Abstract
In this report, the sulfated polysaccharide (SJP) isolated from the sea cucumber Stichopus japonicus can protect PC12 from Na2S2O4-induced hypoxia/reoxygenation (H/R) injury. SJP effectively improves cell viability and reduces extracellular LDH release in PC12 cells after H/R. Moreover, SJP significantly increases SOD activity but decreases MDA levels. Our experiments showed that SJP could significantly reduce cell apoptosis caused by H/R. Our current results demonstrate that SJP suppressed the activation of MAPKs, resulting in a significant decrease in Bax/Bcl-2 ratio, cleaved caspase-3/caspase-3, p53 phosphorylation, and cytochrome c release in a concentration-dependent manner. MAPK is closely related to H/R injury. SJP inhibited JNK1/2 and p38 MAPK activation but did not affect the increased ERK1/2 expression. These results suggested that JNK1/2 and p38 MAPK pathways could be involved in SJP-mediated attenuation of PC12 H/R injury. SJP prevented PC12 H/R injury in a dose-dependent manner, indicating that SJP may be developed as a candidate drug to prevent or treat cerebral ischemia–reperfusion injury.
Keywords: Hypoxia/reoxygenation(H/R), PC12, Na2S2O4, MAPK
Introduction
Hypoxic injury has been implicated in the central nervous system (CNS) pathology of numerous disorders, including stroke, head trauma, neoplasia, vascular malformations, and neurodegenerative diseases (Thompson and Ronaldson 2014). Cerebral ischemia–reperfusion injury results from the permanent reduction in cerebral blood flow that causes nerve cell function disorder, irreversible necrosis, and even death. Reperfusion is currently considered as optimum treatment; however, reperfusion can cause serious brain injuries, such as cerebral edema, cerebral hemorrhage, nerve vascular injury, neuronal necrosis, and inflammatory response failure (Eltzschig and Eckle 2011). Thus far, acceptable methods to treat cerebral ischemia have not yet been developed (Burns and Steinberg 2011). Intensive research has demonstrated that cerebral ischemia–reperfusion injury is associated with cell apoptosis (Hotchkiss et al. 2009; Tang et al. 2010). Hypoxia/reoxygenation (H/R) is also an important physiological factor in ischemia, respiratory disorders, and tumor progression diseases (Sandin et al. 2011). In addition, H/R injury can induce cell damage (Sun et al. 2014).
Cerebral ischemia–reperfusion injury activates cell mitogen-activated protein kinases (MAPKs), resulting in neuronal apoptosis (Kong et al. 2014). p38 kinase, c-Jun N-terminal kinases (JNKs), and extracellular signal-regulated kinases (ERKs) are three identified subfamilies of MAPKs (Cargnello and Roux 2011). ERK promotes cell survival, whereas JNK and p38 lead to cell death (Kyriakis and Avruch 2012). Activated p38 MAPK participates in the regulation of neuronal death that resulted from hypoxia/glucose (Lu et al. 2011). The inhibition of sustained ERK1/2 activation may partially contribute to neuroprotection achieved against H/R injury (Vartiainen et al. 2003). p-ERK, p-JNK, and p-p38 promote cell death or survival, depending on additional factors and interaction with other proteins after focal cerebral ischemia occurs (Ferrer et al. 2003). MAPK is also closely related to cerebral ischemia–reperfusion injury.
Stichopus japonicus is a species of sea cucumber that elicits nutritional, medicinal, and pharmacological effects on human. Furthermore, sea cucumber has been commonly used as traditional food and folk medicine (Bordbar et al. 2011). Sulfated polysaccharide (SJP) exhibits a number of unique biological and pharmacological activities, including anticoagulant (Suzuki et al. 1991), antithrombotic (Zancan and Mourao 2004), antitumor (Lu and Wang 2009), and anti-osteoarthritis (Kariya et al. 2004). Clinical tests have confirmed that SJP is a good treatment for patients suffering from ischemic stroke and ischemic myocardium (Li et al. 2000). As such, SJP is a potential substance that can be applied to treat cerebral ischemia–reperfusion injury. However, the regulatory mechanism remains unclear.
It is well known that neuronal pheochromocytoma (PC12) cell line possesses the phenotypic and physiological properties of sympathetic neurons (Grau and Greene 2012). Therefore, PC12 is often used as neuronal developmental model. In this report, we used PC12 to evaluate the effect of the SJP isolated from the sea cucumber S. japonicus in preventing the H/R injury.
Experimental Procedures
Source of SJP
SJP was isolated from the sea cucumber S. japonicus from Weihai Marine Aquaculture WulingDe Holy Margin Co. at Weihai in the Shandong Province in China, as described previously (Sheng et al. 2012). The weight average molecular weight of SJP was 1.79 × 105 Da as determined by HPSEC analysis. SJP was dissolved in PBS for cell incubation.
Cell Culture
PC12 cells were purchased from Chinese academy sciences (Shanghai, China). Briefly, PC12 cells were cultured in DMEM containing 10 % fetal bovine serum (FBS; Hyclone), 0.37 % NaHCO3, 100 U/ml penicillin, and 100 mg/ml streptomycin, in a humidified atmosphere with 5 % CO2 at 37 °C.
Cells were cultured to 70–80 % confluence, and then treated with 10 mmol/l Na2S2O4 for 2 h, and then were cultured in a humidified atmosphere with 5 % CO2 at 37 °C for 24 h. After H/R, cells were maintained in complete medium with 100 μg/ml (0.56 μM), 300 μg/ml (1.68 μM), and 500 μg/ml (2.79 μM) SJP (diluted from a 500 μg/ml stock solution in PBS) or MAPK inhibitors, 15 μM SB203580 (abcam), 15 μM SP600125 (abcam), and 15 μM U0126 (abcam) for 24 h. The control cells were incubated without SJP and Na2S2O4.
Cell Viability Assay
Cell viability assay was performed according to MTT test. PC12 cells were plated in 96-well plates at a density of 5000 cells/well. When cells were grown to 70–80 % confluence, H/R injury protocol was performed. Then MTT solution at a dilution of 1/10 with 10 % FBS DMEM was added to each well and the cells were incubated for 4 h at 37 °C. Absorbance at 570 nm was measured with a microplate reader (Biotek, MQX200).
LDH, SOD, and MDA Assays
The PC12 LDH, SOD, and MDA levels were measured using commercial kits (NJJC Bio), according to the manufacturer’s instructions.
Hoechst33342/PI Assay
Cell apoptosis was analyzed by staining with Hoechst33342 and propidium iodide (PI) staining. PC12 cells were collected, washed in PBS, and resuspended in 0.8–1 ml of cell staining buffer containing 5 μl of Hoechst33342 and 5 μl of PI incubated for 20–30 min at 4 °C in the dark, washed in PBS, and smeared. The red fluorescence and the blue fluorescence were observed under the fluorescence microscope.
Measurement of Mitochondrial Membrane Potential
The fluorescent, lipophilic, and cationic probe, JC-1 (Beyotime), was employed to measure the mitochondrial membrane potential (Δψm) of PC12 cells according to the manufacturer’s instructions. PC12 cells were plated in 96-well plates at a density of 5000 cells/well. When cells were grown to 70–80 % confluence, 10 mmol/l Na2S2O4 was performed. Then cells were incubated with JC-1 staining solution for 20 min at 37 °C. The fluorescence was detected with a FACS caliber (Becton–Dickinson). The wavelengths of excitation and emission were 490 and 535 nm for detection of monomeric form of JC-1. 525 and 590 nm were used to detect aggregation of JC-1. The ratio of ‘red’ to ‘green’ fluorescence represented Δψm of PC12 cells.
Western Blot Analysis
Cells were homogenized in RIPA lysis buffer containing protease inhibitor PMSF. Mitochondrial and cytosolic proteins were isolated using the Mitochondria/Cytosol Fractionation Kit according to the manufacturer’s protocol (BioVision). Total protein was measured by BCA (Pierce) and size separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis. Proteins were blotted to polyvinylidene difluoride membranes (Amersham Biosciences). Blots were incubated with antibodies against HIF-1α (Cell Signaling), Bax (abcam), Bcl-2 (abcam), cleaved caspase-3 (Cell Signaling), caspase-3 (abcam), p-p53 (abcam), p53 (abcam), cytochrome c (abcam), p-p38 (abcam), p38 (abcam), p-JNK1/2 (abcam), JNK1/2 (abcam), p-ERK1/2 (Cell Signaling), ERK1/2 (Cell Signaling), and β-actin (abcam). Goat anti-rabbit IgG and goat anti-mouse IgG (abcam) were added and the blots were developed with Immobilon Western Chemiluminescent HRP Substrate (Millipore).
Statistical Analyses
All results are expressed as mean ± SEM. For multiple comparisons, the statistical analysis was performed by using one-way ANOVA followed by the Tukey’s multiple comparison tests. Results were considered significant when P < 0.05.
Results
Cell Viability was Assayed Using MTT
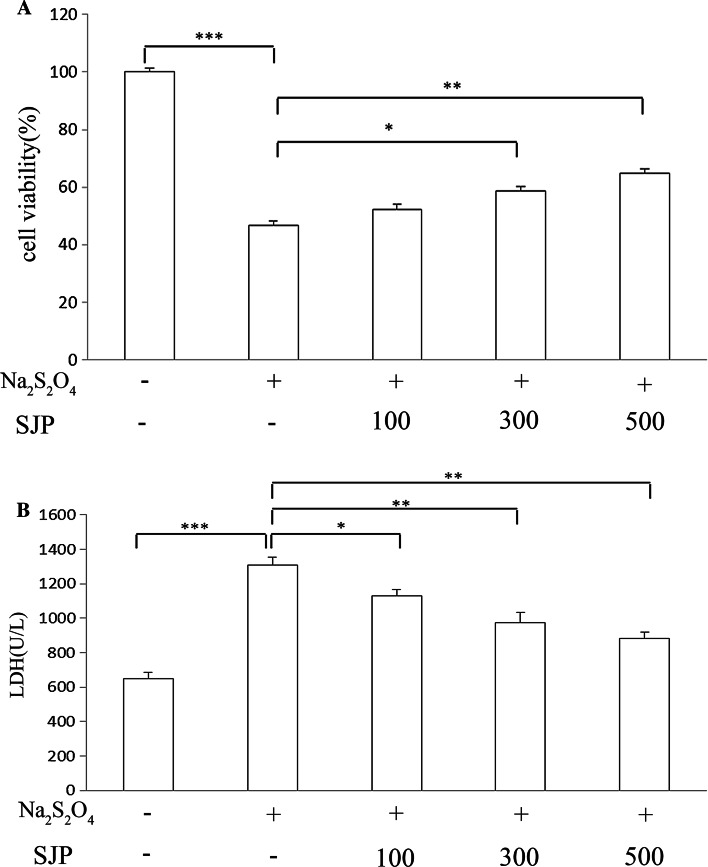
The viability of PC12 cells treated with Na2S2O4 was assessed by MTT assay. Hypoxia induced for 2 h followed by reoxygenation for 24 h significantly decreased cell viability to 46.8 ± 1.4 % (P < 0.001) compared with control cells. SJP (100–500 μg/ml) protected the cells against H/R-induced cytotoxicity; PC12 cells survival was improved as SJP concentration increased, in which 64.7 ± 1.64 % of cells were treated with 500 μg/ml SJP (Fig. 1a). These results indicated that SJP elicited a protective effect against H/R stress on PC12 cells.
Fig. 1.
Effects of SJP on cell viability and LDH in PC12 after H/R. a After PC12 cells were exposed to 2 h of Na2S2O4 and then to 24 h of reoxygenation, cell viability significantly decreased; treatment with SJP (100, 300, and 500 μg/ml) for 24 h significantly increased cell viability. b After PC12 cells were exposed to 2 h of Na2S2O4 and then to 24 h of reoxygenation, LDH level released remarkably increased; treatment with SJP (100, 300, and 500 μg/ml) for 24 h significantly decreased LDH level. Each bar represents the mean ± SEM. n = 3; *P < 0.05, **P < 0.01, ***P < 0.001
Effects of SJP on Extracellular LDH Levels in PC12 Cells
LDH assay was performed to further investigate the protective effects of SJP on H/R injury in PC12 cells. LDH is a stable cytoplasmic enzyme in all cells. LDH is rapidly released in a culture medium when the plasma membrane is damaged. Thus, the increased LDH activity in a culture medium corresponds to the degree of cell necrosis. After the cells were exposed to H/R injury, LDH activity increased to 1310 U/l, indicating that the cells were markedly damaged. After SJP treatment was administered, LDH level induced by H/R was decreased (Fig. 1b). These results suggested that SJP effectively reduced LDH leakage induced by H/R in a concentration-dependent manner.
Effects of SJP on SOD Activity and MDA Concentration in PC12 Cells
H/R significantly decreased SOD activity (Fig. 2a). Oxidative abnormalities were ameliorated by SJP treatment, which was manifested as a significant increase in SOD activity. MDA is widely used as a biomarker of lipid peroxidation. In the present study, H/R significantly increased MDA content (Fig. 2b), which was significantly ameliorated by SJP.
Fig. 2.
Effects of SJP on SOD and MDA levels. a Treatment with SJP (100, 300, and 500 μg/ml) significantly increased SOD activity b and decreased MDA concentration compared with the H/R group
Hoechst33342/PI Assay
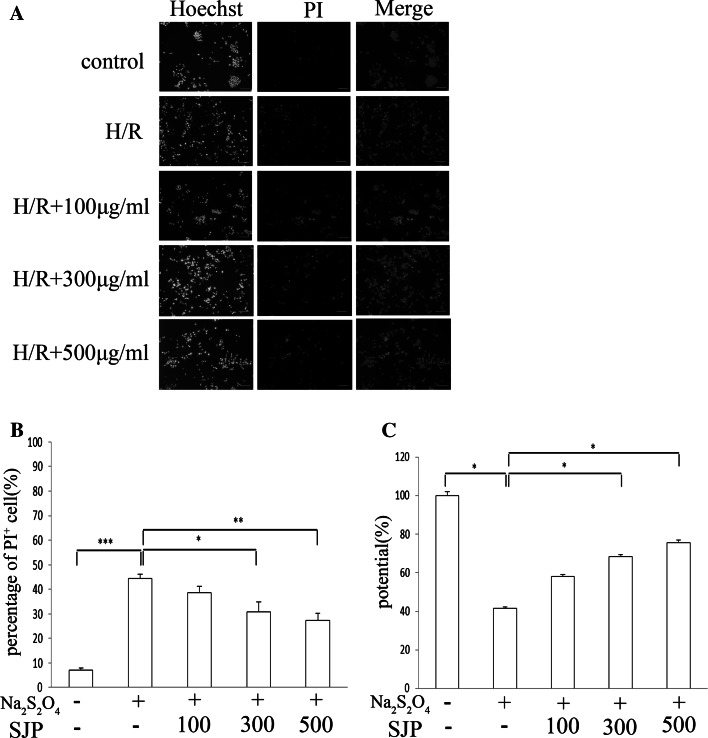
To determine whether or not SJP can block Na2S2O4-induced apoptosis in PC12 cells, we measured the cell apoptotic rate by using a fluorescence microscope after the cells were stained with Hoechst33342 and PI. Na2S2O4 induced apoptosis in PC12 cells, as demonstrated by the decreased number of Hoechst33342−/PI−cells (live cells) and the increased number of Hoechst33342+/PI+ cells (late apoptotic cells). Furthermore, SJP significantly reduced cell apoptosis in a dose-dependent manner (Fig. 3a, b). These results are consistent with those of MTT, LDH, SOD, and MDA assays.
Fig. 3.
SJP treatment decreased the number of apoptotic PC12 cells with Hoechst33342/PI after H/R injury and distribution of Δψm in PC12 cells. a Cell apoptotic rate determined using a fluorescence microscope after staining with Hoechst33342 and PI. Scale bar = 100 µm. b Representative percentages of PI+ are shown. c After SJP treatment (100, 300, and 500 μg/ml) was administered for 24 h, Δψm was measured by flow cytometry. Data are presented as mean ± SEM of at least five visions. n = 3. *P < 0.05, **P < 0.01, ***P < 0.001
Effect of SJP on Δψm in PC12 Cells
We examined the effects of SJP on mitochondrial membrane potential (Δψm) in PC12 cells. The exposure of PC12 cells to H/R resulted in the distribution of Δψm, which was shown as the decreased ratio of red to green fluorescence by JC-1 staining with a flow cytometric procedure. SJP-treated cells demonstrated the distribution of Δψm caused by H/R; this result indicated that SJP could diminish H/R-induced cell apoptosis (Fig. 3c). These results further suggested that the mitochondrion-mediated pathway is involved in SJP-induced apoptosis.
Hypoxia Inducible Factor-1α (HIF-1α) Protein Levels in PC12 Cells
We determined HIF-1α protein levels to confirm the protective effect of SJP on PC12 cells damage caused by Na2S2O4-induced H/R. HIF-1α expression was significantly increased in cells exposed to Na2S2O4 after PC12 H/R injury (Fig. 4). No significant difference was found in HIF-1α expression between SJP-treated groups and H/R group.
Fig. 4.
HIF-1α protein levels in PC12 cells. Expression of HIF-1α in PC12 cells treated with Na2S2O4 by Western blot analysis. Representative blot (up) and quantified protein levels (down) are shown. Each bar represents the mean ± SEM. n = 3; *P < 0.05, **P < 0.01, ***P < 0.001 versus control. # P < 0.05, ## P < 0.01, ### P < 0.001 versus H/R group
Western Blot of Mitochondrial and Cytosolic Cytochrome c (cyto-c)
After hypoxia was induced for 2 h followed by reoxygenation for 24 h, cyto-c was released into the cytosol. SJP suppressed the release of cyto-c into the cytosol in a dose-dependent manner; likewise, SJP increased the levels of cyto-c that remained in the mitochondria in a dose-dependent manner. SJP reduced the ratio of cyto-c between the cytosol and the mitochondria. Compared with 500 μg/ml SJP-treated group, both the p38 inhibitor (SB203580) and the JNK1/2 inhibitor (SP600125) further reduced the ratio of cyto-c. However, the ERK1/2 inhibitor U0126 did not change the effect of SJP on cyto-c release (Fig. 5).
Fig. 5.
SJP prevented apoptosis by maintaining a balanced cytochrome c concentration between the mitochondria and the cytoplasm. Representative Western blots of mitochondrial and cytosolic cytochrome c are shown. Representative blot (up) and quantified protein levels (down) are shown. Each bar represents the mean ± SEM. n = 3; *P < 0.05, **P < 0.01, ***P < 0.001 versus control. # P < 0.05, ## P < 0.01, ### P < 0.001 versus H/R group. △ P < 0.05, △△ P < 0.01, △△△ P < 0.001 versus 500 μg/ml SJP-treated group
Western Blot Analysis of Bax/Bcl-2, cl-casp-3/casp-3, p-p53/β-Actin, and p53/β-Actin
After hypoxia was induced for 2 h and reoxygenation for 24 h, the levels of pro-apoptotic proteins, BCL2-associated X protein (Bax), and cleaved caspase-3 were increased, whereas the levels of anti-apoptotic protein Bcl-2 and caspase-3 were decreased (Fig. 6). SJP reduced the ratios of Bax/Bcl-2 and cl-casp-3/casp-3 to inhibit cell apoptosis. SB203580 and SP600125 further reduced the ratios of Bax/Bcl-2 and cl-casp-3/casp-3 in PC12 cells in contrast with 500 μg/ml SJP-treated group. SJP also inhibited p53 phosphorylation in a dose-dependent manner. Furthermore, SB203580 and SP600125 inhibited p53 phosphorylation. However, U0126 did not affect the ratios of Bax/Bcl-2 and cl-casp-3/casp-3 and p53 phosphorylation. The activation of p53 tumor suppressor is involved in the H/R-induced cell apoptosis, as in p-p53. However, SJP, SB203580, SP600125, and U0126 did not affect p53 expression.
Fig. 6.
SJP prevented PC12 cell apoptosis. Expressions of Bax, Bcl-2, cleaved caspase-3, caspase-3, p-p53, and p53 of PC12 by H/R injury were detected by Western blot analysis. Representative blot (up) and quantified protein levels (down) are shown. Each bar represents the mean ± SEM. n = 3; *P < 0.05, **P < 0.01, ***P < 0.001 versus control. # P < 0.05, ## P < 0.01, ### P < 0.001 versus H/R group. △ P < 0.05, △△ P < 0.01, △△△ P < 0.001 versus 500 μg/ml SJP-treated group
SJP Inhibited PC12 Cells H/R Injury by Activated MAPK Pathways
The MAPK signaling pathway is an important cascade that translates extracellular signals into intracellular activities. To further assess whether SJP treatment modulates MAPK pathways in PC12, we also examined expression of p-p38, p-JNK1/2, and p-ERK1/2 MAPK in Na2S2O4-induced PC12 by Western blot. The phosphorylation of p38, JNK1/2, and ERK1/2 was significantly increased in PC12 cells after H/R. SJP significantly decreased the phosphorylation of p38 and JNK1/2 in PC12 cells in a dose-dependent manner. SJP did not affect the phosphorylation of ERK1/2 after H/R (Fig. 7). These results suggested that SJP prevented H/R injury on PC12 cells via activated MAPK pathways.
Fig. 7.
SJP inhibited MAPK signaling pathways. Expression and phosphorylation levels of p38, JNK, and ERK in PC12 cells by H/R injury were detected by Western blot analysis. Representative blot (up) and quantified protein levels (down) are shown. Each bar represents the mean ± SEM. n = 3; *P < 0.05, **P < 0.01, ***P < 0.001 versus control. # P < 0.05, ## P < 0.01, ### P < 0.001 versus H/R group. △ P < 0.05, △△ P < 0.01, △△△ P < 0.001 versus 500 μg/ml SJP-treated group
Discussion
PC12 is widely used in neurologic research. Sodium hydrosulfite (Na2S2O4) treatment causes hypoxia which can be used as a common oxygen-consuming model. SJP, one of the major bioactive components of S. japonicus, was investigated for its cytoprotection against Na2S2O4-induced H/R on PC12 cells, by focusing on MAPK signaling pathways because these pathways are closely related to apoptotic signaling.
Oxidative stress has been considered as a major factor after H/R injury occurs (Yang et al. 2014; Chen et al. 2011). Oxygen tension regulates apoptosis in PC12 cells (Genetos et al. 2010). Superoxide dismutase (SOD) reflects the level of ischemic tissue damage and plays a beneficial role in the response of the brain to oxidative stress (Li et al. 2015). SOD is also a powerful antioxidant that catalyzes redox reactions by converting superoxide radicals into hydrogen peroxide and oxygen; thus, brain tissues are protected from further oxidative damage (Ciancarelli et al. 2015). Malondialdehyde (MDA), a stable end product of lipid peroxidation, is responsible for ROS-mediated oxidative stress and implicated in disease severity (Naziroglu et al. 2009). In the present study, oxidative injury in PC12 H/R was determined by measuring SOD activity and MDA concentration. SOD activity was significantly decreased in PC12 H/R, and SJP significantly reversed H/R injury; this result indicated that SJP may normalize the redox status of PC12 cells under H/R injury. MDA concentration was significantly increased in PC12 after H/R injury occurred. SJP treatment significantly reduced the H/R-induced MDA increase. In this study, SJP treatment significantly increased SOD activity but decreased MDA levels in the H/R of PC12. Thus, our results revealed the antioxidative activity of SJP after H/R was induced.
H/R not only induces cell apoptosis but also causes cell necrosis. The release of lactate dehydrogenase (LDH) is a biochemical marker of necrotic cell death because of loss of membrane integrity. In the study, we determined the necrosis of PC12 cells during H/R by measuring LDH release. LDH release was increased by H/R injury and reduced by SJP. SJP may also have protected PC12 by reducing H/R-induced necrosis.
HIF is a specific redox-sensitive transcription factor. Reoxygenation following hypoxia results in p53 accumulation (Zhang et al. 2005); cells likely undergo rapid p53-dependent apoptosis (Pires et al. 2010). Physical and functional interactions are observed between HIF-1α and p53 (Poon et al. 2009). In hypoxic neurons, HIF-1α and p53 interact to promote a pathological sequence, resulting in cell death (Dong et al. 2014). In the present study, the protein expression of HIF-1α and p53 significantly increased in the Na2S2O4-induced H/R injury in PC12 cells; however, no difference in HIF-1α and p53 expression was observed after SJP treatment was administered. SJP increased cell survival and decreased cell apoptosis, and these processes were not regulated by HIF-1α and p53.
Neuronal apoptosis is an important event in H/R-induced neuronal injury (Li et al. 2014). The intrinsic pathway used to activate cell death is marked by mitochondrial depolarization and cyto-c release (Cosentino and García-Sáez 2014). PC12 cells are sensitive to mitochondrion-related apoptosis (Wang and Gerdes 2015). SJP modulates the mitochondrial dysfunction by attenuating oxidative stress following H/R insult. H/R injury has been shown to decrease SOD activity and increase MDA levels in PC12 cells. Oxidative stress induces depolarization of the mitochondrial membrane, which eventually increases the level of pro-apoptotic molecules in cells. The first regulatory step in mitochondrial apoptosis is mediated by the Bcl-2 family of proteins. Bax forms oligomers, thereby releasing cyto-c from the intermembrane space of the mitochondria to the cytoplasm; this release likely occurs via a channel formed in the outer mitochondrial membrane. The released cyto-c then activates caspase cascade to induce apoptosis (Hardwick and Soane 2013). Cyto-c accumulates in the cytosol, where it participates in the autoactivation of initiator caspase-9. The activated caspase-9 then proteolytically cleaves and activates executioners, such as caspase-3 (Kurokawa and Kornbluth 2009). The mitochondrial release of cyto-c is associated with the stimulation of caspase-3 (Broughton et al. 2009). Bax is a pro-apoptotic protein that induces cell apoptosis. Bcl-2 is an anti-apoptotic protein and promotes cell survival. Bcl-2 is necessary to regulate caspase-3-mediated apoptosis (Alhosin et al. 2015). Cytosolic cyto-c likely activates caspase-3 that feeds back to the mitochondria (Laethem et al. 2004). In our study, Na2S2O4 induced the distribution of the Δψm in the mitochondria, followed by cyto-c release and caspase-3 activation. SJP reduced Bax expression and increased Bcl-2 expression. SJP suppressed the protein expression of caspase-3 and feeds back to reduce the release of cytochrome c from the mitochondria. The Bax/Bcl-2 ratio contributes to cell survival by regulating the function of the mitochondria. The Bax/Bcl-2 ratio decreased in a dose-dependent manner, as induced by SJP treatment. At the same time, SJP inhibited cyto-c release and caspase-3 activation. SJP protects PC12 cells from apoptosis via modulating mitochondrial-mediated oxidative stress. SJP effectively increased SOD activity and decreased MDA levels and reversed the mitochondrial respiratory dysfunction which subsequently decreased apoptosis induced by H/R injury, indicating that SJP exerts antioxidative activity partially by modulating H/R-induced mitochondrial dysfunctions. Furthermore, SJP significantly decreased the ratios of Bax/Bcl-2 and cl-casp-3/casp-3 and p-p53 and cyto-c phosphorylation, which may be related to MAPK signaling pathway.
MAPK pathways are considered as mediators of cerebral ischemic–reperfusion injury (Kovalska et al. 2012). After the onset of cerebral ischemia reperfusion, oxidative stress activates MAPK signaling cascades that participate in neuronal survival or damage (Nozaki et al. 2001). Our experiments showed that SJP treatment significantly improved cell viability and reduced extracellular LDH level in PC12 cells after H/R. MAPK regulates cell survival or death. Inhibition of MAPK pathways could reduce necrotic PC12 cells in H/R model. The protective effect of SJP was associated with a decrease in LDH level, suggesting that MAPK may participate in the progress of necrotic cell death. Exposing PC12 cells to Na2S2O4 for 2 h and reoxygenation for 24 h caused a rapid increase in the phosphorylation of p38, ERK1/2, and JNK1/2. p38 and JNK1/2 are activated by inflammatory cytokines and environmental stresses, and ERK1/2 is activated by growth factors and cytokines (Johnson and Lapadat 2002). JNKs play a critical role in death receptor-initiated extrinsic pathway and mitochondrial intrinsic apoptotic pathways. JNKs are also involved in apoptotic signaling. The defect in apoptosis can be correlated with the lack of mitochondrial depolarization, cyto-c release, and caspase activation. Moreover, JNK activates the p53 apoptotic pathway (Oleinik et al. 2007). JNK-activated apoptotic pathway involves the p53-mediated upregulation of the pro-apoptotic protein Bax. JNK promotes apoptosis by inhibiting the anti-apoptotic protein Bcl-2 (Nijboer et al. 2013). In many cases, p38-MAPKs are activated simultaneously with JNKs (Werlen et al. 2003). In our study, the phosphorylation of p53 was drastically reduced in cells treated with SJP. SJP inhibited JNK1/2 and p38 MAPK activation but did not affect the increased p-ERK1/2 expression. These results suggested that JNK1/2 and p38 MAPK pathways could be involved in the reduced PC12 H/R injury effects of SJP. SJP protects PC12 cells from apoptosis and necrosis by inhibition of JNK1/2 and p38 MAPK signaling pathway.
In conclusion, our findings suggested that SJP reduced PC12 H/R injury. This protective effect restored neuronal function, promoted oxidative stress balance, and exhibited anti-apoptotic properties. SJP suppressed the activation of MAPKs, resulting in a significant decrease in ratios of Bax/Bcl-2 and cl-casp-3/casp-3, p53 phosphorylation, and cyto-c release in a concentration-dependent manner (Fig. 8). The neuroprotective effects of SJP against H/R injury may be of great importance and contribute to clinical efficacy for the treatment of cerebral ischemic–reperfusion injury.
Fig. 8.
Effects of SJP on PC12 H/R injury are associated with p38 downregulation and JNK1/2 MAPK activation. SJP protected cells against PC12 H/R injury by inhibition of the MAPK signaling pathway. SJP reduced pro-apoptotic Bax expression and increased anti-apoptotic Bcl-2 expression by regulating the function of the mitochondria. SJP inhibited cytochrome c release and caspase-3 activation. SJP suppressed the protein expression of caspase-3 and feeds back to reduce the release of cytochrome c from the mitochondria. SJP significantly decreased p53 phosphorylation and regulated pro-apoptotic Bax expression by nuclear signaling. p38-MAPKs are activated simultaneously with JNKs. These results are related to downregulation of p38 and JNK1/2 MAPK activation
Acknowledgments
This work was supported by Grants from the National Natural Science Foundation of China (No. 81371455).
Conflict of interest
There is no conflict of interest for all authors.
Ethical Standards
The study complies with current ethical consideration.
References
- Alhosin M, León-González AJ, Dandache I, Lelay A, Rashid SK, Kevers C, Pincemail J, Fornecker LM, Mauvieux L, Herbrecht R, Schini-Kerth VB (2015) Bilberry extract (Antho 50) selectively induces redox-sensitive caspase 3-related apoptosis in chronic lymphocytic leukemia cells by targeting the Bcl-2/Bad pathway. Sci Rep 5:8996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordbar S, Anwar F, Saari N (2011) High-value components and bioactives from sea cucumbers for functional foods—a review. Marine Drugs 9:1761–1805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broughton BR, Reutens DC, Sobey CG (2009) Apoptotic mechanisms after cerebral ischemia. Stroke 40:e331–e339 [DOI] [PubMed] [Google Scholar]
- Burns TC, Steinberg GK (2011) Stem cells and stroke: opportunities, challenges and strategies. Expert Opin Biol Ther 11:447–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cargnello M, Roux PP (2011) Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol Mol Biol Rev 75:50–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Yoshioka Y, Kim GS, Jung JE, Okami N, Sakata H, Maier CM, Narasimhan P, Goeders CE, Chan PH (2011) Oxidative stress in ischemic brain damage: mechanisms of cell death and potential molecular targets for neuroprotection. Antioxid Redox Signal 14:1505–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciancarelli I, Massimo CD, Amicis DD, Pistarini C, Ciancarelli MG (2015) Uric Acid and Cu/Zn superoxide dismutase: potential strategies and biomarkers in functional recovery of post-acute ischemic stroke patients after intensive neurorehabilitation. Curr Neurovasc Res 12:120–127 [DOI] [PubMed] [Google Scholar]
- Cosentino K, García-Sáez AJ (2014) Mitochondrial alterations in apoptosis. Chem Phys Lipids 181:62–75 [DOI] [PubMed] [Google Scholar]
- Dong Y, Ito T, Velayo C, Sato T, Iida K, Endo M, Funamoto K, Sato N, Yaegashi N, Kimura Y (2014) Intrauterine ischemic reperfusion switches the fetal transcriptional pattern from HIF-1α- to P53-dependent regulation in the murine brain. PLoS One 9:e110577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eltzschig HK, Eckle T (2011) Ischemia and reperfusion—from mechanism to translation. Nat Med 17:1391–1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer I, Friguls B, Dalfo E, Planas AM (2003) Early modifications in the expression of mitogen-activated protein kinase (MAPK/ERK), stress-activated kinases SAPK/JNK and p38, and their phosphorylated substrates following focal cerebral ischemia. Acta Neuropathol 105:425–437 [DOI] [PubMed] [Google Scholar]
- Genetos DC, Cheung WK, Decaris ML, Leach JK (2010) Oxygen tension modulates neurite outgrowth in PC12 cells through a mechanism involving HIF and VEGF. J Mol Neurosci 40:360–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau CM, Greene LA (2012) Use of PC12 cells and rat superior cervical ganglion sympathetic neurons as models for neuroprotective assays relevant to Parkinson’s disease. Methods Mol Biol 846:201–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick JM, Soane L (2013) Multiple functions of BCL-2 family proteins. Cold Spring Harb Perspect Biol. doi:10.1101/cshperspect.a008722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotchkiss RS, Strasser A, McDunn JE, Swanson PE (2009) Cell death. N Engl J Med 361:1570–1583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson GL, Lapadat R (2002) Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 298:1911–1912 [DOI] [PubMed] [Google Scholar]
- Kariya Y, Mulloy B, Imai K, Tominaga A, Kaneko T, Asari A, Suzuki K, Masuda H, Kyogashima M, Ishii T (2004) Isolation and partial characterization of fucan sulfates from the body wall of sea cucumber Stichopus japonicus and their ability to inhibit osteoclastogenesis. Carbohydr Res 339:1339–1346 [DOI] [PubMed] [Google Scholar]
- Kong LL, Wang ZY, Han N, Zhuang XM, Wang ZZ, Li H, Chen NH (2014) Neutralization of chemokine-like factor 1, a novel C-C chemokine, protects against focal cerebral ischemia by inhibiting neutrophil infiltration via MAPK pathways in rats. J Neuroinflamm 11:112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalska M, Kovalska L, Pavlikova M, Janickova M, Mikuskova K, Adamkov M, Kaplan P, Tatarkova Z, Lehotsky J (2012) Intracellular signaling MAPK pathway after cerebral ischemia-reperfusion injury. Neurochem Res 37:1568–1577 [DOI] [PubMed] [Google Scholar]
- Kurokawa M, Kornbluth S (2009) Caspases and kinases in a death grip. Cell 138:838–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakis JM, Avruch J (2012) Mammalian MAPK signal transduction pathways activated by stress and inflammation: a 10-year update. Physiol Rev 92:689–737 [DOI] [PubMed] [Google Scholar]
- Laethem AV, Kelst SV, Lippens S, Declercq W, Vandenabeele P, Janssens S, Vandenheede JR, Garmyn M, Agostinis P (2004) Activation of p38 MAPK is required for Bax translocation to mitochondria, cytochrome c release and apoptosis induced by UVB irradiation in human keratinocytes. FASEB J 18:1946–1948 [DOI] [PubMed] [Google Scholar]
- Li Z, Wang H, Li J, Zhang G, Gao C (2000) Basic and clinical study on the antithrombotic mechanism of glycosaminoglycan extracted from sea cucumber. Chin Med J 113:706–711 [PubMed] [Google Scholar]
- Li X, Zhao Y, Liu P, Zhu X, Chen M, Wang H, Lu D, Qi R (2014) Senegenin inhibits hypoxia/reoxygenation-induced neuronal apoptosis by upregulating RhoGDIalpha. DOI, Mol Neurobiol. doi:10.1007/s12035-014-8948-6 [DOI] [PubMed] [Google Scholar]
- Li L, Zhu K, Liu Y, Wu X, Wu J, Zhao Y, Zhao J (2015) Targeting thioredoxin-1 with siRNA exacerbates oxidative stress injury after cerebral ischemia/reperfusion in rats. Neuroscience 284:815–823 [DOI] [PubMed] [Google Scholar]
- Lu Y, Wang BL (2009) The research progress of antitumorous effectiveness of Stichopus japonicus acid mucopolysaccharide in north of China. Am J Med Sci 337:195–198 [DOI] [PubMed] [Google Scholar]
- Lu Q, Rau TF, Harris V, Johnson M, Poulsen DJ, Black SM (2011) Increased p38 mitogen-activated protein kinase signaling is involved in the oxidative stress associated with oxygen and glucose deprivation in neonatal hippocampal slice cultures. Eur J Neurosci 34:1093–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naziroglu M, Kutluhan S, Uguz AC, Celik O, Bal R, Butterworth PJ (2009) Topiramate and vitamin E modulate the electroencephalographic records, brain microsomal and blood antioxidant redox system in pentylentetrazol-induced seizure of rats. J Membr Biol 229:131–140 [DOI] [PubMed] [Google Scholar]
- Nijboer CH, Bonestroo HJ, Zijlstra J, Kavelaars A, Heijnen CJ (2013) Mitochondrial JNK phosphorylation as a novel therapeutic target to inhibit neuroinflammation and apoptosis after neonatal ischemic brain damage. Neurobiol Dis 54:432–444 [DOI] [PubMed] [Google Scholar]
- Nozaki K, Nishimura M, Hashimoto N (2001) Mitogen-activated protein kinases and cerebral ischemia. Mol Neurobiol 23:1–19 [DOI] [PubMed] [Google Scholar]
- Oleinik NV, Krupenko NI, Krupenko SA (2007) Cooperation between JNK1 and JNK2 in activation of p53 apoptotic pathway. Oncogene 26:7222–7230 [DOI] [PubMed] [Google Scholar]
- Pires IM, Bencokova Z, Milani M, Folkes LK, Li JL, Stratford MR, Harris AL, Hammond EM (2010) Effects of acute versus chronic hypoxia on DNA damage responses and genomic instability. Cancer Res 70:925–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon E, Harris AL, Ashcroft M (2009) Targeting the hypoxia-inducible factor (HIF) pathway in cancer. Expert Rev Mol Med 11:e26 [DOI] [PubMed] [Google Scholar]
- Sandin A, Dagnell M, Gonon A, Pernow J, Stangl V, Aspenström P, Kappert K, Ostman A (2011) Hypoxia followed by re-oxygenation induces oxidation of tyrosine phosphatases. Cell Signal 23:820–826 [DOI] [PubMed] [Google Scholar]
- Sheng X, Li M, Song S, Zhang N, Wang Y, Liang H, Wang W, Ji A (2012) Sulfated polysaccharide isolated from the sea cucumber Stichopus japonicus promotes neurosphere migration and differentiation via up-regulation of N-cadherin. Cell Mol Neurobiol 32:435–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Li YZ, Ding YH, Wang J, Geng J, Yang H, Ren J, Tang JY, Gao J (2014) Neuroprotective effects of gallic acid against hypoxia/reoxygenation-induced mitochondrial dysfunctions in vitro and cerebral ischemia/reperfusion injury in vivo. Brain Res 1589:126–139 [DOI] [PubMed] [Google Scholar]
- Suzuki N, Kitazato K, Takamatsu J, Saito H (1991) Antithrombotic and anticoagulant activity of depolymerized fragment of the glycosaminoglycan extracted from Stichopus japonicus selenka. Thromb Haemost 65:369–373 [PubMed] [Google Scholar]
- Tang Z, Arjunan P, Lee C, Li Y, Kumar A, Hou X, Wang B, Wardega P, Zhang F, Dong L, Zhang Y, Zhang SZ, Ding H, Fariss RN, Becker KG, Lennartsson J, Nagai N, Cao Y, Li X (2010) Survival effect of PDGF-CC rescues neurons from apoptosis in both brain and retina by regulating GSK3beta phosphorylation. J Exp Med 207:867–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson BJ, Ronaldson PT (2014) Drug delivery to the ischemic brain. Adv Pharmacol 71:165–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vartiainen N, Goldsteins G, Keksa-Goldsteine V, Chan PH, Koistinaho J (2003) Aspirin inhibits p44/42 mitogen-activated protein kinase and is protective against hypoxia/reoxygenation neuronal damage. Stroke 34:752–757 [DOI] [PubMed] [Google Scholar]
- Wang X, Gerdes HH (2015) Transfer of mitochondria via tunneling nanotubes rescues apoptotic PC12 cells. Cell Death Differ. doi:10.1038/cdd [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werlen G, Hausmann B, Naeher D, Palmer E (2003) Signaling life and death in the thymus: timing is everything. Science 299:1859–1863 [DOI] [PubMed] [Google Scholar]
- Yang ZB, Tan B, Li TB, Lou Z, Jiang JL, Zhou YJ, Yang J, Luo XJ, Peng J (2014) Protective effect of vitexin compound B-1 against hypoxia/reoxygenation-induced injury in differentiated PC12 cells via NADPH oxidase inhibition. Naunyn Schmiedebergs Arch Pharmacol 387:861–871 [DOI] [PubMed] [Google Scholar]
- Zancan P, Mourao PA (2004) Venous and arterial thrombosis in rat models: dissociation of the antithrombotic effects of glycosaminoglycans. Blood Coagul Fibrinolysis 15:45–54 [DOI] [PubMed] [Google Scholar]
- Zhang X, Li J, Sejas DP, Pang Q (2005) Hypoxia-reoxygenation induces premature senescence in FA bone marrow hematopoietic cells. Blood 106:75–85 [DOI] [PubMed] [Google Scholar]