ABSTRACT
Healthcare workers (HCWs) are trusted sources of information for vaccination and their attitude toward vaccination is thus critical. We aimed to synthesize existing literature on healthcare workers’ HPV vaccine confidence and their practices of recommending this vaccine. We conducted a systematic literature review and meta-analysis, with the search conducted last in March 2024. For the inclusion criteria, the studies needed to include healthcare worker practices or behaviors on recommending the HPV vaccination. Seventy-three articles were included. The proportions of HCWs recommending varied considerably by region and gender of the recipient, but there was no statistically significant difference in income level or pre- or post-HPV vaccine introduction into the national vaccination program. The main barriers to recommending HPV vaccination were concerns around safety and efficacy, cost, parental concerns, and systemic barriers. The results illustrate the importance of contextually adapted approaches to improving vaccine acceptance and recommendation.
KEYWORDS: Human papillomavirus, healthcare workers, vaccine confidence, recommendation behavior, systematic review, meta-analysis
Introduction
Human papilloma virus (HPV)-related cancers affect approximately 625,600 women and 69,400 men per year, with cervical cancer being the most common HPV-related cancer.1 Despite being preventable, cervical cancer remains one of the leading causes of cancer-related deaths among women worldwide.2,3 In 2020, the global incidence rate of cervical cancer reached 13.3 cases and resulted in 7.2 deaths per 100,000 women-years4 with significantly higher incidence and mortality rates in low- and middle-income countries (LMICs).5,6 The World Health Organization (WHO) advocates two primary prevention strategies for cervical cancer: the widespread adoption of the HPV vaccine and cervical cancer screening programs.7
Although the HPV vaccine has demonstrated efficacy in protection against major HPV strains associated with cervical cancer,2,8,9 global vaccination coverage remains low.10 In 2021, an estimated 12.2% vaccine coverage was reported amongst 15-year-old females globally, ranging from 51.5% coverage in high-income countries to 11.3% in low-middle-income countries.10 This low coverage has been attributed to the limited availability of the vaccine given its high cost in some settings, but also due to safety concerns about the vaccine among adolescents, parents, and communities.11
MacDonald et al. define vaccine hesitancy as a “delay in acceptance or refusal of vaccines, despite vaccine available services” and is a complex and context-specific phenomenon.12 Vaccine hesitancy has been defined as both a behavior and a state of indecisiveness, which is characterized by the WHO 3C model: Confidence, Complacency, and Convenience.12,13 Confidence refers to trust in the vaccine’s safety, efficacy, and the system delivering the vaccine, healthcare workers (HCWs), and policy makers.a Complacency denotes the perception of a low risk of disease and the belief that vaccination is unnecessary, while convenience involves factors such as vaccine availability, affordability, and willingness-to-pay for vaccines.12 Understanding these dimensions for different contexts and vaccines is fundamental to increasing coverage.
Healthcare workers (HCWs) are considered trusted sources of information about vaccines,14,15 play a vital role in shaping public perceptions, and are influential in improving vaccine uptake.15–17 However, globally, declining vaccine confidence among HCWs, is a major public health concern.17,18 Previous reviews have examined HCWs perceptions and knowledge on vaccines14 and their recommendation quality for adolescents in the USA.19 To expand on this literature, we aimed to review existing literature measuring HCW practices on recommending the HPV vaccine globally. By synthesizing and critically evaluating available studies, we aim to elucidate the factors influencing HCW recommendations for the HPV vaccine, providing insights for global efforts aimed at enhancing vaccine confidence and uptake.
Materials and methods
We conducted a systematic literature review based on the following research question: What are health care workers’ recommendation behaviors for the human papilloma virus (HPV) vaccine? The full systematic review protocol is available in Appendix 1.
Search strategy
A literature search was performed in June 2023 and re-run in March 2024, in the following databases: Medline, Web of Science, CABI: CAB Abstracts, and Global Health and Sociological Abstracts and as a complementary search Publicly Available Content database was used. The search strategy was developed in Medline (Ovid) in collaboration with librarians at the Karolinska Institutet University Library. For each search concept Medical Subject Headings (MeSH-terms) and free-text terms were identified. The search was then translated, in part using Polyglot Search Translator,20 into the other databases. The strategies were peer reviewed by another librarian prior to execution.
Key search terms used were (immunization, immunization programs, exp vaccination, exp vaccines) and (anxiety, awareness, behavior, choice behavior, communication barriers, health knowledge, attitude, and practice, intention) and (health personnel, benchmarking, health care surveys, quality assurance, health care, survey and questionnaire). A de-duplication process was done using the method described by Bramer et al. (2016) and DOIs were compared to avoid duplicate articles.21 The full search strategy is available in Appendix 2.
Eligibility criteria
We included original peer-reviewed articles that focused on vaccine hesitancy/confidence, behavior, or attitudes, with data on HPV vaccine recommendation behavior or practices. The population group studied were HCWs, which includes physicians, nurses, pharmacists, and healthcare administrators.22 Dentists and students were excluded since they are not directly involved in vaccination. There were no language restrictions applied, and the databases were searched from inception.
Selection process
We used Rayyan.ai23 software for the screening process which also allowed for blinding. In the first round of screening, EG created a shortlist including any articles measuring HCW vaccine confidence/hesitancy/acceptance. The articles from this shortlist were rescreened in February 2024 to double check for any potentially missed articles. Then, two researchers (EG and KA) did a blinded title and abstract, and then full-text screening. Any discrepancies in screening between the researchers were discussed with the wider team for final inclusion. More information is provided in Appendix 1.
Data extraction
We employed a two-tiered approach for data extraction and analysis. First, we collected descriptive data from each study, including publication year, study design (e.g., cross-sectional, mixed methods, or qualitative), sample size, participant demographics (such as type of healthcare worker), and geographical location (country and WHO region). The data was divided so that two researchers (KA and EG) extracted data and conducted the quality assessment on two-thirds of the articles, and another two researchers (DB and JS) did the remaining third. This allowed for all the articles to be extracted and screened by two researchers. The entire process was blinded among all the researchers until after extraction and quality assessment was completed on all articles.
Data was extracted using a form based on the research question and guidance from the JBI systematic review framework.24 For the meta-analysis, we extracted data on the study country and used World Bank income-level definitions to categorize by income, if the publication was before or after the official inclusion of the HPV vaccine in the study setting’s national guidelines, and data on total sample size, total recommending, and total willingness to recommend (including sub-groups for boys and girls). We accounted for variation in measurement tools by including any item on HCW recommendation (should, would, or do) and distinguishing between actual recommendation behaviors (practical application) and those assessing willingness to recommend (intent or inclination). Where data differentiated recommendation practices or willingness by gender or age, we further separated these into distinct sub-analyses.
Quality assessment
The quality assessment tools used were the JBI cross-sectional study25 and JBI qualitative study,26 depending on the study design. No articles were excluded on quality concerns only. However, we did take note of articles that conducted some form of validation or reliability check on the tool utilized, and that adjusted appropriately for confounders.
Data analysis
We first described key study characteristics using means and proportions and conducted a narrative synthesis of the principal findings concerning HCW attitudes and behaviors related to recommending the HPV vaccine. This step involved summarizing the main outcomes, identifying reported barriers and facilitators to vaccine recommendation and examining any additional relevant information.
We then performed a meta-analysis including all studies that reported quantitative measures of either past recommendation practices or current willingness to recommend the HPV vaccine. These papers used a variety of measures. We defined recommending behavior and willingness to recommend as binary variables. Questions about how often (i.e. always/never) or how strongly HCWs recommend the HPV vaccine were used to assess recommending behaviors, while willingness to recommend was defined as an intent or interest in recommending HPV. We used binary measures/responses to obtain the proportion of HCWs’ recommendation behavior or willingness when a binary measure was used directly. If the authors generated a binary outcome from a scale (i.e., Likert scale) we used that, and if there no binary measure was provided in the article, we derived a binary from the top two to three responses (i.e. Always, Almost Always, Sometimes or Strongly Agree, Agree, Neutral).
To accommodate potential variability among the studies, we applied random-effects meta-analyses, using the metaprop command in Stata SE18,27 which estimates a pooled proportion and exact binomial confidence intervals. When studies reported multiple age groups, we used the mean proportion for our calculations. We also conducted sub-group analyses by study design (limited to cross-sectional and mixed methods), the income status of the study setting (according to the World Bank classification), WHO region, and the publication date in relation to when the HPV vaccine was officially included in the study countries national guidelines. These sub-groups were selected due to their potential influence on HPV vaccine uptake and coverage, and completeness of reporting.
Results
Overall 10,877 articles were returned from the search, 73 articles were selected for final inclusion, and 65 were included in the meta-analysis (Figure 1). Sixty-five of the articles used a cross-sectional design,28–91 four used mixed-methods,44,92–94 and four had a qualitative study design.95–98 All the articles were published since 2005, which is 1 year prior to when the HPV vaccine first became available in the USA.99 The sample sizes in the cross-sectional studies varied widely with a mean of 525 and median of 245 participants (range: 9–5270). The vast majority of studies were conducted in high-income countries with 73% (53/73) articles in high-income countries,29–31,34,35,37–39,41,43–51,54–67,69,70,72–74,79,81,83,85–94,97,98,100 11 in upper-middle,33,36,40,53,71,75–78,82,84 8 in lower-middle,28,32,42,52,68,80,95,96 and none in a low-income country, as illustrated in Figure 2. Notably, 41% (30/73) of the studies were done in the USA, which is where the HPV vaccine was first introduced in the world.30,31,35,38,39,41,43–45,49,55,56,62,63,65,67,70,74,79,81,83,86,88–91,94,97, 100 A full summary of the included 73 articles is presented in Appendix 3.
Figure 1.
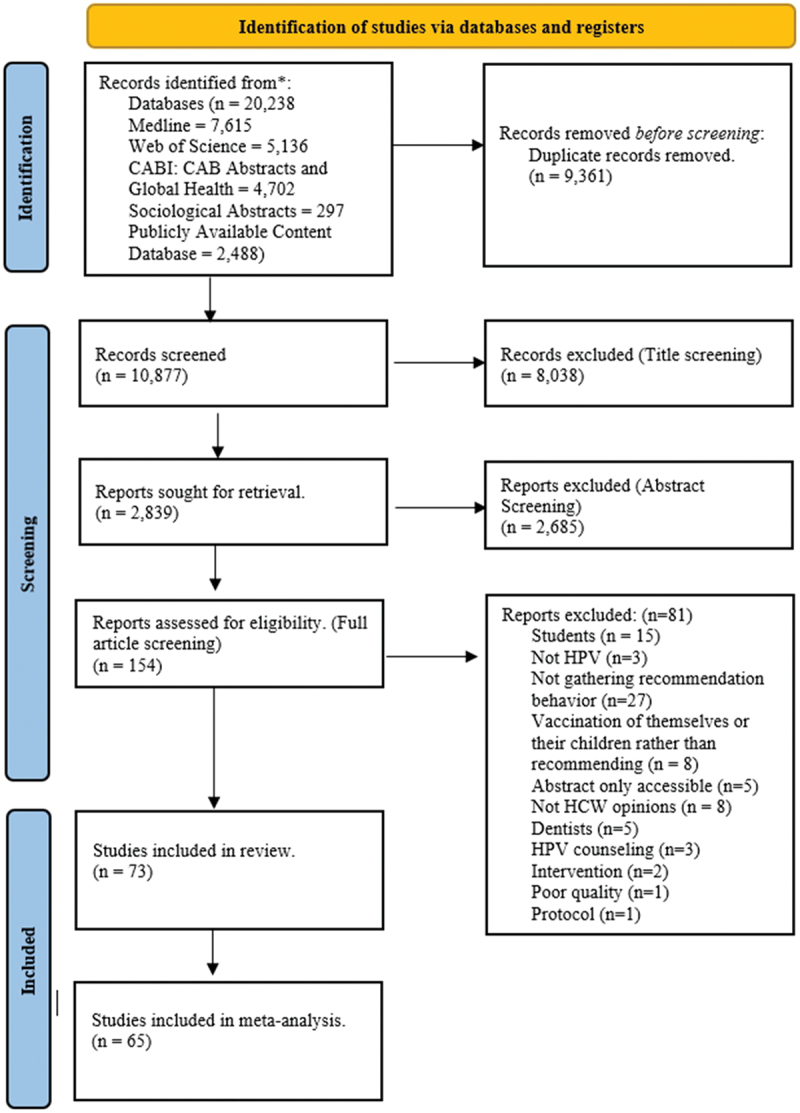
PRISMA flow diagram for systematic review article inclusion.
Figure 2.
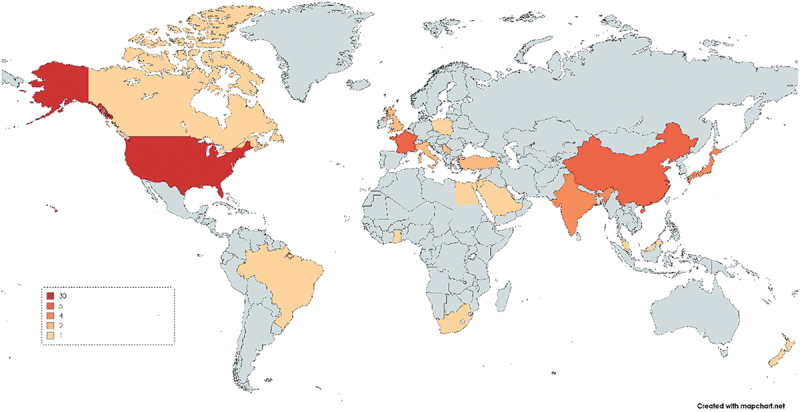
Global map with number of articles per country (MapChart)*.
*One article80 had participants from 23 African countries, but since the specific countries were not named by the authors this article was not included in this map.
Meta-analyses of recommendation behavior
From the meta-analysis results, we observed substantial heterogeneity in the proportion of HCW who reported previously recommending the HPV vaccine (or expressing a strong recommendation) across the included studies. Figure 3 presents the overall Forest plot for HCWs’ HPV recommendation behavior, with 35 articles presenting this data; the pooled proportion was 60% (95% CI: 0.50, 0.69). However, the proportion varied considerably, ranging from nearly 100%,52,92 to less than 20% of HCWs recommending the HPV vaccine.29,38 The use of different measures and target populations may have contributed to this variability along with different levels of hesitancy.
Figure 3.
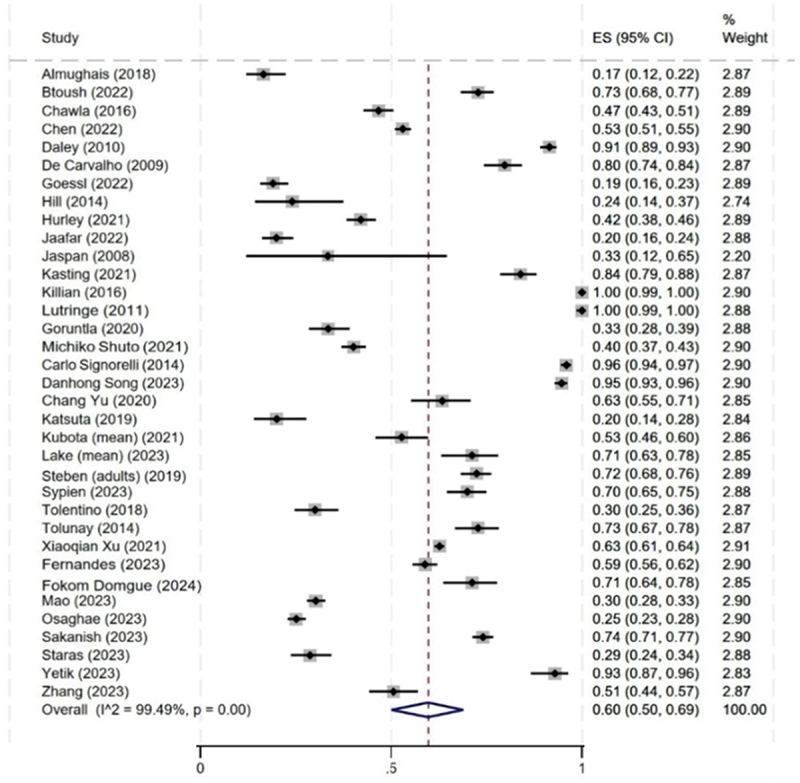
Proportion of healthcare workers recommendation behavior (practice) for both boys and girls (n = 35).
We found a higher overall proportion of HCWs expressing willingness to recommend the HPV vaccine (0.73 [95% CI: 0.65, 0.80]) compared to those who reported having already recommended it (0.60 [95% CI: 0.50, 0.69]). Twenty-four studies assessed HCWs’ willingness (or intent) to recommend the vaccine, and the findings are presented in Figure 4. Across these studies, more HCWs cited a willingness to recommend than those who strongly recommended in practice.29,33,40,42,53,68–71,76,78,84,100
Figure 4.
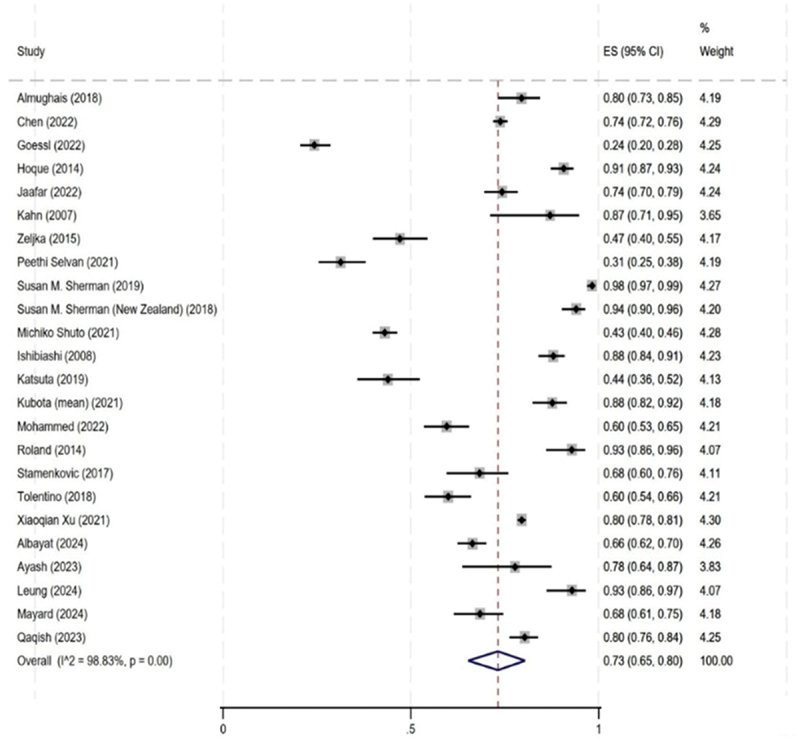
Proportion of healthcare workers recommendation willingness (intent) for both boys and girls (n = 24).
The sub-group analyses illustrate some of the factors behind this wide variation in results – Figure 5. Ninety-three percent (95% CI: 0.80, 0.99) of HCWs from the European region reported that they are recommending the HPV vaccine, whereas in the Eastern Mediterranean region only around 19% (95% CI: 0.16, 0.22) are recommending the HPV vaccine. Appendix 4 illustrates willingness to recommend by subgroups (region, income level, and HPV introduction). There was no significant difference in recommendation behavior by income status (low income = 0.42, upper middle income = 0.60 and high income = 0.62) or pre-post introduction (pre-introduction = 0.58 and post-introduction = 0.61). Meanwhile, there was a greater proportion of HCWs who have recommended to girls compared with boys (in studies where this was assessed separately), 58% (95% CI: 0.40, 0.75) and 29% (95% CI: 0.18, 0.41), respectively (Appendix 5).
Figure 5.
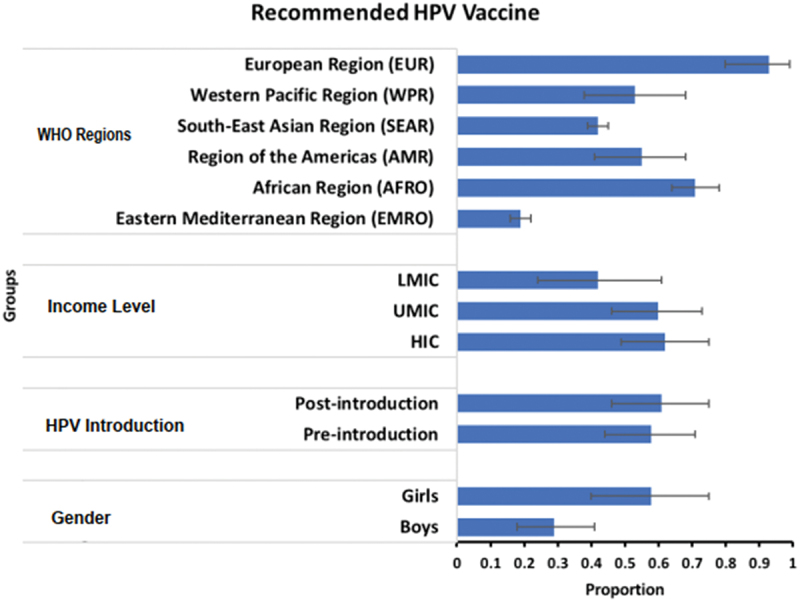
Bar chart of overall proportion for the sub-analyses of WHO region, income level, HPV introduction time, and gender.
Facilitators and barriers
To understand what influences HCWs’ recommendation behavior, we analyzed the studies for reported facilitators and barriers to HCWs confidence in recommending the HPV vaccine. Fifty-five of the studies gathered information specifically on barriers as part of the study.28,30,31,33–37,39,42–45,47–59,61–64,66,69–78,80,82–84,86,91–98,100,101 Figure 6 illustrates the main barriers cited by the HCWs in the included studies. The most cited barriers to recommending were concerns about the efficacy or safety of the vaccine,31,33,34,36,42,44,45,51–54,56,57,59–61,63,64,66,69,73,75,78,82,84,91,93,95,96 the cost of the HPV vaccine,28,34,35,39,42,43,45,49,52,53,55,56,61–63,69,71–73,75,80,94,96,97 and concerns about parental vaccine hesitancy.28,35,43,44,47,49,51,55,60,69–72,74,76,82,91–94,96–98,100
Figure 6.
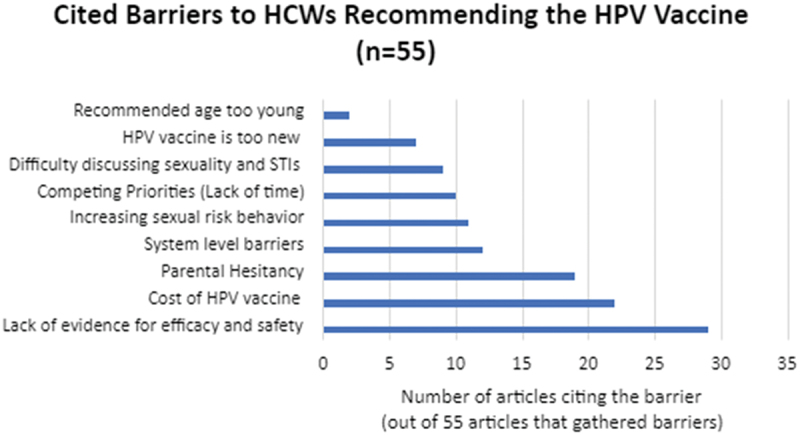
Cited barriers to recommending HPV (n=55).
Facilitators were not explicitly gathered by the articles, but several presented some demographic characteristics associated with HCWs who were willing to recommend the HPV vaccine. Among the relevant studies shown in Appendix 6, 19/21 found female providers versus male providers,28,29,31,33–35,48,53,69,72–75,80,82,86,88,89,95 17/17 found those with higher knowledge scores,28,29,31,45,46,48,61,73,74,78,79,82,84,87,89,95,100 10/13 found younger providers versus older,29,33,44,52,68,69,86,88,92,100 8/9 found OB/GYNs versus other physician groups,42,54,64,73,75,78,82,89 and 4/4 found urban providers versus rural were more likely recommend.38,51,77,86
Quality of included studies
Thirty-nine out of 65 (60%) of the studies that used a cross-sectional design reported a form of psychometric validity or reliability testing of the survey tool used.29,31,34,35,37,45–47,49–52,56,57,59,61,62,64,65,67–76,78–80,84,86,88,91,94,100 These studies either conducted some form of validation testing on the survey questions used or used a survey tool that has previously been validated to measure vaccine hesitancy and provider recommendation behavior. One of the limitations is that there is not a widely accepted or common survey tool that is validated to assess provider willingness to recommend vaccines.102
Another important quality assessment is to determine if the studies adjusted for confounders. Out of the 65 cross-sectional studies, 23 studies32,33,39,41,43,50,52,54–57,59,60,65,72–75,77,84,86,94,103 did not fully control for confounders, based on the research team’s quality assessments (available in Appendix 7). For the qualitative and mixed-methods studies, they generally fulfilled the quality assessment criteria, but only one study revealed the role of researcher within the study.98
Discussion
We aimed to review existing literature exploring HCW behavior toward recommending the HPV vaccine. 73 articles that measured HCW attitudes toward recommending the HPV vaccine were included in the review. We found significant differences in HCW recommendation practice across the WHO regions from over 90% in the European region to less than 30% in the EMRO. There was also a statistically significant difference in HCWs recommending to girls compared with boys. While other the factors of income and HPV vaccine introduction did not demonstrate any statistical significance. Additionally, there are more HCWs that indicated a willingness to recommend the HPV vaccine compared to HCWs who have previously recommended it. This illustrates a gap in HCW workers’ intention and actual behavior. Similarly, a 2021 systematic review of 17 vaccines including HPV vaccine, found that there is a significant prevalence of HCW vaccine hesitancy related to inadequate knowledge, low confidence, and low self-vaccination.14 It is critical to examine and address this hesitancy as HCWs are trusted information sources and can influence patient or parental vaccine decision-making.14,16 These systematic reviews and meta-analysis results indicate mixed levels of HCW HPV vaccine confidence and that it varies by geographic location and gender of the vaccine recipient.
A deeper analysis shows the ways the 3Cs of confidence, complacency, and convenience impact HCWs’ recommendations. One notable barrier to HCW vaccine confidence was concerns around parental hesitancy. A report by the European Centre for Disease Control provides resources on strategies to address parental hesitancy or refusal.104 Inclusion of these strategies, such as a framework for communicating,105 motivational interviewing,106 and the SARAH (Strategies and resources to assist hesitant parents with vaccination) method,107 into HCW vaccine training could alleviate some of these concerns. This should focus on building confidence by addressing concerns about vaccine safety, efficacy, and side effects through trusted expert-led education and open communication.
Another confidence-related barrier was that HCWs were slightly more hesitant in settings where the HPV vaccine was not yet introduced into the official vaccine schedule. A few studies also mentioned the HPV vaccine being “too new” as a barrier to recommending the vaccine. Larson et al. report that there are noted population level increases in vaccine hesitancy surrounding new policies or newly reported vaccine risks, and they draw particular attention to hesitancy surrounding the MMR, COVID-19, and HPV vaccines.65 However, the difference in proportion was very small, and so it is important to consider the barriers to HCWs recommending during both the introduction of the HPV vaccine and even years later. Additionally, similar to the findings from Kong et al. in the United States,19 higher knowledge levels were associated with a higher likelihood to recommend. These illustrate the importance of ensuring good HCW vaccine education as a method for improving general population vaccine uptake.
There also appeared to be limiting factors around the convenience of recommending the vaccine due to competing interests. In several articles, the HCWs mentioned a lack of time as a limitation to recommending the HPV vaccine. This indicates that the HPV vaccine may not be of a high priority within their workflow due to logistical constraints. A few other studies have similarly shown that HCWs do not feel they have adequate time or resources to address vaccine hesitancy.14,108,109 This could be a possible explanation for the notable gap between intention to recommend and actual vaccination practice. Thus, streamlining vaccination access by offering on-site clinics, reducing administrative burdens associated with vaccination, being well trained in vaccine education, and having prepared information resources could improve convenience for HCWs.
The studies also revealed challenges relating to system-level concerns around cost, cold-chain management, and access to the HPV vaccine. In a qualitative study in Nigeria,110 a similar result was found that HCWs had lower confidence due to the precarious nature of the health system. Thus, our findings suggest a multifaceted approach is needed while addressing HCW vaccine hesitancy, similar to parental hesitancy.
Additionally, the group analyses of the meta-analysis revealed stark variation in vaccine confidence by region. Particularly, complacency and cultural barriers around HPV vaccination seems to be a concern in the EMRO region. In this region, there was a notably lower proportion of HCWs recommending the vaccine compared with the other WHO regions. Among the nine studies conducted in the EMRO region, six reported that HCWs cited cultural reasons or difficulty discussing sexuality and sexually transmitted diseases as a barrier to recommending.28,42,68,69,75,84 In another review on the impact of Islam on HPV vaccine acceptability in the Middle East and North Africa, Hamdi explains that countries in this region often have more traditional norms and, thus, it is thought that if the tenets of Islam regarding sexuality are followed then the risk of HPV or other sexually transmitted diseases (STDs) is very low.111 This contributes to a false belief, due to limited reporting, that STDs are rare in this region.111
These preconceived judgments that HPV and other STDs are not relevant make the HCWs become complacent in this region. Our results similarly illustrate that HCWs in the EMRO region felt that the HPV vaccine was not needed in their setting68,69,84,85 or mentioned other cultural or religious barriers to recommending the HPV vaccine.28,42,68,69,75,84 Especially, as the sociosexual behaviors begin to drastically change in the region (more youth participating in premarital sexual behaviors and getting married at older ages) and the subsequent increased risk of STD and HPV transmission in the region, there is an urgent need to have culturally relevant strategies to improve vaccine acceptance among HCWs.111
Additionally, Western Pacific and South-Eastern Asia Regions also both had slightly below half of HCWs recommending the HPV vaccine overall seemingly due to cultural confidence and complacency-related concerns. An article from Wong et al., explains that there are many social and cultural norms influencing HPV vaccine uptake in Asia, such as: parental concerns around the vaccine increasing sexual risk behavior, complacency due to religious beliefs to only engage in marital sexual activity, stigma of promiscuity associated around taking the vaccine, beliefs in local remedies, the vaccine being considered non-halal, and concerns around the rise of manufacturing from India and China.112 Similarly to the EMRO region, Wong et al., recommend that social-culturally sensitive approaches using community and religious leaders are needed to better overcome vaccine hesitancy in Asia.112
Contributing to the lack of confidence and trust in the vaccine and the institutions that prompt it, the Japanese government suspended the recommendation of the HPV vaccine between July 2013 and November 2021 due to highly publicized alleged adverse events, which sowed public distrust of the vaccine.113–115 Among the four articles from Japan in our review, none of them had more than 50% always/often recommending the HPV vaccine.54,59,64,87 Thus, in Japan, it is particularly important to disseminate accurate information about cervical cancer, HPV, and the HPV vaccine14,104–110,114
Gaps in literature
Overall, we found strong evidence on HCW HPV vaccine hesitancy in some regions, but some gaps remain. The literature covers many different types of healthcare workers and patient groups but lacks socioeconomic diversity. Currently, there are very few studies (8/73) in LMICs. This could be because many LMICs have not yet or recently introduced the HPV vaccine into their routine immunization programs. Since 2019, 52 countries have introduced the HPV vaccine, many of which are LMICs,116 thus we would anticipate fewer studies in these countries.21,26,36,46,59 However, this could also be due to limited resources or disease burden prioritization in these areas117 hence further research should be done in LMICs particularly because we found an indication of high HCW HPV vaccine hesitancy, and because of the higher incidence rate of cervical cancer and lower vaccination uptake in those countries.7
Limitations
There are some limitations to our study. First, due to the large number of studies found in the search, two sets of researchers conducted the extraction and quality assessment. This could have led to some variety in the results, but we ensured that at least two researchers extracted data and conducted a quality assessment on each article included in the review. Second, for the meta-analysis the articles did not have consistent tools or items that they measured, and thus the proportions were calculated from a diversity of measures.118
Lastly, the systematic review demonstrates that only slightly over half (39/67) of all the included studies utilizing a survey or questionnaire described a clear tool validation and reliability procedure. The studies were either not conducting validity testing or not using an existing validated tool, or not presenting it in the articles, which may diminish the trustworthiness of the results presented. Utilizing well-validated tools is critical for ensuring that the tools are reliable, accurate, and effective ways to measure HCW vaccine confidence and sentiments on recommendation.118 Thus, we recommend that future studies on HCW vaccine confidence attempt to use a standardized and validated survey tool.
Conclusion
HCWs are critical for supporting vaccine acceptance and uptake as they are one of the most trusted sources of vaccine information.
It is valuable to use this existing research to help inform efforts to improve HCW HPV recommendation practice. Our results show that there are notable levels of HPV vaccine hesitancy among HCWs, but HCWs’ recommendation behaviors vary based on many factors, including geographic region of practice and concerns around parental hesitancy. This indicates a need for more contextual relevant approaches to addressing HCW vaccine hesitancy among HCWs. Additionally, efforts need to address HPV vaccine literacy around concerns of safety and efficacy about the HPV vaccine, and other systemic-level changes (cost, stocking issues, physical accessibility) are important to ensure HCWs are confident and able to recommend the HPV vaccine.
Supplementary Material
Acknowledgments
We would like to acknowledge the Karolinska Institute Librarians (Narcisa Hannerz) who supported search strategy and conducted the search and duplicate deletion process in collaboration with the research team.
Biographies
Damola Bakare is a dynamic and accomplished professional with a dual background in public health and biochemistry, holding a master’s in public health (MPH) and a Bachelor of Science in Biochemistry. Currently, he is a dedicated team member of the MOXY-IR Project in Kano State, Nigeria, actively contributing to program planning, implementation, and evaluation to enhance healthcare delivery and outcomes. His expertise in quantitative data management enables meticulous analysis and interpretation of complex data, crucial for evidence-based decision-making and strategic program development, including assessing intervention effectiveness and identifying areas for improvement. As Data Manager for the INSPIRING Project in Jigawa State, he excels in data collection, cleaning, and analysis, transforming raw data into actionable insights that shape future strategies. In addition to his analytical abilities, he leverages strong communication and interpersonal skills to forge meaningful partnerships and educate stakeholders on public health issues, driving collaborative change.
Elisa Gobbo is a doctoral student at the Karolinska Institutet in Stockholm, Sweden. She completed her master’s in health economics, management, and policy in 2023. Her focus is on implementation science, sustainable adolescent health, and vaccine hesitancy.
Kofoworola Olamide Akinsola is a public health researcher at Oxygen for Life Initiative, UCH, in Ibadan, Nigeria, where she manages various research projects. She holds a Master of Public Health in Field Epidemiology from the University of Ibadan, Nigeria. Her research focuses on global health, health services, and health systems.
Ayobami Bakare is a community health physician at the University College Hospital, Ibadan, Nigeria, and a doctoral student at the Karolinska Institutet, Sweden. Dr. Bakare’s research area is focused on child health with particular interest in vaccine, oxygen, and nutrition.
Julius Salako is a highly driven public health expert with an extensive professional background in health initiatives, health services analysis, and health system enhancement. He holds a master’s degree in public health (MPH) and my focus lies in research and developing interventions at both facility and community levels to enhance human health. Over the past 5 years, he has served as a field researcher overseeing evaluation activities at the state level for various health projects in Nigeria, particularly in the North Central and Northwest regions of Nigeria. In this role, he has managed the day-to-day operations of multiple research endeavors and ensured strict adherence to project research protocols.
Claudia Hanson is a Professor in Implementation Science and Perinatal Epidemiology. She is trained as a physician and epidemiologist, and her key interest lies in implementation science and health systems research. She leads and co-leads several projects in Africa, Asia, and Latin America to improve access and quality of health care.
Sibylle Herzig van Wees is an Associate Professor at the Karolinska Institutet at the department of Global Public Health. She is a global behavioral and implementation science researcher working with mixed methods. Currently, she researches vaccine confidence, develops interventions to strengthen vaccine confidence and evaluates these interventions in Sweden, Nigeria, and Ethiopia. She is currently PI on several projects, including: a Forte funded project that investigates vaccine hesitancy in Sweden (2021–2023).
Adegoke Falade is a paediatric pulmonologist at the University of Ibadan, Ibadan, Nigeria. For 3 decades, he has led and collaborated with international experts in conducting clinical research projects involving children, especially Nigerian under-5s and neonates. Professor Falade led a quasi-prospective study titled: Pneumonia hospitalizations and mortality in children 3 - 24-month-old in Nigeria from 2013 to 2020: Impact of pneumococcal conjugate vaccine ten valent (PHiD-CV-10), which was published in Hum Vaccin Immunother, 2023 Dec 31;19(1):2162289. doi: 10.1080/21645515.2022.2162289. Epub 2023 Jan 3. PMID: 36597576.
Carina King is an infectious disease epidemiologist at the Department of Global Public Health, Karolinska Institutet. Her research primarily focuses on evaluating interventions to reduce child mortality and improve primary care case management for pneumonia in low-resource contexts.
Funding Statement
The project was supported by The Swedish Research Council 2022-00756. Vetenskapsrådet [2022-00756].
Note
Vaccine hesitancy, vaccine confidence, and vaccine acceptance are used interchangeably throughout the literature, but do have varying definitions and focuses. For the purpose of this paper, we will use the term vaccine confidence.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Disclaimers
The submitted article are the research teams own and not an official position of the institution or funder.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2024.2402122
References
- 1.World Health Organization. World Health Organization . Human papillomavirus and cancer. 2023. [accessed 2023 Nov 21]. https://www.who.int/news-room/fact-sheets/detail/human-papilloma-virus-and-cancer.
- 2.Burmeister CA, Khan SF, Schäfer G, Mbatani N, Adams T, Moodley J, Prince S.. Cervical cancer therapies: current challenges and future perspectives. Tumour Virus Res. 2022. June;13:200238. doi: 10.1016/j.tvr.2022.200238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buskwofie A, David-West G, Clare CA. A review of cervical cancer: incidence and disparities. J Natl Med Assoc. 2020. Apr;112(2):229–13. doi: 10.1016/j.jnma.2020.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Singh D, Vignat J, Lorenzoni V, Eslahi M, Ginsburg O, Lauby-Secretan B, Arbyn M, Basu P, Bray F, Vaccarella S. Global estimates of incidence and mortality of cervical cancer in 2020: a baseline analysis of the WHO global cervical cancer elimination initiative. Lancet Glob Health [Internet]. 2023. Feb 1 [cited 2023 Nov 6]. 11(2):e197–206. http://www.thelancet.com/article/S2214109X22005010/fulltext. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. [2018 Nov 12];68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 6.Torre LA, Islami F, Siegel RL, Ward EM, Jemal A. Global cancer in women: burden and trends. Cancer Epidemiol, Biomarker & Prev. [2017 Apr 1];26(4):444–457. doi: 10.1158/1055-9965.EPI-16-0858. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization . Global strategy to accelerate the elimination of cervical cancer as a public health problem [internet]. Geneva; 2020. [cited 2023 Nov 6]. http://apps.who.int/bookorders. [Google Scholar]
- 8.Kamolratanakul S, Pitisuttithum P. Human papillomavirus vaccine efficacy and effectiveness against cancer. Vaccines (Basel) [Internet]. 2021. Dec 1 [cited 2024 June 7];9(12). Available from:/pmc/articles/PMC8706722/ 10.3390/vaccines9121413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ananina OA, Kolomiets LA, Zhuykova LD, Churuksaeva ON, Chernyshova AL, Villert AB, Pikalova LV, Mosolkov VY. Efficacy of HPV vaccine in preventing cervical cancer in the Tomsk region. Opuholi ženskoj reproduktivnoj sistemy [Internet] 2023. June 26 [accessed 2024 June 10];19(1):120–128. https://ojrs.abvpress.ru/ojrs/article/view/1083. [Google Scholar]
- 10.Spayne J, Hesketh T. Estimate of global human papillomavirus vaccination coverage: analysis of country-level indicators. BMJ Open [Internet]. 2021. [accessed 2023 Sep 7];11(9):52016. 10.1136/bmjopen-2021-052016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cadeddu C, Castagna C, Sapienza M, Lanza TE, Messina R, Chiavarini M, Ricciardi, W, De Waure, C. Understanding the determinants of vaccine hesitancy and vaccine confidence among adolescents: a systematic review. Vol. 17. Philadelphia, PA: Human Vaccines and Immunotherapeutics. Taylor and Francis Ltd.; 2021. p. 4470–4486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.MacDonald NE, SAGE Working Group on Vaccine Hesitancy . Vaccine hesitancy: definition, scope and determinants. Vaccine. 2015. Aug;33(34):4161–4. doi: 10.1016/j.vaccine.2015.04.036. [DOI] [PubMed] [Google Scholar]
- 13.Bussink-Voorend D, Hautvast JLA, Vandeberg L, Visser O, Hulscher MEJL. A systematic literature review to clarify the concept of vaccine hesitancy. Nat Hum Behav [Internet]. 2022. Dec 1 [accessed 2023 Jul 4];6(12):1634–1648. https://pubmed.ncbi.nlm.nih.gov/35995837/. [DOI] [PubMed] [Google Scholar]
- 14.Lin C, Mullen J, Smith D, Kotarba M, Kaplan SJ, Tu P. Healthcare providers’ vaccine perceptions, hesitancy, and recommendation to patients: a systematic review. Vaccines (Basel). [2021 Jul 1];9(7):713. doi: 10.3390/vaccines9070713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen AK, Browne S, Srivastava T, Michel JJ, Tan ASL, Kornides ML. Factors influencing parental and individual COVID-19 vaccine decision making in a pediatric network. Vaccines (Basel). [2022 Aug 8];10(8):1277. doi: 10.3390/vaccines10081277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vorsters A, Bonanni P, Maltezou HC, Yarwood J, Brewer NT, Xavier Bosch F, Hanley S, Cameron R, Franco EL, Arbyn M, et al. The role of healthcare providers in HPV vaccination programs – a meeting report. Papillomavirus Res. 2019. [accessed 2023 Oct 23];8:100183. doi: 10.1016/j.pvr.2019.100183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paterson P, Meurice F, Stanberry LR, Glismann S, Rosenthal SL, Larson HJ. Vaccine hesitancy and healthcare providers. Vaccine. [2016 Dec 20];34(52):6700–6. doi: 10.1016/j.vaccine.2016.10.042. [DOI] [PubMed] [Google Scholar]
- 18.de Araújo TM, Souza F, Pinho PDS, Werneck GL. Beliefs and sociodemographic and occupational factors associated with vaccine hesitancy among health workers. Vaccines (Basel) [Internet]. 2022. Dec 1 [accessed 2024 June 7];10(12). https://pubmed.ncbi.nlm.nih.gov/36560423/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kong WY, Oh NL, Kennedy KL, Carlson RB, Liu A, Ozawa S, Brewer NT, Gilkey MB, et al. Identifying healthcare professionals with lower human papillomavirus (HPV) vaccine recommendation quality: a systematic review. J Adolesc Health. [2024 May 1]; 74(5):868–77. doi: 10.1016/j.jadohealth.2023.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clark JM, Sanders S, Carter M, Honeyman D, Cleo G, Auld Y, Booth D, Condron P, Dalais C, Bateup S, et al. Improving the translation of search strategies using the polyglot search Translator: a randomized controlled trial. J Med Lib Assoc. [2020 Apr 1];108(2). doi: 10.5195/jmla.2020.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bramer WM, Giustini D, de Jonge GB, Holland L, Bekhuis T. De-duplication of database search results for systematic reviews in EndNote. J Med Libr Assoc. 2016. Jul;104(3):240–243. doi: 10.3163/1536-5050.104.3.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.OSHA (Occupational Safety and Health Administration). US Department of Labor . Healthcare - overview. 2023. [accessed 2023 Oct 15]. https://www.osha.gov/healthcare.
- 23.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Systematic Rev. 2016;5(1):210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Santos WD, Secoli SR, Püschel VDA. The Joanna Briggs institute approach for systematic reviews. Rev Lat Am Enfermagem. [2018 Nov 14];26. doi: 10.1590/1518-8345.2885.3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moola S, Munn Z, Tufanaru C, Arinataris E, Sfetc R, Currie M, Lisy K, Qureshi R, Mattis P, Mu P. Chapter 7: systematic reviews of etiology and risk. In: Aromataris E, Munn Z. editors. JBI manual for evidence synthesis. JBI; 2020. [Google Scholar]
- 26.Lockwood CM, Munn Z, Porritt K. Qualitative research synthesis: methodological guidance for systematic reviewers utilizing meta-aggregation. Int J Evid Based Healthcare. 2015;13(3):179–187. doi: 10.1097/XEB.0000000000000062. [DOI] [PubMed] [Google Scholar]
- 27.Nyaga VN, Arbyn M, Aerts M. Metaprop: a stata command to perform meta-analysis of binomial data. Arch Public Health. 2014. Nov 10 [accessed 2024 June 7];72(1):1–0. 10.1186/2049-3258-72-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abi Jaoude J, Saad H, Farha L, Dagher H, Khair D, Kaafarani MA, Jamaluddine Z, Cherfan P. Barriers, attitudes and clinical approach of Lebanese physicians towards HPV vaccination; a cross- sectional study. Asian Pac J Cancer Prev. 2019;20(10):3181–3187. doi: 10.31557/APJCP.2019.20.10.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Almughais ES, Alfarhan A, Salam M. Awareness of primary health care physicians about human papilloma virus infection and its vaccination: a cross-sectional survey from multiple clinics in Saudi Arabia. Infect Drug Resist. 2018;11:2257–2267. doi: 10.2147/IDR.S179642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ayres S, Gee A, Kim S, Hashibe M, Praag A, Kaiser D, Chang C-P, Brandt HM, Kepka D. Human papillomavirus vaccination knowledge, barriers, and recommendations among healthcare provider groups in the Western United States. J Canc Educ. [2022 Dec 1];37(6):1816–1823. doi: 10.1007/s13187-021-02047-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Btoush R, Kohler RK, Carmody DP, Hudson SV, Tsui J. Factors that influence healthcare provider recommendation of HPV vaccination. Am J Health Promot. [2022 Sep 1];36(7):1152–1161. doi: 10.1177/08901171221091438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chawla PC, Chawla A, Chaudhary S. Knowledge, attitude & practice on human papillomavirus vaccination: a cross-sectional study among healthcare providers. Indian J Med Res. [2016 Nov 1];144(NOVEMBER):741–749. doi: 10.4103/ijmr.IJMR_1106_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen S, Mei C, Huang W, Liu P, Wang H, Lin W, Yuan S, Wang Y. Human papillomavirus vaccination related knowledge, and recommendations among healthcare providers in Southern China: a cross-sectional survey. BMC Women’s Health. [2022 Dec 1];22(1). doi: 10.1186/s12905-022-01728-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu C, Chen L, Ruan G, An J, Sun P. Evaluation of knowledge and attitude toward hpv and vaccination among medical staff, medical students, and community members in Fujian province. Risk Manag Healthc Policy. 2020;13:989–997. doi: 10.2147/RMHP.S243048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Daley MF, Crane LA, Markowitz LE, Black SR, Beaty BL, Barrow J, Babbel C, Gottlieb SL, Liddon N, Stokley S, et al. Human papillomavirus vaccination practices: a survey of US physicians 18 months after licensure. Pediatrics. 2010;126(3):425–433. doi: 10.1542/peds.2009-3500. [DOI] [PubMed] [Google Scholar]
- 36.de Carvalho NS, Teixeira LM, Pradel EM, Gabardo J, Joly C, Urbanetz AA. Vaccinating against HPV: physicians’ and medical students’ point of view. Vaccine. [2009 May 5];27(20):2637–2640. doi: 10.1016/j.vaccine.2009.02.046. [DOI] [PubMed] [Google Scholar]
- 37.Dufour L, Carrouel F, Dussart C. Human papillomaviruses in adolescents: knowledge, attitudes, and practices of pharmacists regarding virus and vaccination in France. Viruses. [2023 Mar 1];15(3):778. doi: 10.3390/v15030778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goessl CL, Christianson B, Hanson KE, Polter EJ, Olson SC, Boyce TG, Dunn D, Williams CL, Belongia EA, McLean HQ, et al. Human papillomavirus vaccine beliefs and practice characteristics in rural and urban adolescent care providers. BMC Public Health. [2022 Dec 1];22(1). doi: 10.1186/s12889-022-13751-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hill M, Okugo G. Emergency medicine physician attitudes toward HPV vaccine uptake in an emergency department setting. Hum Vaccin Immunother. [2014 Sep 1];10(9):2551–2556. doi: 10.4161/21645515.2014.970933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoque ME, Monokoane S, Van Hal G. Knowledge of and attitude towards human papillomavirus infection and vaccines among nurses at a tertiary hospital in South Africa. J Obstet Gynaecol (Lahore). 2014. Feb;34(2):182–186. doi: 10.3109/01443615.2013.861395. [DOI] [PubMed] [Google Scholar]
- 41.Hurley LP, O’Leary ST, Markowitz LE, Crane LA, Cataldi JR, Brtnikova M, Beaty BL, Gorman C, Meites E, Lindley MC, et al. US primary care physicians’ viewpoints on HPV vaccination for adults 27 to 45 years. J Am Board Fam Med. [2021 Jan 1];34(1):162–170. doi: 10.3122/jabfm.2021.01.200408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jaafar I, Atallah D, Mirza F, Abu Musa A, El-Kak F, Seoud M. Determinants of human papillomavirus vaccine recommendation among middle eastern and Lebanese healthcare providers. Clin Epidemiol Glob Health. [2022 Sep 1];17:101092. doi: 10.1016/j.cegh.2022.101092. [DOI] [Google Scholar]
- 43.Jaspan DM, Dunton CJ, Cook TL. Acceptance of human papillomavirus vaccine by gynecologists in an Urban setting. J Low Genit Tract Dis. 2008;12(2):118–121. doi: 10.1097/LGT.0b013e31815d9639. [DOI] [PubMed] [Google Scholar]
- 44.Kahn JA, Rosenthal SL, Tissot AM, David M, Bernstein I, Wetzel C. Factors influencing pediatricians’ intention to recommend human papillomavirus vaccines. Ambul PEDIATRICS. 2007;7(5). doi: 10.1016/j.ambp.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 45.Kasting ML, Head KJ, Demaria AL, Neuman MK, Russell AL, Robertson SE, Rouse, CE, Zimet, GD. A National survey of Obstetrician/Gynecologists’ knowledge, attitudes, and beliefs regarding adult human papillomavirus vaccination. J Women’s Health Mary Ann Liebert Inc. 2021;30:1476–1484. doi: 10.1089/jwh.2020.8727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khamisy-Farah R, Endrawis M, Odeh M, Tuma R, Riccò M, Chirico F, Bragazzi NL. Knowledge of human papillomavirus (HPV), attitudes, and practices towards anti-hpv vaccination among Israeli nurses. J Canc Educ [Internet]. 2023. Aug 1 [accessed 2023 Aug 17];38(4):1391–1396. 10.1007/s13187-023-02281-0. [DOI] [PubMed] [Google Scholar]
- 47.Killian M, Detoc M, Berthelot P, Charles R, Gagneux-Brunon A, Lucht F, Pulcini, C, Barbois, S, Botelho-Nevers, E. Vaccine hesitancy among general practitioners: evaluation and comparison of their immunisation practice for themselves, their patients and their children. Eur J Clin Microbiol Infect Dis. [2016 Nov 1];35(11):1837–1843. doi: 10.1007/s10096-016-2735-4. [DOI] [PubMed] [Google Scholar]
- 48.Lee YY, Wang Z, Angelillo IF. Facilitators and barriers for healthcare providers to recommend HPV vaccination to attendees of public sexually transmitted diseases clinics in Hong Kong, China. PLoS One. [2019 Jan 1];14(1):e0209942. doi: 10.1371/journal.pone.0209942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McCave EL. Influential factors in HPV vaccination uptake among providers in four states. J Community Health. [2010 Dec 1];35(6):645–652. doi: 10.1007/s10900-010-9255-4. [DOI] [PubMed] [Google Scholar]
- 50.Merriel SWD, Flannagan C, Kesten JM, Shapiro GK, Nadarzynski T, Prue G. Knowledge and attitudes of general practitioners and sexual health care professionals regarding human papillomavirus vaccination for young men who have sex with men. Int J Environ Res Public Health. [2018 Jan 18];15(1):151. doi: 10.3390/ijerph15010151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Napolitano F, Navaro M, Vezzosi L, Santagati G, Angelillo IF, Spearman P. Primary care pediatricians’ attitudes and practice towards hpv vaccination: a nationwide survey in Italy. PLoS One. Public Library of Science; 2018;13(3):e0194920. doi: 10.1371/journal.pone.0194920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goruntla N, Bhupalam P, Narayana G, Suchitra J, Kavya Suma G, Deepthi G, Jyothi CD, Kumar BP. Physician’s knowledge, attitude, and practice towards human papilloma virus (HPV) vaccine recommendation in Anantapur District, Andhra Pradesh, India. Arch pharm pract [Internet]. 2020; https://www.researchgate.net/publication/343151439.
- 53.Nikolic Z, Matejic B, Kesic V, Eric Marinkovic J, Jovic Vranes A. Factors influencing the recommendation of the human papillomavirus vaccine by Serbian pediatricians. J Pediatr Adolesc Gynecol. [2015 Feb 1];28(1):12–18. doi: 10.1016/j.jpag.2014.01.107. [DOI] [PubMed] [Google Scholar]
- 54.Nishioka H, Onishi T, Kitano T, Takeyama M, Imakita N, Kasahara K, Kawaguchi, R, Masaki, JA, Nogami, K. A survey of healthcare workers’ recommendations about human papillomavirus vaccination. Clin Exp Vaccine Res. [2022 May 1];11(2):149–154. doi: 10.7774/cevr.2022.11.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Petrusek J, Thorpe E, Britt CJ. HPV vaccination practices and attitudes among primary care physicians since FDA approval to age 45. Am J Otolaryngology - Head And Neck Med And Surg. [2020 Nov 1];41(6):102685. doi: 10.1016/j.amjoto.2020.102685. [DOI] [PubMed] [Google Scholar]
- 56.Selvan P, Kearney M, Cognetti D, Massey P, Leader A. Exploring knowledge and attitudes about human papillomavirus vaccination among school nurses in an urban school district. J Sch Health. [2021 Feb 1];91(2):125–132. doi: 10.1111/josh.12981. [DOI] [PubMed] [Google Scholar]
- 57.Sherman SM, Cohen CR, Denison HJ, Bromhead C, Patel H. A survey of knowledge, attitudes and awareness of the human papillomavirus among healthcare professionals across the UK. Eur J Public Health. Oxford University Press; 2020;30:10–16. doi: 10.1093/eurpub/ckz113. [DOI] [PubMed] [Google Scholar]
- 58.Sherman SM, Bartholomew K, Denison HJ, Patel H, Moss EL, Douwes J, Bromhead C. Knowledge, attitudes and awareness of the human papillomavirus among health professionals in New Zealand. PLOS ONE [Internet]. 2018. Dec 1 [accessed 2023 Nov 21];13(12). Available from:/pmc/articles/PMC6312361/ e0197648. doi: 10.1371/journal.pone.0197648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shuto M, Kim Y, Okuyama K, Ouchi K, Ueichi H, Nnadi C, Larson HJ, Perez G, Sasaki S. Understanding confidence in the human papillomavirus vaccine in Japan: a web-based survey of mothers, female adolescents, and healthcare professionals. Hum Vaccin Immunother. 2021;17(9):3102–3112. doi: 10.1080/21645515.2021.1918042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Signorelli C, Odone A, Pezzetti F, Spagnoli F, Visciarelli S, Ferrari A, Camia P, Latini C, Ciorba V, Agodi A, Barchitta M. Infezione da Papillomavirus umano e vaccinazione: conoscenze e ruolo dei medici di medicina generale Human Papillomavirus infection and vaccination: knowledge and attitudes of Italian general practitioners. Epidemiol Prev. 2014;38:88–92. PMID: 25759351. [PubMed] [Google Scholar]
- 61.Song D, Liu P, Wu D, Zhao F, Wang Y, Zhang Y. Knowledge and attitudes towards human papillomavirus vaccination (HPV) among healthcare providers involved in the governmental free HPV vaccination program in Shenzhen, Southern China. Vaccines (Basel). [2023 May 1];11(5):997. doi: 10.3390/vaccines11050997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Soon R, Rose M, Dela I, Drph C, Tsark JU, Chen JJ, Braun, KL. A survey of Physicians’ attitudes and practices about the human papillomavirus (HPV) vaccine in Hawaiʻi. Hawaiʻi J Med Public Health. 2015;74:234–241. [PMC free article] [PubMed] [Google Scholar]
- 63.Ishibashi KL, Koopmans J, Curlin FA, Alexander KA, Ross LF. Paediatricians’ attitudes and practices towards HPV vaccination. Acta Paediatrica Int J Paediatr. 2008. Nov;97(11):1550–1556. doi: 10.1111/j.1651-2227.2008.00958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Katsuta T, Moser CA, Offit PA, Feemster KA. Japanese physicians’ attitudes and intentions regarding human papillomavirus vaccine compared with other adolescent vaccines. Papillomavirus Res. [2019 June 1];7:193–200. doi: 10.1016/j.pvr.2019.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Roland KB, Benard VB, Greek A, Hawkins NA, Saraiya M. Primary care providers human papillomavirus vaccine recommendations for the medically underserved: a pilot study in U.S. federally qualified health centers. Vaccine. 2014;32(42):5432–5435. doi: 10.1016/j.vaccine.2014.07.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kubota M, Kondo K, Tomiyoshi Y, Fukushima W. Survey of pediatricians concerning the human papillomavirus vaccine in Japan: positive attitudes toward vaccination during the period of proactive recommendation being withheld. Hum Vaccin Immunother. 2022;18(6). doi: 10.1080/21645515.2022.2131337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lake P, Fuzzell L, Brownstein NC, Fontenot HB, Michel A, McIntyre M, Whitmer A, Rossi SL, Perkins RB, Vadaparampil ST, et al. HPV vaccine recommendations by age: a survey of providers in federally qualified health centers. Hum Vaccin Immunother. 2023;19(1). doi: 10.1080/21645515.2023.2181610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mohamed ML, Tawfik AM, Mohammed GF, Elotla SF. Knowledge, attitude, and practice of cervical cancer screening, and HPV vaccination: a Cross-sectional study among obstetricians and gynecologists in Egypt. Matern Child Health J. [2022 Mar 1];26(3):565–574. doi: 10.1007/s10995-021-03352-8. [DOI] [PubMed] [Google Scholar]
- 69.Albayat SS, Mundodan JM, Elmardi K, Hasnain S, Khogali H, Baaboura R, Al-Romaihi HE, AlKubaisi NJ, Bougmiza MI. Knowledge, attitude, and practices regarding human papilloma virus vaccination among physicians in Qatar. Women’s Health. 2024. Jan 1;20. doi: 10.1177/17455057241227360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Riedesel JM, Rosenthal SL, Zimet GD, Bernstein DI, Huang B, Lan D, Kahn JA. Attitudes about human papillomavirus vaccine among family physicians. J Pediatr Adolesc Gynecol. 2005. Dec. 18(6):3919–38. doi: 10.1016/j.jpag.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 71.Stamenkovic Z, Matejic B, Djikanovic B, Zaric M. Gynecologists’ knowledge, attitudes, and intentions toward human papillomavirus vaccination in Serbia. J Low Genit Tract Dis [Internet]. 2017. Jan 1 [accessed 2024 Mar 13];21(1):9–11. https://journals.lww.com/jlgtd/fulltext/2017/01000/gynecologists__knowledge,_attitudes,_and.3.aspx. [DOI] [PubMed] [Google Scholar]
- 72.Steben M, Durand N, Guichon JR, Greenwald ZR, McFaul S, Blake J. A national survey of Canadian physicians on HPV: knowledge, barriers, and preventive practices. J Obstet And Gynaecol Canada. [2019 May 1];41(5):599–607.e3. doi: 10.1016/j.jogc.2018.09.016. [DOI] [PubMed] [Google Scholar]
- 73.Sypień P, Marek W, Zielonka TM. Awareness and attitude of Polish gynecologists and General practitioners towards human papillomavirus vaccinations. Healthcare (Basel) [Internet]. 2023. Apr 1 [accessed 2024 Mar 13];11(8). https://pubmed.ncbi.nlm.nih.gov/37107910/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tolentino V, Unni E, Montuoro J, Bezzant-Ogborn D, Kepka D. Utah pharmacists’ knowledge, attitudes, and barriers regarding human papillomavirus vaccine recommendation. J Am Pharmacists Assoc. [2018 Jul 1];58(4):S16–S23. doi: 10.1016/j.japh.2018.04.014. [DOI] [PubMed] [Google Scholar]
- 75.Tolunay O, Celik U, Karaman SS, Celik T, Resitoglu S, Donmezer C, Aydin F, Baspinar H, Mert MK, Samsa H, et al. Awareness and attitude relating to the human papilloma virus and its vaccines among pediatrics, obstetrics and gynecology specialists in Turkey. Asian Pac J Cancer Prev [Internet]. 2014. [accessed 2024 Mar 13];15(24):10723–10728. https://pubmed.ncbi.nlm.nih.gov/25605165/. [DOI] [PubMed] [Google Scholar]
- 76.Wong MCS, Lee A, Ngai KLK, Chor JCY, Chan PKS, He Y. Knowledge, attitude, practice and barriers on vaccination against human papillomavirus infection: a cross-sectional study among primary care physicians in Hong Kong. PLOS ONE. [2013 Aug 21];8(8):e71827. doi: 10.1371/journal.pone.0071827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wong LP, Edib Z, Alias H, Mohamad Shakir SM, Raja Muhammad Yusoff RNA, Sam IC, Zimet GD. A study of physicians’ experiences with recommending HPV vaccines to adolescent boys. J Obstet Gynaecol (Lahore). [2017 Oct 3];37(7):937–943. doi: 10.1080/01443615.2017.1317239. [DOI] [PubMed] [Google Scholar]
- 78.Xu X, Wang Y, Liu Y, Yu Y, Yang C, Zhang Y, Hong Y, Wang Y, Zhang X, Bian R, et al. A nationwide post-marketing survey of knowledge, attitudes and recommendations towards human papillomavirus vaccines among healthcare providers in China. Prev Med (Baltim) [Internet]. 2021. [accessed 2024 Mar 13]; 146:106484. 10.1016/j.ypmed.2021.106484. [DOI] [PubMed] [Google Scholar]
- 79.Fernandes A, Wang D, Domachowske JB, Suryadevara M. HPV vaccine knowledge, attitudes, and practices among New York State medical providers, dentists, and pharmacists. Hum Vaccin Immunother. 2023;19(2). doi: 10.1080/21645515.2023.2219185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fokom Domgue J, Dille I, Kapambwe S, Yu R, Gnangnon F, Chinula L, Murenzi G, Mbatani N, Pande M, Sidibe F, et al. HPV vaccination in Africa in the COVID-19 era: a cross-sectional survey of healthcare providers’ knowledge, training, and recommendation practices. Front Public Health. 2024;12:12. doi: 10.3389/fpubh.2024.1343064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Leung SOA, Villa A, Duffey-Lind E, Welch K, Jabaley T, Hammer M, Feldman S. An interactive educational tool to improve human papillomavirus vaccine knowledge and recommendation among nurses. J Canc Educ. [2023 Dec 1];38(6):1880–1886. doi: 10.1007/s13187-023-02352-2. [DOI] [PubMed] [Google Scholar]
- 82.Mao Y, Zhao Y, Zhang L, Li J, Abdullah AS, Zheng P, Wang F. Frequency of health care provider recommendations for HPV vaccination: a survey in three large cities in China. Front Public Health. 2023;11:11. doi: 10.3389/fpubh.2023.1203610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Maynard G, Akpan IN, Meadows RJ, Fulda KG, Patel DA, Leidner V, Taskin T, Gehr AW, Lu Y, Matches S, et al. Evaluation of a human papillomavirus vaccination training implementation in clinical and community settings across different clinical roles. Transl Behav Med [Internet]. 2024. Apr 1 [accessed 2024 May 8];14(4):249–256. https://pubmed.ncbi.nlm.nih.gov/38459904/. [DOI] [PubMed] [Google Scholar]
- 84.Qaqish A, Abdo N, Abbas MM, Saadeh N, Alkhateeb M, Msameh R, Tarawneh S, Al-Masri M. Awareness and knowledge of physicians and residents on the non-sexual routes of human papilloma virus (HPV) infection and their perspectives on anti-hpv vaccination in Jordan. PLOS ONE [Internet]. 2023. Oct 1 [accessed 2024 May 6];18(10). e0291643. https://pubmed.ncbi.nlm.nih.gov/37819974/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yetik I, Tanoglu FB, Pasin O, Cetin C, Ozcan P. Knowledge levels and community guidance of doctors working in family health centers on HPV screening and HPV vaccination. J Obstet Gynaecol Res [Internet]. 2023. Oct 1 [accessed 2024 May 6];49(10):2519–2527. https://pubmed.ncbi.nlm.nih.gov/37515522/. [DOI] [PubMed] [Google Scholar]
- 86.Osaghae I, Chido-Amajuoyi OG, Khalifa BAA, Shete S. Barriers and determinants of consistent offering of HPV vaccination by healthcare facilities. Hum Vaccin Immunother [Internet]. 2023. [accessed 2024 May 6];19(2). Available from:/pmc/articles/PMC10583630/ 10.1080/21645515.2023.2264596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sakanishi Y, Takeuchi J, Suganaga R, Nakayama K, Nishioka Y, Chiba H, Kishi T, Machino A, Mastumura M, Okada T and Suzuki T. Original research: association between administration or recommendation of the human papillomavirus vaccine and primary care physicians’ knowledge about vaccination during proactive recommendation suspension: a nationwide cross-sectional study in Japan. BMJ Open [Internet]. 2023. Nov 22 [accessed 2024 May 6];13(11):74305. Available from:/pmc/articles/PMC10668282/ 10.1136/bmjopen-2023-074305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Staras SAS, Salloum RG, Osegueda E, Bylund CL, Chi X, Mohan V, Sage E, Huo T, Young A, Thompson LA. North-central Florida clinicians’ human papillomavirus vaccine recommendation priorities and practices for 11- to 12-year-olds: a discrete choice experiment. J Adolesc Health [Internet]. 2023. Jul 1 [accessed 2024 May 6];73(1):172–180. https://pubmed.ncbi.nlm.nih.gov/37029049/. [DOI] [PubMed] [Google Scholar]
- 89.Zhang C, Greengold J, Tackett S, Lentz C, Bennett W, McGuire M. Evaluation of online educational curriculum on HPV vaccination practices among adult primary care providers. BMC Med Educ [Internet]. 2023. Dec 1 [accessed 2024 May 6];23(1):1–8. https://bmcmededuc.biomedcentral.com/articles/10.1186/s12909-023-04807-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gilkey MB, Malo TL, Shah PD, Hall ME, Brewer NT. Quality of physician communication about human papillomavirus vaccine: findings from a national survey. Cancer Epidemiol Biomarker Prev. [2015 Nov 1];24(11):1673–1679. doi: 10.1158/1055-9965.EPI-15-0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Thaker J, Albers AN, Newcomer SR. Nurses’ perceptions, experiences, and practices regarding human papillomavirus vaccination: results from a cross-sectional survey in Montana. BMC Nurs [Internet]. 2023. Dec 1 [accessed 2024 May 6];22(1). Available from:/pmc/articles/PMC10278302/ 10.1186/s12912-023-01379-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lutringer-Magnin D, Kalecinski J, Barone G, Leocmach Y, Regnier V, Jacquard AC, Soubeyrand B, Vanhems P, Chauvin F, Lasset C, et al. Human papillomavirus (HPV) vaccination: perception and practice among French general practitioners in the year since licensing. Vaccine. [2011 Jul 18];29(32):5322–5328. doi: 10.1016/j.vaccine.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 93.Lasset C, Kalecinski J, Régnier V, Barone G, Leocmach Y, Vanhems P, Chauvin, F and Lutringer-Magnin, D. Practices and opinions regarding HPV vaccination among French general practitioners: evaluation through two cross-sectional studies in 2007 and 2010. Int J Public Health. 2014;59(3):519–528. doi: 10.1007/s00038-014-0555-9. [DOI] [PubMed] [Google Scholar]
- 94.Ayash C, Raad N, Finik J, Taoube J, Gorayeb S, Abouhala S, Nourredine S, Jdid M, Aragones A, Gany FM, et al. Perspectives on human papilloma virus vaccination barriers, knowledge and beliefs, and practices: providers serving Arab-American populations. J Community Health. [2024 Feb 1];49(1):127–38. doi: 10.1007/s10900-023-01248-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Agyei-Baffour P, Asare M, Lanning B, Koranteng A, Millan C, Commeh ME, Montealegre JR, Mamudu HM. Human papillomavirus vaccination practices and perceptions among Ghanaian healthcare providers: a qualitative study based on multitheory model. PLOS ONE. 2020. Oct [2020 Oct 1];15(10):e0240657. doi: 10.1371/journal.pone.0240657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Krupp K, Marlow LAV, Kielmann K, Doddaiah N, Mysore S, Reingold AL, Madhivanan, P. Factors associated with intention-to-recommend human papillomavirus vaccination among physicians in Mysore, India. J Adolesc Health. 2010. Apr; 46(4):379–84. doi: 10.1016/j.jadohealth.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 97.Moya EM, Garcia A, Joyce Ponder A, Frietze G. Addressing knowledge gaps: the key role of community health workers and healthcare providers in human papillomavirus prevention and vaccine uptake in a border community. Front Public Health. 2023;11:11. doi: 10.3389/fpubh.2023.1243539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tron A, Schlegel V, Pinot J, Bruel S, Ecollan M, Bel J, Rossignol L, Gauchet A, Gagneux-Brunon A, Mueller J and Banaszuk AS. Barriers and facilitators to the HPV vaccine: a multicenter qualitative study of French general practitioners. Arch Public Health [Internet]. 2024. Dec 1 [accessed 2024 May 6];82(1). https://pubmed.ncbi.nlm.nih.gov/38178269/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Markowitz LE, Gee J, Chesson H, Stokley S. Ten Years of human papillomavirus vaccination in the United States. Acad Pediatr. [2018 Mar 1];18(2):S3–10. doi: 10.1016/j.acap.2017.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kahn JA, Zimet GD, Bernstein DI, Riedesel JM, Lan D, Huang B, Rosenthal, SL. Pediatricians’ intention to administer human papillomavirus vaccine: the role of practice characteristics, knowledge, and attitudes. J Adolesc Health. 2005. Dec;37(6):502–10. doi: 10.1016/j.jadohealth.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 101.Badalyan AR, Hovhannisyan M, Ghavalyan G, Ter-Stepanyan MM, Cave R, Cole J, Farlow AWK, Mkrtchyan HV. Knowledge, attitude, and practice of physicians regarding vaccinations in Yerevan, Armenia: a case study of hpv. Vaccines (Basel). [2021 Oct 1];9(10):1188. doi: 10.3390/vaccines9101188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Akinsola KO, Bakare AA, Gobbo E, King C, Hanson C, Falade A, Herzig van Wees S. A systematic review of measures of healthcare workers’ vaccine confidence. Hum Vaccin Immunother [Internet]. 2024. [accessed 2024 Aug 6];20(1). https://pubmed.ncbi.nlm.nih.gov/38506574/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Diaz Rijo J, Magri J, Stoner A, Carlson L, Fradua K, Carroll L, Redden D. An evaluation of knowledge and comfort in discussing the human papillomavirus (HPV) vaccine among a sample of physicians practicing in South Carolina. Cureus. [2023 Sep 14.] doi: 10.7759/cureus.45247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.European Centre for Disease Prevention and Control . Catalogue of interventions addressing vaccine hesitancy. Stockholm: ECDC; 2017. [Google Scholar]
- 105.Danchin M, Nolan T. A positive approach to parents with concerns about vaccination for the family physician Background. Australian Fam Physicians. 2014;43(10):690–4. [PubMed] [Google Scholar]
- 106.Gagneur A. ClinicalTrails.gov. Evaluation of an Intervention PROMOting VAccination in Maternity in Quebec. 2021. [accessed 2023 Dec 18]. https://clinicaltrials.gov/study/NCT02666872.
- 107.National Centre for Immunisation Research and Survelliance. NCIRS . SARAH Project. accessed 2023 Dec 18]. http://www.ncirs.edu.au/research/social-research/sarah-project/. [Google Scholar]
- 108.Karafillakis E, Dinca I, Apfel F, Cecconi S, Wűrz A, Takacs J, Suk J, Celentano LP, Kramarz P, Larson HJ, et al. Vaccine hesitancy among healthcare workers in Europe: A qualitative study. Vaccine. 2016. Sep;34(41):5013–20. doi: 10.1016/j.vaccine.2016.08.029. [DOI] [PubMed] [Google Scholar]
- 109.Verger P, Botelho-Nevers E, Garrison A, Gagnon D, Gagneur A, Gagneux-Brunon A, Dubé, E. Vaccine hesitancy in health-care providers in Western countries: a narrative review. Expert Rev Vaccines. [2022 Jul 3];21(7):909–27. doi: 10.1080/14760584.2022.2056026. [DOI] [PubMed] [Google Scholar]
- 110.Bakare AAB, Gobbo E, Akinsola KO, King C, Salakof J, Bakare D. “It’s easy to deal with the vaccines you know than the ones you don’t know”: a qualitative study on healthcare workers’ vaccine confidence in Nigeria. 2023.
- 111.Hamdi S. The impact of teachings on sexuality in Islam on HPV vaccine acceptability in the Middle East and North Africa region. J Epidemiol Global Health; Elsevier Ltd 2018;7(S1):S17–22. doi: 10.1016/j.jegh.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wong LP, Wong PF, Megat Hashim MMAA, Han L, Lin Y, Hu Z, Zhao Q, Zimet GD. Multidimensional social and cultural norms influencing HPV vaccine hesitancy in Asia. Hum Vaccin Immunother [Internet]. 2020. Jul 2 [accessed 2024 May 8];16(7):1611. Available from:/pmc/articles/PMC7482900/ 10.1080/21645515.2020.1756670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Larson HJ, Wilson R, Hanley S, Parys A, Paterson P. Tracking the global spread of vaccine sentiments: the global response to Japan’s suspension of its HPV vaccine recommendation. Hum Vaccin Immunother [Internet]. 2014. Sep 1 [accessed 2024 May 8];10(9):2543–50. https://pubmed.ncbi.nlm.nih.gov/25483472/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Namba M, Kaneda Y, Kotera Y. Breaking down the stigma: reviving the HPV vaccination trust in Japan. Oxford J Med [Internet]. 2023. [cited 2024 May 8];116(11):895–6. doi: 10.1093/qjmed/hcad146. [DOI] [PubMed] [Google Scholar]
- 115.Haruyama R, Obara H, Fujita N. Japan resumes active recommendations of HPV vaccine after 8·5 years of suspension. Lancet Oncol [Internet]. 2022. [cited 2024 May 8];23(2):197–8. https://www.mhlw.go.jp/content/. [DOI] [PubMed] [Google Scholar]
- 116.World Health Organization . Immunizations, Vaccines, and Biologics. HPV Dashboard. 2023. [cited 2023 Dec 18]. https://www.who.int/teams/immunization-vaccines-and-biologicals/diseases/human-papillomavirus-vaccines-(HPV)/hpv-clearing-house/hpv-dashboard. [Google Scholar]
- 117.Sharma S, Verhagen AP, Elkins M, Brismée JM, Fulk GD, Taradaj J, Steen L, Jette A, Moore A, Stewart A, et al. Research from low-income and middle-income countries will benefit global health and the physiotherapy profession, but it requires support. J Man Manipulative Ther. [2023 Sep 3];31(5):305–310. doi: 10.1080/10669817.2023.2253071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Oduwole E, Pienaar ED, Mahomed H, Wiysonge CS, Casuccio A. Overview of Tools and Measures Investigating Vaccine Hesitancy in a Ten Year Period: A Scoping Review. Vaccines. 2022. [cited 2023 Oct 23];10(8):1198. 10.3390/vaccines10081198. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


