Abstract
Hydrogen sulfide (H2S) has been recently categorized as a gasotransmitter, and it may be involved in the pathology of Alzheimer’s disease. However, whether H2S induces amyloid precursor protein (APP) processing remains unknown. In the present study, we tested the ability of H2S to mediate APP processing in SH-SY5Y human neuroblastoma cells. We treated SH-SY5Y human neuroblastoma cells with a range of sodium hydrosulfide (H2S donor) concentrations. Western blot analysis showed that H2S increased the generation of C83 and decreased the production of C99. Meanwhile, H2S increased the levels of a disintegrin and metalloprotease 10 (ADAM10) mRNA and protein, but had no effect on TNF-α-converting enzyme (TACE, also known as ADAM17) mRNA and protein levels. H2S also induced a significant decrease of extracellular amyloid-β42 (Aβ42). Furthermore, SH-SY5Y human neuroblastoma cells were assayed for activation of the phosphoinositide 3-kinase (PI3-K) pathway. H2S activated the PI3-K pathway. Using specific inhibitor of PI3-K, we determined that the effects of H2S on APP processing and Aβ42 were blocked by LY 294002 (PI3-K inhibitor). These data indicate that H2S can induce APP processing, and this effect is dependent on activation of the PI3-K signaling pathway.
Keywords: Amyloid-β, Alzheimer’s disease, A disintegrin and metalloprotease 10, Amyloid precursor protein, Hydrogen sulfide, Phosphoinositide 3-kinase, TNF-α-converting enzyme
Introduction
It has been traditionally suggested that hydrogen sulfide (H2S) is a poisonous gas that smells like rotten eggs (Milby and Baselt 1999). However, in recent years, H2S was classified as a gasotransmitter like nitric oxide (NO) and carbon monoxide (CO) (Olson and Donald 2009). H2S is endogenously produced in mammalian cells by pyridoxa-5′-phosphate-dependent enzymes such as cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE), which use l-cysteine as the dominant substrate (Wang 2002). 3-Mercaptopyruvate sulfurtransferase was recently added to the H2S producing enzymes (Shibuya et al. 2009). Increasing evidence demonstrates that endogenous H2S plays a pivotal role in some pathological and physiological conditions such as vasorelaxation (Bhatia 2005), heart protection (Pan et al. 2006), and neuroregulation (Kimura 2002). The H2S donor, sodium hydrosulfide (NaHS), elevates N-methyl d-aspartate (NMDA) receptor-mediated responses and promotes the induction of hippocampal long-term potentiation (LTP) at concentrations of 10–130 µM (Abe and Kimura 1996). In addition, the potential physiological functions of H2S in the brain may include calcium homeostasis, inhibition of oxidative stress, and regulation of neurotransmission (Qu et al. 2008). These physiological and pathological processes are closely associated with Alzheimer’s disease (AD). Therefore, H2S may be involved in AD pathologies.
A major histopathological hallmark in AD is the accumulation and deposition of amyloid-β (Aβ) in the brain. Aβ derives from the amyloid precursor protein (APP), which is a type-1 integral membrane protein with 770 amino acid residues. Also, APP is expressed in far-ranging tissues with unknown functions (Sardana et al. 2004). Proteolytic processing of APP is mediated by two alternative pathways: the amyloidogenic pathway (β-secretase pathway) or the non-amyloidogenic pathway (α-secretase pathway). Proteolysis at the β-site, which is in the N-terminus of the Aβ domain, results in the shedding of sAPPβ and the production of a membrane-bound 99 amino acid fragment (C99). C99 can be further cleaved by γ-secretase to generate Aβ (Haass et al. 1992; Shoji et al. 1992). Cleavage at the α-site, localized within the Aβ domain, results in the secretion of sAPPα and the formation of a membrane-bound 83 amino acid fragment (C83), which can be further cleaved by γ-secretase (Esch et al. 1990; Sisodia et al. 1990). Proteolysis occurs within the Aβ sequence; therefore, α-secretase processing does not produce Aβ (Esch et al. 1990; Sisodia et al. 1990). Both a disintegrin and metalloprotease(ADAM10) and TNF-a-converting enzyme (TACE, also known as ADAM17) are known to possess α-secretase activity (Allinson et al. 2003; Asai et al. 2003). Accumulated Aβ antagonizes neuronal cells and leads to neuronal cells death. Additionally, Aβ also promotes the phosphorylation of tau protein, which causes neurofibrillary tangles. Therefore, the suppression of Aβ production, the promotion of Aβ degradation, or the clearance of deposited Aβ is significant for the prevention and treatment of AD (Harada et al. 2006). A shift in APP processing toward the α-secretase pathway instead of the β-secretase pathway is a potent method to block Aβ production before its deposition. Therefore, exploration of novel approaches to shift APP processing toward the non-amyloidogenic pathway may potentially lead to better prevention and treatment for AD.
Thus far, no information exists regarding the potential role of H2S in APP processing in vitro. The above-mentioned roles of H2S raise the possibility that H2S may be able to modulate APP processing. Therefore, we tested the ability of H2S to regulate APP processing in SH-SY5Y human neuroblastoma cells. Our findings show that H2S enhances C83 and ADAM10 productions and reduces the generations of C99 and Aβ42. We also demonstrate that the activity of phosphatidylinositol 3-kinase (PI3-K) may be necessary for these actions of H2S.
Materials and Methods
Materials
All culture media, supplements, and fetal bovine serum (FBS) were purchased from GIBCO-BRL (USA). SH-SY5Y human neuroblastoma cells were purchased from ATCC (Manassas, VA). Antibodies against, Akt and phospho-Akt, were purchased from Santa Cruz Biotechnology (CA, USA). AHP538, an antibody against APP, C99, and C83, was obtained from Serotec (UK). LY 294002 was purchased from Calbiochem (USA). Aβ42 ELISA kits were obtained from BioSource. NaHS was obtained from Sigma (St Louis, MO, USA). H2S and inhibitor solution were prepared as previously described (Cai et al. 2007).
Cell Culture and Treatments
SH-SY5Y human neuroblastoma cells were routinely maintained at 37 °C in a 5 % CO2 incubator in 1640 medium supplemented with 10 % FBS, penicillin (100 IU/mL), and streptomycin (100 IU/mL). SH-SY5Y human neuroblastoma cells were plated at 7.6 × 105 in 100 mm dishes and cultured for 48 h. On the third day, the medium was removed, and the cells were washed twice with phosphate-buffered saline (PBS). Next, the cells were exposed to serum-free culture medium for 24 h, and experimental treatments were performed in serum-free culture medium, and the cells were incubated for 2 or 16 h. SH-SY5Y human neuroblastoma cells were treated with the H2S donor, NaHS, at concentrations of 10, 25, and 50 µM. For inhibition studies, LY294002 (25 or 50 µM), a specific PI3-K inhibitor, was administered 30 min prior to NaHS treatment (50 µM). Each experiment was repeated three times.
Quantitative RT-PCR (QPCR)
Expressions of ADAM10 and ADAM17 were analyzed by QPCR. We averaged expression of β-actin as internal reference genes to normalize input cDNA. QPCR was performed using the Brilliant SYBR Green QPCR Core Reagent Kit in an Mx4000 Multiplex Quantitative PCR System (Stratagene, La Jolla, CA), and 40 ng of cDNA was used per reaction. The comparative 2−ΔΔCT method was used to compute relative expression values.
Western Blotting
Abovementioned cells were lysed with 1 × sodium dodecyl sulfate (SDS) sample buffer (62.5 mmol/L Tris–HCl [pH 6.8 at 25 °C], 2 % (w/v) SDS, 10 % glycerol, 50 mmol/L DTT). Protein concentration was determined using bicinchoninic acid (BCA) reagent. Next, 30 μg of protein was separated by 10 % SDS–polyacrylamide gel electrophoresis and transferred to a polyvinyl difluoride (PVDF) membrane. After blocking with Tris buffered saline and Tween 20 (TBST) containing 5 % milk for 1 h, the membrane was incubated with antibodies against Akt, phospho-Akt, AHP538 or β-actin overnight at 4 °C. After incubation with horseradish peroxidase conjugated secondary antibody for 1 h, SuperSignal West Pico Chemiluminescent Substrate was used for detection.
ELISA
Secreted Aβ42 levels in the cell culture media were quantitatively measured using colorimetric enzyme-linked immunosorbent assay (ELISA) kits according to the protocol provided by the manufacturer. Culture medium was collected and cleared by centrifugation (14,000×g for 10 min at 4 °C), and 100 µL sample of medium was used for Aβ42 measurement.
Statistical Analysis
All data are expressed as mean ± standard error (SE). Differences between groups were analyzed by ANOVA followed by the Student Newman–Keuls (S–N–K) test. Statistically significant differences were defined as p < 0.05.
Results
H2S Stimulated C83 Production and Inhibited C99 Generation, But it did not Alter APP Levels
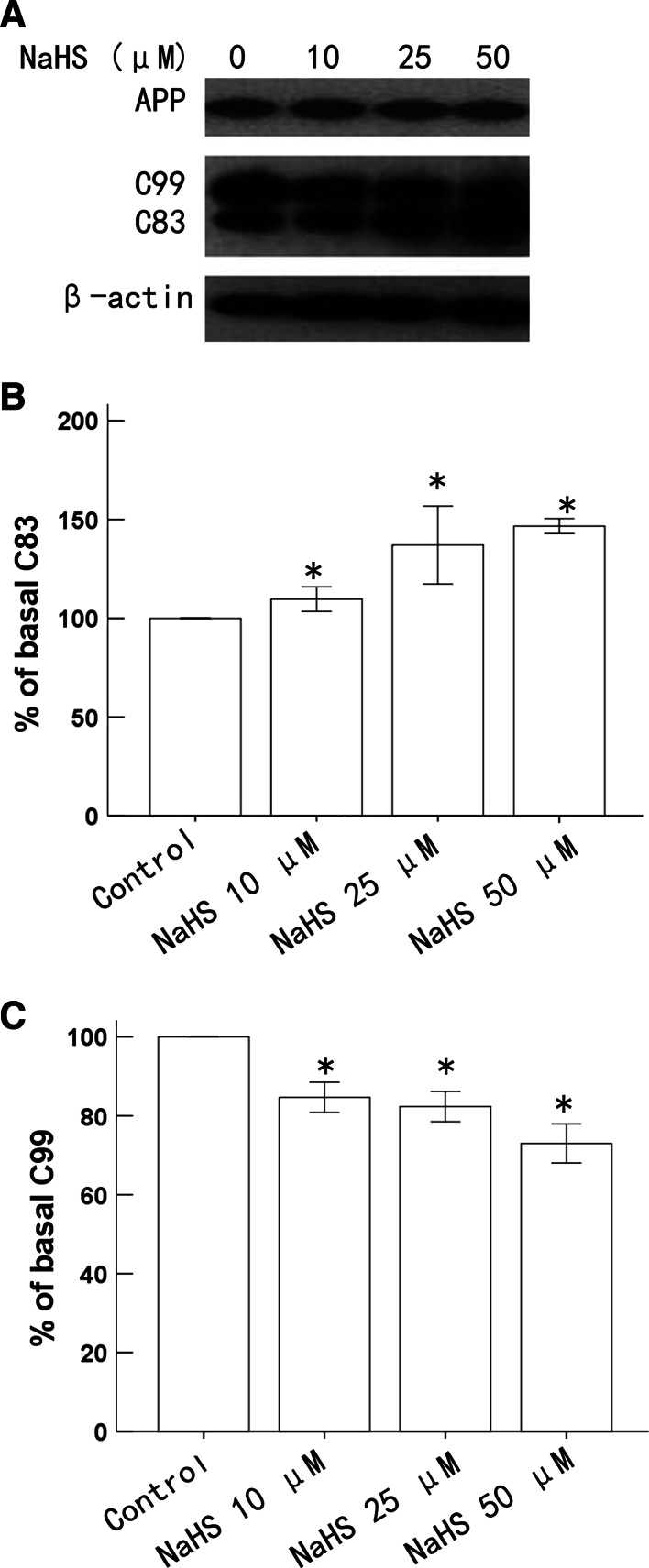
To examine if H2S affects APP processing, SH-SY5Y human neuroblastoma cells were treated with increasing concentrations of NaHS for 16 h, which resulted in a concentration-dependent increase in C83 production (Fig. 1a, b). The concentration of 50 µM NaHS resulted in an approximately 1.47-fold increase in C83 compared with the basal level (Fig. 1b). Differences were observed between concentrations of 10, 25, and 50 µM NaHS. C99 generation gradually decreased at concentrations of 10, 25, and 50 µM NaHS (Fig. 1a, c). Furthermore, NaHS treatment for 16 h did not affect the levels of APP (Fig. 1a).
Fig. 1.
Effects of H2S on APP processing. SH-SY5Y human neuroblastoma cells were treated with 10, 25, and 50 µM NaHS for 16 h. a Cells were harvested and subjected to western blot analysis for APP, C83, and C99. b, c Densitometric analysis of western blots is expressed as the percent of basal level. Results are expressed as the mean ± SE of three independent experiments. *p < 0.05, compared with control group
H2S Increased the Levels of ADAM10 mRNA and Protein, but did not Modify the Expressions of ADAM17 mRNA and Protein
ADAM10 and ADAM17, which play a pivotal role in α-secretase pathway, were monitored. SH-SY5Y human neuroblastoma cells were treated with NaHS (at concentrations of 10, 25, 50 µM). ADAM10 and ADAM17 mRNA and protein levels were examined by QPCR and Western blot, respectively. We found that ADAM10 mRNA and protein levels increased in NaHS-treated cells compared with control cells (Fig. 2a, c, and d). However, ADAM17 mRNA and protein levels were not modified (Fig. 2b, c). The effect of NaHS on ADAM10 appeared to be dose dependent.
Fig. 2.
Effects of H2S on cellular levels of ADAM10 and ADAM17 mRNA and protein. SH-SY5Y human neuroblastoma cells were treated with 10, 25, 50 µM NaHS for 16 h. a QPCR was performed to quantify ADAM10 mRNA relative expressions, and H2S decreased ADAM10 mRNA levels. b QPCR was performed to quantify ADAM17 mRNA relative expressions, and H2S did not alter ADAM17 mRNA levels. c Representative Western blot showing ADAM10 and ADAM17 bands. d Densitometric analysis of Western blots is expressed as the percent of basal levels of ADAM10. Results are expressed as the mean ± SE of three independent experiments. *p < 0.05, compared with control group
H2S Activated the PI3-K/Akt Pathway, Which Mediated the Effect of H2S on APP Processing
To explore the molecular mechanisms of H2S-mediated APP processing, lysates obtained from 50 µM NaHS-treated cells were subjected to western blot analysis for phosphorylated or total Akt. Akt is a PI3-K downstream effector. Treatment with 50 µM NaHS for 2 h caused a significant increase of phosphorylated Akt (Fig. 3a, b). To investigate if the PI3-K/Akt pathway is involved in H2S-induced processing of APP, cells were pre-incubated with LY294002, a selective inhibitor of PI3-K (25 or 50 µM), for 30 min before treatment with 50 µM NaHS. LY294002 is no toxic to SH-SY5Y cells (data not shown). As shown in Fig. 3a, b, Akt phosphorylation was abolished by LY294002 after cells were treated 50 µM NaHS for 2 h. We also found that LY294002 suppressed the effects of H2S on C83 and C99 after cells were treated 50 µM NaHS for 16 h (Fig. 3c, d, and e).
Fig. 3.
Effects of the PI3-K/Akt pathway on APP processing in NaHS-treated cells. a Western blotting reveals that Akt is phosphorylated by 50 µM NaHS at 2 h, and this induction is blocked when cells are treated with LY294002 for 30 min prior to the addition of NaHS. b Densitometric analysis of western blots of pAkt is expressed as the percent of basal level. c A Western blot probing for C83, C99, and APP was performed in SH-SY5Y human neuroblastoma cells in the presence or absence of LY294002 and NaHS (50 µM). β-actin was used as the loading control. d, e Densitometric analysis of western blots of C83 or C99 is expressed as the percent of basal level. Results are expressed as the mean ± SE of three independent experiments. *p < 0.05, compared with NaHS 50 µM group
PI3-K/Akt Signaling Pathway Also Regulated the Effect of H2S on ADAM10
To determine if H2S regulated the levels of ADAM10 mRNA and protein in SH-SY5Y human neuroblastoma cells through the PI3-K/Akt pathway, SH-SY5Y human neuroblastoma cells were treated with NaHS (50 µM) in the presence and absence of the PI3-K inhibitor LY294002 (25 or 50 µM). Pre-treatment of SH-SY5Y human neuroblastoma cells with 25 or 50 µM LY294002 for 30 min prior to the addition of NaHS blocked the NaHS –induced increase of ADAM10 mRNA and protein (Fig. 4a, b and c). These findings indicate that PI3-K/Akt signaling pathway may involve in the effect of H2S on ADAM10.
Fig. 4.
Effects of PI3-K/Akt pathway on ADAM10 in NaHS-treated cells. a Effects of H2S on ADAM10 mRNA levels reversed by LY294002. b Representative Western blot showing ADAM10 bands. c Densitometric analysis of Western blots is expressed as the percent of basal levels. Results are expressed as the mean ± SE of three independent experiments. *p < 0.05, compared with NaHS 50 µM group
Effects of H2S and PI3-K/Akt Signaling Pathway on Aβ42 Levels
To investigate the effects of H2S and PI3-K/Akt signaling pathways on Aβ42 levels, conditioned medium was analyzed for Aβ42 by a high sensitive sandwich enzyme-linked immunosorbent assay (ELISA, Fig. 5). After treatment with NaHS, the levels of extracellular Aβ42 decreased by 20 %. The effect of PI3-K inhibitors was also analyzed, and in the presence of 25 or 50 µM LY 294002 the above phenomena were reversed.
Fig. 5.
Production of Aβ42 is reduced by NaHS treatment and reversed by LY294002. Levels of Aβ42 were determined using a sandwich-ELISA. Data represent mean ± SE from 12 cultures derived from three independent experiments. *p < 0.05, compared with NaHS 50 µM group
Discussion
In neuronal cells, H2S enhances the NMDA receptor-mediated response and facilitates LTP by activating the cyclic adenosine monophosphate (cAMP) pathway (Abe and Kimura 1996; Kimura 2000). In addition, H2S protects neurons from oxidative stress by activating ATP-dependent K+ (K ATP) and Cl− channels and by increasing the levels of glutathione (GSH) (Kimura and Kimura 2004; Kimura et al. 2006). Oxidative stress has been shown to play a prominent role in the progression of AD (Smith et al. 1998), and treatment with antioxidant agents has been suggested to ameliorate cognitive dysfunction (Kontush 2001). In glia and other cells, H2S has a positive effect on intracellular calcium concentration homeostasis and protects against inflammation via various mechanisms such as inhibiting the p38 MAPK and activating the ERK-NF-κB pathway (Hu et al. 2007; Zhi et al. 2007). Inflammation has also been found to play a pivotal role in the development of AD (McGeer and McGeer 2003), and anti-inflammatory mechanisms are beneficial to halt AD progression (Brinton 1999). Although there is no direct evidence that H2S levels are reduced in AD, it has been demonstrated that CBS deficiency leads to mental retardation and increases the levels of homocysteine (Hcy) (Mudd et al. 1985). Hcy was implicated in eliciting neuronal apoptosis and in the elevation of neuronal vulnerability to excitotoxicity via DNA damage, the activation of poly (adenosine diphosphate-ribose) polymerase, and the phosphorylation of p53 (Kruman et al. 2000). In addition, hyperhomocyteinemia (hyperHcy) is one of the important risk factors in AD because it facilitates hippocampal neuron damage induced by Aβ in cell cultures. Also, hyperHcy promotes Aβ production induced by the stress protein Herp (homocysteine-induced endoplasmic reticulum protein) through an interaction with both presenilin 1 and 2 (Sai et al. 2002). Furthermore, in AD brains, S-adenosyl-methionine (SAM), a CBS activator, is significantly reduced (Morrison et al. 1996). Therefore, we hypothesize that H2S may play an important role in the pathogenesis of AD. However, no information exists concerning the relationship between H2S and APP processing in vitro.
Previous studies showed that APP processing was affected by many factors such as insulin (Solano and Sironi 2000), insulin-like growth factor-1 (IGF-1) (Adlerz et al. 2007), brain-derived neurotrophic factor (BDNF), retinoic acid (RA) (Holback et al. 2005), epidermal growth factor (EGF), and nerve growth factor (NGF) (Refolo et al. 1989). In the present study, H2S was administered in the form of 10–50 μM NaHS. We found that NaHS also induced APP processing because secretion of the membrane-bound C-terminal fragment (C83) was increased, whereas the C99 levels decreased. In addition, the levels of ADAM10 mRNA and protein were also increased because of treatment of NaHS. However, it had no effect on ADAM17. Simultaneously, the levels of extracellular Aβ42 reduced after SH-SY5Y human neuroblastoma cells were treated with NaHS. Interestingly, these roles of NaHS were significantly different at 10, 25, and 50 µM NaHS. Thus, 50 µM NaHS was used in the remaining trials. Our results clearly demonstrate that H2S has the ability to shift APP processing toward the non-amyloidogenic pathway instead of the amyloidogenic pathway, and this effect is concentration-dependent. In SH-SY5Y human neuroblastoma cells, we hypothesize that the above capacities of H2S may be achieved by enhancing the expression of ADAM10, but not ADAM17. It is likely that the expressions of ADAM10 and ADAM17 may be tissue/cell type specific.
To investigate the mechanism of the effect of H2S on APP processing, the PI3-K/Akt pathway was analyzed. The normal on and off switching of the PI3-K/Akt pathway is a powerful integrator of physiological responses rudimentary to successful aging (O’ Neill 2013). Aging is the major risk factor for AD. Through the PI3-K/Akt pathway, the integrated coordination of neuronal responses has significant functional impact on key events that go awry in AD, and that includes neurotransmission, neuronal polarity, synaptic plasticity, use-dependent translation, proteostasis, metabolic control and stress responses including DNA repair (O’ Neill 2013). Akt is a primary downstream mediator of the PI3-K pathway. Activated Akt phosphorylates serine or threonine residues of target proteins, protects neurons, and increases neuronal survival (Rodgers and Theibert 2002). In RF/6A endothelial cells, H2S (10–200 μM NaHS) increased Akt phosphorylation and promoted angiogenesis, and these effects were blocked by LY294002 (Cai et al. 2007). After the administration of a single dose of NaHS (10 μM), Akt phosphorylation peaked at 30 min and lasted until 1 h (Cai et al. 2007). In human umbilical vein endothelial cells, NaHS stimulated the phosphorylation of endothelial NO synthase (eNOS) and enhanced NO production, and LY294002 abolished the effects of H2S on eNOS phosphorylation, NO production, cell proliferation and tube formation (Altaany et al. 2013). It is likely that the effects of H2S on PI3-K/Akt signaling pathway may be tissue/cell type specific and/or time-dependent. In this study, however, we showed that Akt phosphorylation increased at 2 h after 50 μM NaHS treatment. At this concentration of NaHS, a significant APP processing effect was induced. LY294002 blocked Akt phosphorylation, the elevation of C83 levels, the reduction of C99 levels, and the increase of ADAM10 mRNA and protein levels after NaHS administration. These data suggest that H2S shifts APP processing toward the α-secretase pathway by activating the PI3-K/Akt pathway in SH-SY5Y human neuroblastoma cells.
Increasing evidence indicates that over-activities of glycogen synthase kinase-3 (GSK-3) are critical for producing AD-related phenotypes such as tau hyperphosphorylation, Aβ production, and synaptic or memory deficits (Phiel et al. 2003; Hong and Lee 1997). Is the down-regulation of GSK-3 (especially, GSK-3beta) signaling involved in H2S-induced APP processing as a downstream mediator of the activated PI3 K/Akt pathway? This remains to be further investigated in our future studies.
H2S is a toxic gas; therefore, we must note its side effects if H2S was to be used to develop novel therapeutic approaches for the treatment of AD. In humans, acute intoxication by H2S causes four dose-dependent symptoms: hyperpnea, apnea, unconsciousness or knockdown, and death (Pearson et al. 2006). Rats exposed to ≥30 ppm (0.9 mM) H2S show a decrease in cytochrome oxidase activity (Dorman et al. 2002). Our present study showed the effects of H2S on APP processing in SH-SY5Y human neuroblastoma cells can be observed at physiologically relevant dosages (10–50 μM).
In conclusion, the present study shows that H2S induces APP processing in vitro. The PI3-K signaling pathway may play an important role in this response, and this effect of H2S may be explored to develop novel approaches for the treatment of AD. The actions of H2S on the PI3-K signaling pathway will be the direction of future research.
Acknowledgments
This study was supported by Scientific and Technological Research Program of Chongqing Municipal Education Commission (KJ1400227) and National Natural Science Foundation of China (NSFC) (81271222).
Conflict of interests
The authors declare that they have no competing interests.
Abbreviations
- Aβ
Amyloid-β
- AD
Alzheimer’s disease
- ADAM10
A disintegrin and metalloprotease 10
- APP
Amyloid precursor protein
- C83
A membrane-bound 83 amino acid fragment
- C99
A membrane-bound 83 amino acid fragment
- H2S
Hydrogen sulfide
- NaHS
Sodium hydrosulfide
- PI3-K
Phosphoinositide 3-kinase
- TACE(ADAM17)
TNF-α-converting enzyme
Contributor Information
Hua Zhang, Phone: +86-23-89012009, Email: zhanghua0303@hotmail.com.
Yong Yan, Email: yongyanpro@hotmail.com.
References
- Abe K, Kimura H (1996) The possible role of hydrogen sulfide as an endogenous neuromodulator. J Neurosci 16:1066–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adlerz L, Holback S, Multhaup G, Iverfeldt K (2007) IGF-1-induced processing of the amyloid precursor protein family is mediated by different signaling pathways. J Biol Chem 282:10203–10209 [DOI] [PubMed] [Google Scholar]
- Allinson TM, Parkin ET, Turner AJ, Hooper NM (2003) ADAMs family members as amyloid precursor protein alpha-secretases. J Neurosci Res 74:342–352 [DOI] [PubMed] [Google Scholar]
- Altaany Z, Yang G, Wang R (2013) Crosstalk between hydrogen sulfide and nitric oxide in endothelial cells. J Cell Mol Med 17:879–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai M, Hattori C, Szabó B, Sasagawa N, Maruyama K, Tanuma S, Ishiura S (2003) Putative function of ADAM9, ADAM10, and ADAM17 as APP alphasecretase. Biochem Biophys Res Commun 301:231–235 [DOI] [PubMed] [Google Scholar]
- Bhatia M (2005) Hydrogen sulfide as a vasodilator. IUBMB Life 57:603–606 [DOI] [PubMed] [Google Scholar]
- Brinton RD (1999) A women’s health issue: Alzheimer’s disease and strategies for maintaining cognitive health. Int J Fertil Womens Med 44:174–185 [PubMed] [Google Scholar]
- Cai WJ, Wang MJ, Moore PK, Jin HM, Yao T, Zhu YC (2007) The novel proangiogenic effect of hydrogen sulfide is dependent on Akt phosphorylation. Cardiovasc Res 76:29–40 [DOI] [PubMed] [Google Scholar]
- Dorman DC, Moulin FJ, McManus BE, Mahle KC, James RA, Struve MF (2002) Cytochrome oxidase inhibition induced by acute hydrogen sulfide inhalation: correlation with tissue sulfide concentrations in the rat brain, liver, lung, and nasal epithelium. Toxicol Sci 65:18–25 [DOI] [PubMed] [Google Scholar]
- Esch FS, Keim PS, Beattie EC, Blacher RW, Culwell AR, Oltersdorf T, McClure D, Ward PJ (1990) Cleavage of amyloid beta peptide during constitutive processing of its precursor. Science 248:1122–1124 [DOI] [PubMed] [Google Scholar]
- Haass C, Schlossmacher MG, Hung AY, Vigo-Pelfrey C, Mellon A, Ostaszewski BL, Lieberburg I, Koo EH, Schenk D, Teplow DB, Selkoe DJ (1992) Amyloid beta-peptide is produced by cultured cells during normal metabolism. Nature 359:322–325 [DOI] [PubMed] [Google Scholar]
- Harada H, Tamaoka A, Ishii K, Shoji S, Kametaka S, Kametani F, Saito Y, Murayama S (2006) Beta-site APP cleaving enzyme 1 (BACE1) is increased in remaining neurons in Alzheimer’s disease brains. Neurosci Res 54:24–29 [DOI] [PubMed] [Google Scholar]
- Holback S, Adlerz L, Iverfeldt K (2005) Increased processing of APLP2 and APP with concomitant formation of APP intracellular domains in BDNF and retinoic acid-differentiated human neuroblastoma cells. J Neurochem 95:1059–1068 [DOI] [PubMed] [Google Scholar]
- Hong M, Lee VM (1997) Insulin and insulin-like growth factor-1 regulate tau phosphorylation in cultured human neurons. J Biol Chem 272:19547–19553 [DOI] [PubMed] [Google Scholar]
- Hu LF, Wong PT, Moore PK, Bian JS (2007) Hydrogen sulfide attenuates lipopolysaccharide-induced inflammation by inhibition of p38 mitogen-activated protein kinase in microglia. J Neurochem 100:1121–1128 [DOI] [PubMed] [Google Scholar]
- Kimura H (2000) Hydrogen sulfide induces cyclic AMP and modulates the NMDA receptor. Biochem Biophys Res Commun 267:129–133 [DOI] [PubMed] [Google Scholar]
- Kimura H (2002) Hydrogen sulfide as a neuromodulator. Mol Neurobiol 26:13–19 [DOI] [PubMed] [Google Scholar]
- Kimura Y, Kimura H (2004) Hydrogen sulfide protects neurons from oxidative stress. FASEB J 18:1165–1167 [DOI] [PubMed] [Google Scholar]
- Kimura Y, Dargusch R, Schubert D, Kimura H (2006) Hydrogen sulfide protects HT22 neuronal cells from oxidative stress. Antioxid Redox Signal 8:661–670 [DOI] [PubMed] [Google Scholar]
- Kontush A (2001) Amyloid-beta: an antioxidant that becomes a pro-oxidant and critically contributes to Alzheimer’s disease. Free Radic Biol Med 31:1120–1131 [DOI] [PubMed] [Google Scholar]
- Kruman II, Culmsee C, Chan SL, Kruman Y, Guo Z, Penix L, Mattson MP (2000) Homocysteine elicits a DNA damage response in neurons that promotes apoptosis and hypersensitivity to excitotoxicity. J Neurosci 20:6920–6926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer EG, McGeer PL (2003) Inflammatory processes in Alzheimer’s disease. Prog Neuropsychopharmacol Biol Psychiatry 27:741–749 [DOI] [PubMed] [Google Scholar]
- Milby TH, Baselt RC (1999) Hydrogen sulfide poisoning: clarification of some controversial issues. Am J Ind Med 35:192–195 [DOI] [PubMed] [Google Scholar]
- Morrison LD, Smith DD, Kish SJ (1996) Brain S-adenosylmethionine levels are severely decreased in Alzheimer’s disease. J Neurochem 67:1328–1331 [DOI] [PubMed] [Google Scholar]
- Mudd SH, Skovby F, Levy HL, Pettigrew KD, Wilcken B, Pyeritz RE, Andria G, Boers GH, Bromberg IL, Cerone R, Fowler B, Gröbe H, Schmidt H, Schweitzer L (1985) The natural history of homocystinuria due to cystathionine beta-synthase deficiency. Am J Hum Genet 37:1–31 [PMC free article] [PubMed] [Google Scholar]
- O’ Neill C (2013) PI3-kinase/Akt/mTOR signaling: impaired on/off switches in aging, cognitive decline and Alzheimer’s disease. Exp Gerontol 48:647–653 [DOI] [PubMed] [Google Scholar]
- Olson KR, Donald JA (2009) Nervous control of circulation–the role of gasotransmitters, NO, CO, and H2S. Acta Histochem 111:244–256 [DOI] [PubMed] [Google Scholar]
- Pan TT, Feng ZN, Lee SW, Moore PK, Bian JS (2006) Endogenous hydrogen sulfide contributes to the cardioprotection by metabolic inhibition preconditioning in the rat ventricular myocytes. J Mol Cell Cardiol 40:119–130 [DOI] [PubMed] [Google Scholar]
- Pearson RJ, Wilson T, Wang R (2006) Endogenous hydrogen sulfide and the cardiovascular system-what’s the smell all about? Clin Invest Med 29:146–150 [PubMed] [Google Scholar]
- Phiel CJ, Wilson CA, Lee VM, Klein PS (2003) GSK-3alpha regulates production of Alzheimer’s disease amyloid-beta peptides. Nature 423:435–439 [DOI] [PubMed] [Google Scholar]
- Qu K, Lee SW, Bian JS, Low CW, Wong PTH (2008) Hydrogen sulfide: neurochemistry and neurobiology. Neurochem Int 52:155–165 [DOI] [PubMed] [Google Scholar]
- Refolo LM, Salton SR, Anderson JP, Mehta P, Robakis NK (1989) Nerve and epidermal growth factors induce the release of the Alzheimer amyloid precursor from PC 12 cell cultures. Biochem Biophys Res Commun 164:664–670 [DOI] [PubMed] [Google Scholar]
- Rodgers EE, Theibert AB (2002) Functions of PI 3-kinase in development of the nervous system. Int J Dev Neurosci 20:187–197 [DOI] [PubMed] [Google Scholar]
- Sai X, Kawamura Y, Kokame K, Yamaguchi H, Shiraishi H, Suzuki R, Suzuki T, Kawaichi M, Miyata T, Kitamura T, De Strooper B, Yanagisawa K, Komano H (2002) Endoplasmic reticulum stress-inducible protein, Herp, enhances presenilin-mediated generation of amyloid beta-protein. J Biol Chem 277:12915–12920 [DOI] [PubMed] [Google Scholar]
- Sardana V, Xu B, Zugay-Murphy J, Chen Z, Sardana M, Darke PL, Munshi S, Kuo LC (2004) A general procedure for the purification of human beta-secretase expressed in Escherichia coli. Protein Expr Purif 34:190–196 [DOI] [PubMed] [Google Scholar]
- Shibuya N, Tanaka M, Yoshida M, Ogasawara Y, Togawa T, Ishii K, Kimura H (2009) 3-Mercaptopyruvate sulfurtransferase produces hydrogen sulfide and bound sulfane sulfur in the brain. Antioxid Redox Signal 11:703–714 [DOI] [PubMed] [Google Scholar]
- Shoji M, Golde TE, Ghiso J, Cheung TT, Estus S, Shaffer LM, Cai XD, McKay DM, Tintner R, Frangione B, Younkin SG (1992) Production of the Alzheimer amyloid beta protein by normal proteolytic processing. Science 258:126–129 [DOI] [PubMed] [Google Scholar]
- Sisodia SS, Koo EH, Beyreuther K, Unterbeck A, Price DL (1990) Evidence that beta-amyloid protein in Alzheimer’s disease is not derived by normal processing. Science 248:492–495 [DOI] [PubMed] [Google Scholar]
- Smith MA, Hirai K, Hsiao K, Pappolla MA, Harris PL, Siedlak SL, Tabaton M, Perry G (1998) Amyloid-beta deposition in Alzheimer transgenic mice is associated with oxidative stress. J Neurochem 70:2212–2215 [DOI] [PubMed] [Google Scholar]
- Solano DC, Sironi M (2000) BonfiniC, Solerte SB, Govoni S, Racchi M. Insulin regulates soluble amyloid precursor protein release via phosphatidyl inositol 3 kinase-dependent pathway. FASEB J 14:1015–1022 [DOI] [PubMed] [Google Scholar]
- Wang R (2002) Two’s company, three’s a crowd: can H2S be the third endogenous gaseous transmitter? FASEB J. 16:1792–1798 [DOI] [PubMed] [Google Scholar]
- Zhi L, Ang AD, Zhang H, Moore PK, Bhatia M (2007) Hydrogen sulfide induces the synthesis of proinflammatory cytokines in human monocyte cell line U937 via the ERK-NF-kappaB pathway. J Leukoc Biol 81:1322–1332 [DOI] [PubMed] [Google Scholar]