Abstract
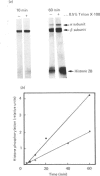
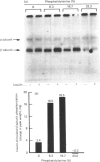
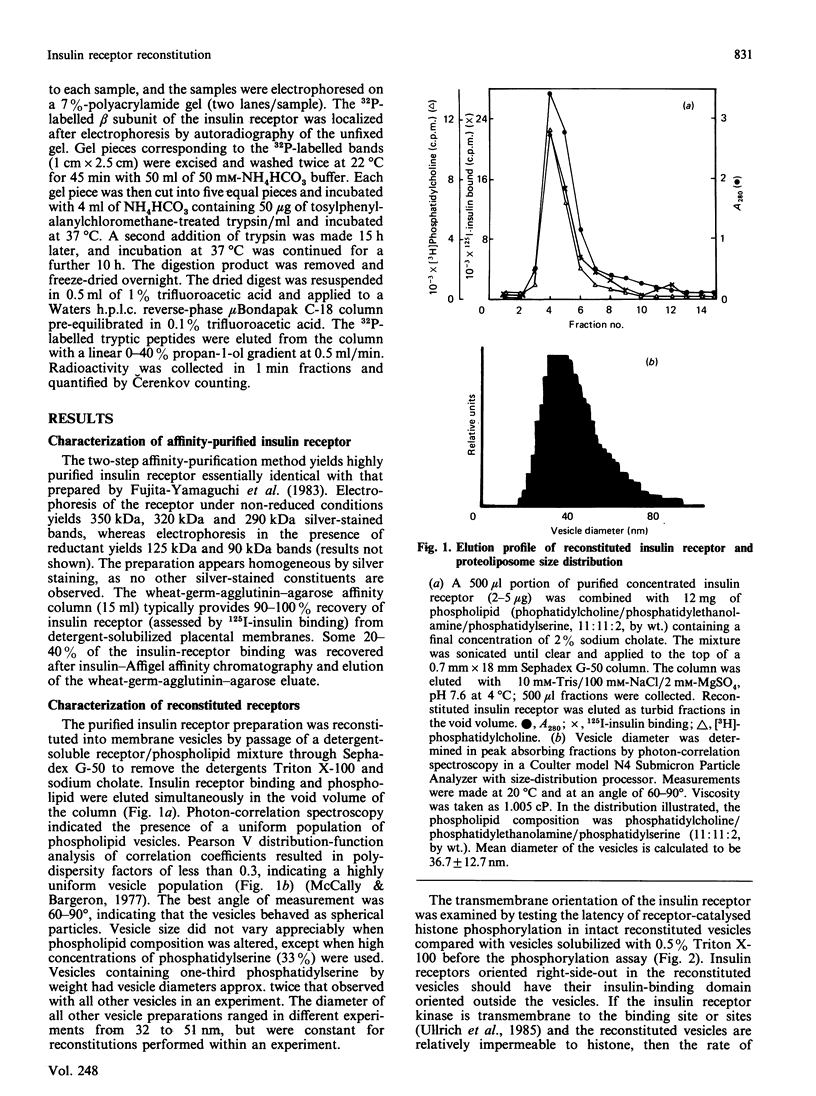
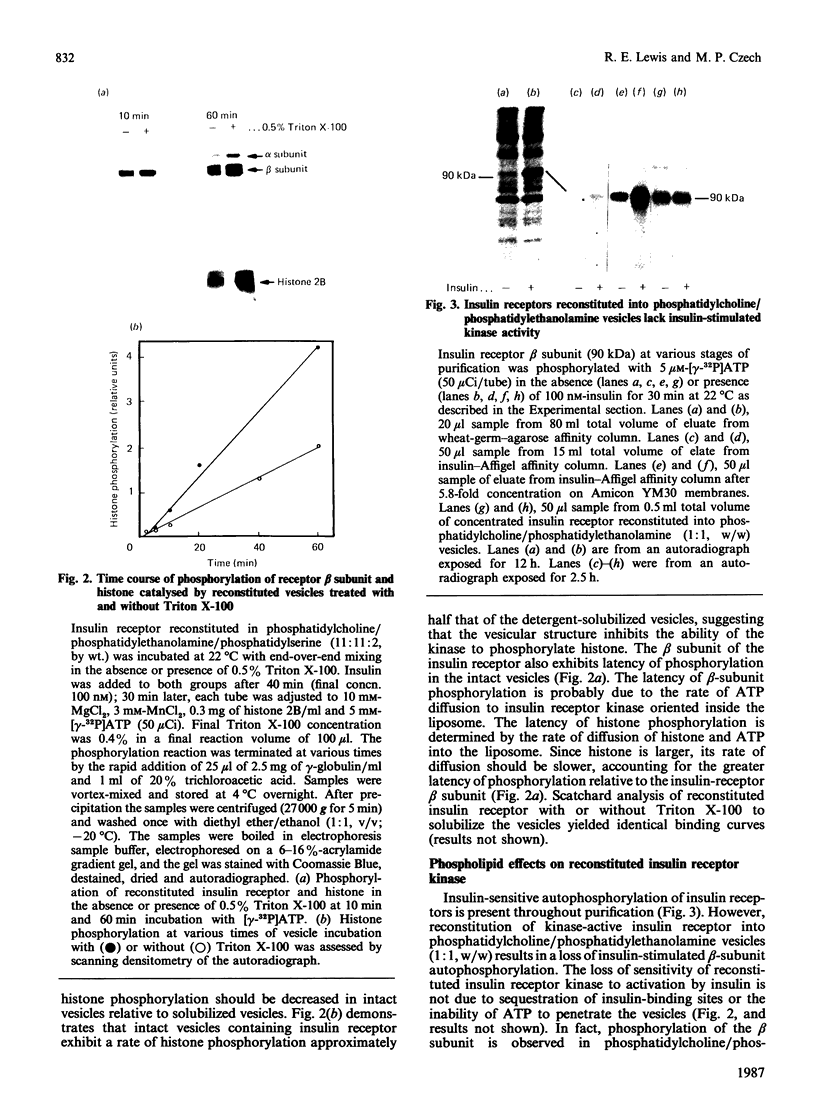
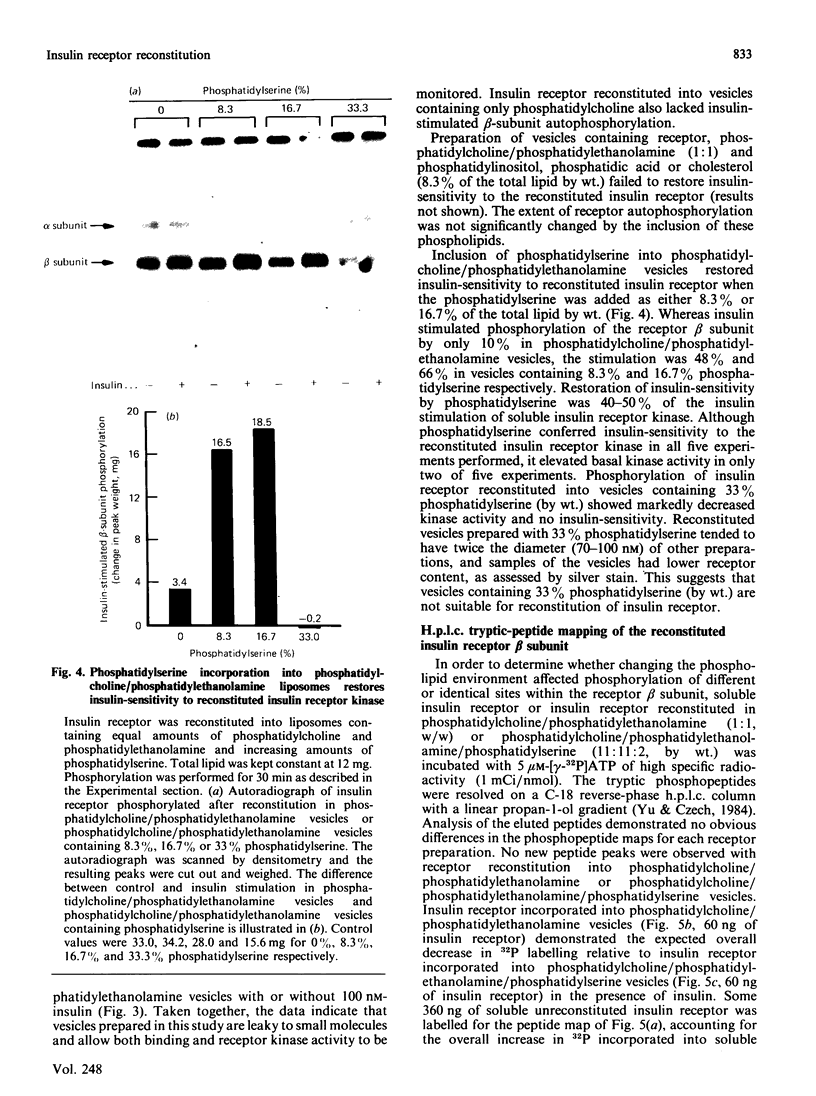
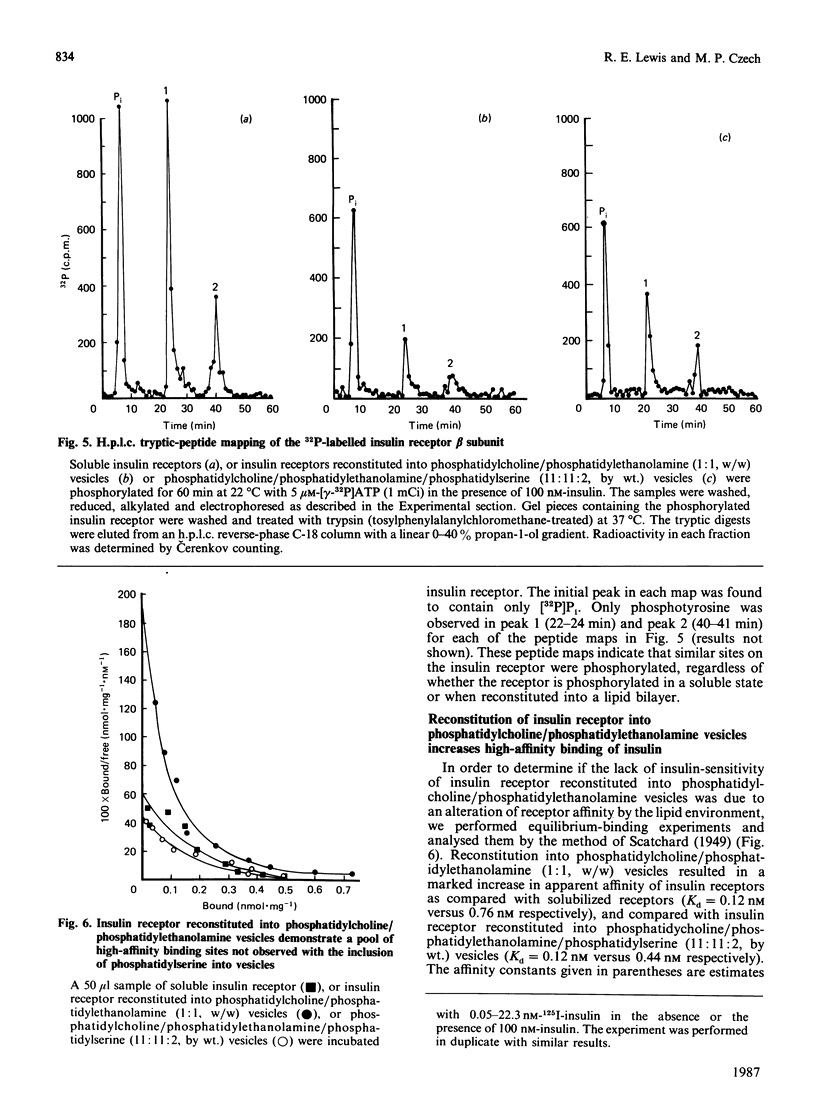
Insulin receptor kinase, affinity-purified by adsorption and elution from immobilized insulin, is stimulated 2-3-fold by insulin in detergent solution. Reconstitution of the receptor kinase into leaky vesicles containing phosphatidylcholine and phosphatidylethanolamine (1:1, w/w) by detergent removal on Sephadex G-50 results in the complete loss of receptor kinase sensitivity to activation by insulin. Insulin receptors in these vesicles also exhibit an increase in their apparent affinity for 125I-insulin (Kd = 0.12 nM versus 0.76 nM). Inclusion of 8.3-16.7% phosphatidylserine into the reconstituted vesicles restores 40-50% of the insulin-sensitivity to the receptor kinase. An elevated apparent affinity for 125I-insulin of insulin receptors in vesicles containing phosphatidylcholine and phosphatidylethanolamine is also restored to the value observed in detergent solution by the inclusion of phosphatidylserine in the reconstituted system. The effect of phosphatidylserine on insulin receptor kinase appears specific, because cholesterol, phosphatidylinositol and phosphatidic acid are all unable to restore insulin-sensitivity to the receptor kinase. Autophosphorylation sites on the insulin receptor as analysed by h.p.l.c. of tryptic 32P-labelled receptor phosphopeptides are not different for insulin receptors autophosphorylated in detergent solution or for the reconstituted vesicles in the presence or absence of phosphatidylserine. These data indicate that the phospholipid environment of insulin receptors can modulate its binding and kinase activity, and phosphatidylserine acts to restore insulin-sensitivity to the receptor kinase incorporated into phosphatidylcholine/phosphatidylethanolamine vesicles.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avruch J., Nemenoff R. A., Blackshear P. J., Pierce M. W., Osathanondh R. Insulin-stimulated tyrosine phosphorylation of the insulin receptor in detergent extracts of human placental membranes. Comparison to epidermal growth factor-stimulated phosphorylation. J Biol Chem. 1982 Dec 25;257(24):15162–15166. [PubMed] [Google Scholar]
- Brunner J., Skrabal P., Hauser H. Single bilayer vesicles prepared without sonication. Physico-chemical properties. Biochim Biophys Acta. 1976 Dec 2;455(2):322–331. doi: 10.1016/0005-2736(76)90308-4. [DOI] [PubMed] [Google Scholar]
- Cerione R. A., Staniszewski C., Benovic J. L., Lefkowitz R. J., Caron M. G., Gierschik P., Somers R., Spiegel A. M., Codina J., Birnbaumer L. Specificity of the functional interactions of the beta-adrenergic receptor and rhodopsin with guanine nucleotide regulatory proteins reconstituted in phospholipid vesicles. J Biol Chem. 1985 Feb 10;260(3):1493–1500. [PubMed] [Google Scholar]
- Cerione R. A., Strulovici B., Benovic J. L., Lefkowitz R. J., Caron M. G. Pure beta-adrenergic receptor: the single polypeptide confers catecholamine responsiveness to adenylate cyclase. Nature. 1983 Dec 8;306(5943):562–566. doi: 10.1038/306562a0. [DOI] [PubMed] [Google Scholar]
- Czech M. P. The nature and regulation of the insulin receptor: structure and function. Annu Rev Physiol. 1985;47:357–381. doi: 10.1146/annurev.ph.47.030185.002041. [DOI] [PubMed] [Google Scholar]
- Ebina Y., Ellis L., Jarnagin K., Edery M., Graf L., Clauser E., Ou J. H., Masiarz F., Kan Y. W., Goldfine I. D. The human insulin receptor cDNA: the structural basis for hormone-activated transmembrane signalling. Cell. 1985 Apr;40(4):747–758. doi: 10.1016/0092-8674(85)90334-4. [DOI] [PubMed] [Google Scholar]
- Eytan G. D., Racker E. Selective incorporation of membrane proteins into proteoliposomes of different compositions. J Biol Chem. 1977 May 25;252(10):3208–3213. [PubMed] [Google Scholar]
- Fujita-Yamaguchi Y., Choi S., Sakamoto Y., Itakura K. Purification of insulin receptor with full binding activity. J Biol Chem. 1983 Apr 25;258(8):5045–5049. [PubMed] [Google Scholar]
- Ginsberg B. H., Brown T. J., Simon I., Spector A. A. Effect of the membrane lipid environment on the properties of insulin receptors. Diabetes. 1981 Sep;30(9):773–780. doi: 10.2337/diab.30.9.773. [DOI] [PubMed] [Google Scholar]
- Gould R. J., Ginsberg B. H., Spector A. A. Lipid effects on the binding properties of a reconstituted insulin receptor. J Biol Chem. 1982 Jan 10;257(1):477–484. [PubMed] [Google Scholar]
- Harrison L. C., Itin A. Purification of the insulin receptor from human placenta by chromatography on immobilized wheat germ lectin and receptor antibody. J Biol Chem. 1980 Dec 25;255(24):12066–12072. [PubMed] [Google Scholar]
- Häring H. U., Kasuga M., Kahn C. R. Insulin receptor phosphorylation in intact adipocytes and in a cell-free system. Biochem Biophys Res Commun. 1982 Oct 29;108(4):1538–1545. doi: 10.1016/s0006-291x(82)80082-x. [DOI] [PubMed] [Google Scholar]
- Ito S., Richert N., Pastan I. Phospholipids stimulate phosphorylation of vinculin by the tyrosine-specific protein kinase of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4628–4631. doi: 10.1073/pnas.79.15.4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs S., Cuatrecasas P. Insulin receptors. Annu Rev Pharmacol Toxicol. 1983;23:461–479. doi: 10.1146/annurev.pa.23.040183.002333. [DOI] [PubMed] [Google Scholar]
- Kasuga M., Karlsson F. A., Kahn C. R. Insulin stimulates the phosphorylation of the 95,000-dalton subunit of its own receptor. Science. 1982 Jan 8;215(4529):185–187. doi: 10.1126/science.7031900. [DOI] [PubMed] [Google Scholar]
- Kasuga M., Zick Y., Blith D. L., Karlsson F. A., Häring H. U., Kahn C. R. Insulin stimulation of phosphorylation of the beta subunit of the insulin receptor. Formation of both phosphoserine and phosphotyrosine. J Biol Chem. 1982 Sep 10;257(17):9891–9894. [PubMed] [Google Scholar]
- Mandersloot J. G., Roelofsen B., de Gier J. Phosphatidylinositol as the endogenous activator of the (Na+ + K+)-ATPase in microsomes of rabbit kidney. Biochim Biophys Acta. 1978 Apr 20;508(3):478–485. doi: 10.1016/0005-2736(78)90093-7. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Ochoa E. L., Dalziel A. W., McNamee M. G. Reconstitution of acetylcholine receptor function in lipid vesicles of defined composition. Biochim Biophys Acta. 1983 Jan 5;727(1):151–162. doi: 10.1016/0005-2736(83)90379-6. [DOI] [PubMed] [Google Scholar]
- Palatini P., Dabbeni-Sala F., Pitotti A., Bruni A., Mandersloot J. C. Activation of (Na+ + K+)-dependent ATPase by lipid vesicles of negative phospholipids. Biochim Biophys Acta. 1977 Apr 1;466(1):1–9. doi: 10.1016/0005-2736(77)90203-6. [DOI] [PubMed] [Google Scholar]
- Petruzzelli L. M., Ganguly S., Smith C. J., Cobb M. H., Rubin C. S., Rosen O. M. Insulin activates a tyrosine-specific protein kinase in extracts of 3T3-L1 adipocytes and human placenta. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6792–6796. doi: 10.1073/pnas.79.22.6792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike L. J., Kuenzel E. A., Casnellie J. E., Krebs E. G. A comparison of the insulin- and epidermal growth factor-stimulated protein kinases from human placenta. J Biol Chem. 1984 Aug 10;259(15):9913–9921. [PubMed] [Google Scholar]
- Radany E. W., Bellet R. A., Garbers D. L. The incorporation of a purified, membrane-bound form of guanylate cyclase into phospholipid vesicles and erythrocytes. Biochim Biophys Acta. 1985 Feb 14;812(3):695–701. doi: 10.1016/0005-2736(85)90263-9. [DOI] [PubMed] [Google Scholar]
- Roelofsen B., van Deenen L. L. Lipid requirement of membrane-bound ATPase. Studies on human erythrocyte ghosts. Eur J Biochem. 1973 Dec 3;40(1):245–257. doi: 10.1111/j.1432-1033.1973.tb03192.x. [DOI] [PubMed] [Google Scholar]
- Rosen O. M., Herrera R., Olowe Y., Petruzzelli L. M., Cobb M. H. Phosphorylation activates the insulin receptor tyrosine protein kinase. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3237–3240. doi: 10.1073/pnas.80.11.3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth R. A., Cassell D. J. Insulin receptor: evidence that it is a protein kinase. Science. 1983 Jan 21;219(4582):299–301. doi: 10.1126/science.6849137. [DOI] [PubMed] [Google Scholar]
- Shanahan M. F., Czech M. P. Purification and reconstitution of the adipocyte plasma membrane D-glucose transport system. J Biol Chem. 1977 Dec 10;252(23):8341–8343. [PubMed] [Google Scholar]
- Stadtmauer L. A., Rosen O. M. Phosphorylation of exogenous substrates by the insulin receptor-associated protein kinase. J Biol Chem. 1983 Jun 10;258(11):6682–6685. [PubMed] [Google Scholar]
- Sweet L. J., Wilden P. A., Spector A. A., Pessin J. E. Incorporation of the purified human placental insulin receptor into phospholipid vesicles. Biochemistry. 1985 Nov 5;24(23):6571–6580. doi: 10.1021/bi00344a040. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Bell J. R., Chen E. Y., Herrera R., Petruzzelli L. M., Dull T. J., Gray A., Coussens L., Liao Y. C., Tsubokawa M. Human insulin receptor and its relationship to the tyrosine kinase family of oncogenes. 1985 Feb 28-Mar 6Nature. 313(6005):756–761. doi: 10.1038/313756a0. [DOI] [PubMed] [Google Scholar]
- Yu K. T., Czech M. P. Tyrosine phosphorylation of the insulin receptor beta subunit activates the receptor-associated tyrosine kinase activity. J Biol Chem. 1984 Apr 25;259(8):5277–5286. [PubMed] [Google Scholar]
- Zick Y., Kasuga M., Kahn C. R., Roth J. Characterization of insulin-mediated phosphorylation of the insulin receptor in a cell-free system. J Biol Chem. 1983 Jan 10;258(1):75–80. [PubMed] [Google Scholar]