5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosenblum C I, Stollar V. SVMPA, a mutant of sindbis virus resistant to mycophenolic acid and ribavirin, shows an increased sensitivity to chick interferon. Virology. 1999;259:228–233. doi: 10.1006/viro.1999.9775. [DOI] [PubMed] [Google Scholar]
- 48.Ryman K, Klimstra W B, Nguyen K B, Biron C A, Johnston R E. Alpha/beta interferon protects adult mice from fatal Sindbis virus infection and is an important determinant of cell and tissue tropism. J Virol. 2000;74:3366–3378. doi: 10.1128/jvi.74.7.3366-3378.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Samuel C E. Antiviral actions of interferon. Interferon-regulated cellular proteins and their surprisingly selective antiviral activities. Virology. 1991;183:1–11. doi: 10.1016/0042-6822(91)90112-o. [DOI] [PubMed] [Google Scholar]
- 50.Schlesinger S, Schlesinger M J. Togaviridae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lipincott-Raven; 1996. pp. 825–841. [Google Scholar]
- 51.Schoepp R J, Johnston R E. Sindbis virus pathogenesis: phenotypic reversion of an attenuated strain to virulence by second-site intragenic suppressor mutations. J Gen Virol. 1993;74:1691–1695. doi: 10.1099/0022-1317-74-8-1691. [DOI] [PubMed] [Google Scholar]
- 52.Sherry B, Torres J, Blum M A. Reovirus induction of and sensitivity to beta interferon in cardiac myocyte cultures correlate with induction of myocarditis and are determined by viral core proteins. J Virol. 1998;72:1314–1323. doi: 10.1128/jvi.72.2.1314-1323.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Simon E H, Kung S, Koh T T, Brandman P. Interferon-sensitive mutants of mengovirus. I. Isolation and biological characterization. Virology. 1976;69:727–736. doi: 10.1016/0042-6822(76)90501-8. [DOI] [PubMed] [Google Scholar]
- 54.Spotts D R, Reich R M, Kalkhan M A, Kinney R M, Roehrig J T. Resistance to alpha/beta interferons correlates with the epizootic and virulence potential of Venezuelan equine encephalitis viruses and is determined by the 5′ noncoding region and glycoproteins. J Virol. 1998;72:10286–10291. doi: 10.1128/jvi.72.12.10286-10291.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Strauss J H, Strauss E G. The alphaviruses: gene expression, replication, and evolution. Microbiol Rev. 1994;58:491–562. doi: 10.1128/mr.58.3.491-562.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tiwari R K, Kusari J, Sen G C. Functional equivalents of interferon-mediated signals needed for induction of an mRNA can be generated by double-stranded RNA and growth factors. EMBO J. 1987;6:3373–3378. doi: 10.1002/j.1460-2075.1987.tb02659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Trgovcich J, Aronson J F, Eldridge J C, Johnston R E. TNF-alpha, interferon and stress response induction as a function of age-related susceptibility to fatal Sindbis virus infection of mice. Virology. 1999;263:339–348. doi: 10.1006/viro.1999.9913. [DOI] [PubMed] [Google Scholar]
- 58.Trgovcich J, Aronson J F, Johnston R E. Fatal Sindbis virus infection of neonatal mice in the absence of encephalitis. Virology. 1996;224:73–83. doi: 10.1006/viro.1996.0508. [DOI] [PubMed] [Google Scholar]
- 59.Walter A E, Turner D H, Kim J, Lyttle M H, Muller P, Mathews D H, Zuker M. Coaxial stacking of helixes enhances binding of oligoribonucleotides and improves predictions of RNA folding. Proc Natl Acad Sci USA. 1994;91:9218–9222. doi: 10.1073/pnas.91.20.9218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang E, Barrera R, Boshell J, Ferro C, Freier J E, Navarro J C, Salas R, Vasquez C, Weaver S C. Genetic and phenotypic changes accompanying the emergence of epizootic subtype IC Venezuelan equine encephalitis viruses from an enzootic subtype ID progenitor. J Virol. 1999;73:4266–4271. doi: 10.1128/jvi.73.5.4266-4271.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weaver S C, Salas R, Rico-Hesse R, Ludwig G V, Oberste M S, Boshell J, Tesh R B. Re-emergence of epidemic Venezuelan equine encephalomyelitis in South America. Lancet. 1996;348:436–440. doi: 10.1016/s0140-6736(96)02275-1. [DOI] [PubMed] [Google Scholar]
- 62.Welsh R M, Sen G C. Nonspecific host responses to viral infections. In: Nathanson N, Ahmed R, Gonzalez-Scarano F, Griffin D E, Holmes K V, Murphy F A, Robinson H L, editors. Viral pathogenesis. Philadelphia, Pa: Lippincott-Raven; 1997. pp. 109–141. [Google Scholar]