Abstract
The accumulation and deposition of β-amyloid peptide (Aβ) in senile plaques and cerebral vasculature is believed to facilitate the progressive neurodegeneration that occurs in the Alzheimer’s disease (AD). The present study sought to elucidate possible effects of baicalin, a natural phytochemical, on Aβ toxicity in a rat model of AD. By morris water maze test, Aβ1–42 injection was found to cause learning and memory deficit in rat, which was effectively improved by baicalin treatment. Besides, histological examination showed that baicalin could attenuate the hippocampus injury caused by Aβ. The neurotoxicity mechanism of Aβ is associated with oxidative stress and apoptosis, as revealed by increased malonaldehyde generation and TUNEL-positive cells. Baicalin treatment was able to increase antioxidant capabilities by recovering activities of antioxidant enzymes (superoxide dismutase, catalase, and glutathione peroxidase) and up-regulating their gene expression. Moreover, baicalin effectively prevented Aβ-induced mitochondrial membrane potential decrease, Bax/Bcl-2 ratio increase, cytochrome c release, and caspase-9/-3 activation. In addition, we found that the anti-oxidative effect of baicalin was associated with Nrf2 activation. In conclusion, baicalin effectively improved Aβ-induced learning and memory deficit, hippocampus injury, and neuron apoptosis, making it a promising drug to preventive interventions for AD.
Keywords: Baicalin, Aβ, Alzheimer’s disease, Learning and memory deficit, Oxidative stress, Apoptosis
Introduction
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder, which is characterized by progressive cognitive dysfunction (Mebane-Sims 2009). It is hypothesized that the accumulation and deposition of the abnormal metabolic products, amyloid-beta peptide (Aβ), plays a key role in the pathogenic mechanism of AD (Yankner et al. 1989; Yankner 1989; Selkoe et al. 1991). The Aβ aggregates located around neurons not only have a direct toxic effect on the neurons, but also make the neurons susceptible to free radicals, nerve toxins, and other harmful factors, which results in widespread degeneration, the loss of functional neurons, and ultimately memory and cognitive dysfunction (Cappai and Barnham 2008; Lauren et al. 2009; Lee et al. 2007). SK-PC-B70M and T-817MA, which protect neurons from Aβ aggregate-induced toxicity, have been shown to have therapeutic potential for the treatment of AD (Hawkes et al. 2009; Sabbagh 2009; Seo et al. 2009). Some of these compounds have entered clinical trials. Therefore, therapeutic strategies aimed at preventing or delaying Aβ-induced toxicity, which might be a reasonable choice against AD.
Baicalin (7-d-glucuronic acid, 5,6-dihydroxyflavone) is a flavone isolated from the Chinese medicinal herb Scutellaria baicalensis Georgi that has been used to treat inflammatory diseases and ischemic stroke for thousands of years in China. Baicalin is found to be a novel and promising therapeutic agent for human central nervous system diseases, because it can pass through the blood–brain barrier into the central nervous system (Tarrago et al. 2008). Therefore, its physiological effects need to be thoroughly elucidated. The neuroprotective effects of baicalin have been described in many experimental models, such as cerebral ischemia (Cheng et al. 2013; Cao et al. 2011; Xue et al. 2010; Zhou et al. 2014), spinal cord injury (Cao et al. 2010), epilepsy (Liu et al. 2012), learning and memory deficits (Lee et al. 2014), and cultured neuron (Zheng et al. 2014; Yin et al. 2011). However, there is little information about the therapeutic effects of baicalin on Aβ-induced toxicity in vivo. Herein, we tested the efficacy of baicalin in an Aβ injection rat model and investigated the potential mechanisms (Fig 1).
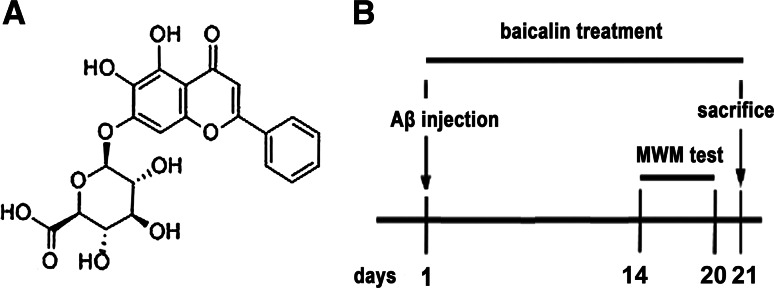
Fig. 1.
a Chemical structure of baicalin. b Diagram of the experimental protocol
Materials and Methods
Ethics Statement
All experiments were executed in accordance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health Publication No. 80-23, revised 1996).
Materials
Baicalin and donepezil were obtained from National Institutes for Food and Drug Control (China). Aβ1–42 was obtained from Sigma Aldrich (USA). β-Actin antibody was obtained from Sungene Biotech (China). Cytochrome c, cleaved caspase-9, cleaved caspase-3, Bax, and Bcl-2 antibodies were obtained from Boster Biotechnology (China). NF E2-related factor 2 (Nrf2) antibody was obtained from Santa Cruz (USA). TUNEL detection kit, rhodamine 123, total superoxide dismutase (SOD) assay kit, catalase (CAT) assay kit, glutathione peroxidase (GPx) assay kit, and malonaldehyde (MDA) assay kit were obtained from Beyotime Biotechnology (China). All the other reagents, unless otherwise stated, were from Beyotime Biotechnology (China).
Model and Drug Treatment
Thirty six adult male Wistar rats weighting 250–280 g were used in these experiments. The rats were divided randomly into six groups (six rats each): the sham vehicle group (normal saline), the model group (Aβ), the B50 group (Aβ+ 50 mg/kg baicalin), the B100 group (Aβ+ 100 mg/kg baicalin), the B200 group (Aβ+ 200 mg/kg baicalin), and the D group (Aβ+ 1 mg/kg donepezil). Donepezil is an FDA-approved drug against AD, and it is selected as the positive control.
Aβ1–42 was dissolved in normal saline at the concentration of 2.5 μg/μL and incubated at 37 °C for 3 days to allow the peptide to aggregate. Rats were anesthetized with 10 % chloral hydrate (4 mL/kg, i.p.) and placed into a stereotaxic apparatus. The coordinates for cannulas implantation into the dorsal hippocampus were anterocaudal: −3.2 mm, lateral: ±1.8 mm (both respect to bregma), and vertical: 7.2 mm (from dura), according to a previous study (Tusi et al. 2011). After the skull was opened, Aβ or normal saline as a control was injected into the left or right hippocampus (4 μL/side) within 8 min through the guide cannulas. The needle was left in place for another 5 min before it was slowly withdrawn.
After Aβ injection, rats were administered with baicalin (50, 100 or 200 mg/kg; i.p.) or donepezil (1 mg/kg) once a day for consecutive 21 days. The sham and model group received the same volume of normal saline. Twenty-one days after Aβ injection, rats were sacrificed. Left brain was fixed in formalin and right brain was used to separate hippocampus. Once separated, the hippocampus was immediately frozen in liquid nitrogen and stored at −80 °C.
Morris Water Maze Test
The morris water maze test was performed based on previous reports with minor adjustment (Song et al. 2013; Han et al. 2011; Qin et al. 2013). The maze consisted of a black circular pool (1.6 cm diameter and 50 cm height) was divided into four quadrants and filled with water (23 ± 1 °C) to a depth of 50 cm. A platform (10 cm in diameter) was fixed in the center of one quadrant and submerged 2 cm below the surface of the water hidden from the rat’s view. On the 14th day after Aβ injection, two training trials each day were conducted for six consecutive days. For each trial, the animals (six rats in each group) were placed in the pool at one starting location and were allowed to search for the platform for 90 s. If a rat did not find the platform after 90 s, it would be guided to the platform and was recorded as 90 s. The rats remained on the platform for 20 s before being returned to the cage. The average escape latency of each rat in the two trials was counted as the individual result of a rat per day. One day after the training period, the probe trial was performed. The platform was removed from the tank and they were allowed to swim freely for 120 s. The time spent in the target quadrant and the number of rats crossing the platform was recorded.
Histological Studies
Brain tissue samples were fixed in 10 % buffered formalin and then processed for paraffin sectioning. The paraffin-embedded brains were cut into serial sections (5 μm), each representing distinct antero-posterior levels of the hippocampus. After stained with hematoxylin and eosin, sections were evaluated under a light microscope (Olympus, Japan).
TUNEL Assay
TdT-mediated dUTP nick-end labeling (TUNEL) detection of apoptotic cells was performed in formalin-fixed, paraffin-embedded brain tissue sections according to the manufacturer’s recommended protocol (Beyotime Biotechnology, China). Sections were observed under a confocal microscopy (Olympus Fluoview-10).
Assay of Oxidative Biochemical Parameters
Hippocampus tissue samples were homogenized in RIPA lysis buffer. The mixture was centrifuged at 12,000×g for 30 min at 4 °C. The supernatant was collected and used for the experiments. The activities of total SOD, CAT, and GPx and the levels of MDA were determined using commercially available colorimetric assay kits (Beyotime Biotechnology, China).
Measurement of Mitochondrial Membrane Potential
Isolation of mitochondria from hippocampus was performed using commercially available kits (Beyotime Biotechnology, China). Rhodamine 123 (Rh-123) can enter the mitochondrial matrix and cause photoluminescent quenching that is dependent on mitochondrial membrane potential. The mitochondrial membrane potential (ΔΨm) was measured using Rh-123 in a fluorometer (Chen et al. 2013). Isolated mitochondria (1 mg/mL) was incubated with Rh-123 (5 mg/L) for 30 min at 37 °C in the dark and washed three times with PBS. Fluorescence emission intensity was measured on a microplate reader (Ex = 488 nm, Em = 510 nm) and expressed as the percentage of control group.
Western Blotting Analysis
Total proteins, mitochondrial proteins, cytosolic proteins, or nuclear proteins from hippocampus tissues were extracted using commercially available kits (Beyotime Biotechnology, China). Samples (30 μg) were separated on SDS-PAGE gel and electrophoretically transferred onto polyvinylidene fluoride (PVDF) membranes. After rinsed in Tris-buffer saline (TBST), the membranes were incubated with specific antibody (for β-actin, 1:1,000 dilution; Nrf2, 1:500 dilution; cytochrome c, cleaved caspase-9, caspase -3, Bax, Bcl-2, 1:250 dilution). After washing with TBST for three times, the membranes were incubated with appropriate secondary antibodies conjugated to horseradish peroxidase. The protein bands were visualized by ECL detection kit.
Real-Time Fluorescent Quantitative PCR and PCR Analysis
Specific primers for CAT, Mn/SOD, GPx, and β-actin were designed using Primer Premier software (PREMIER Biosoft International, USA) based on rattus norvegicus sequences (Table 1). Total RNA was isolated from hippocampus tissues using the TRIzol reagent according to the manufacturer’s instructions (Takara, Japan) and transcribed into cDNA using a reverse transcription kit (Takara, Japan). RNA concentrations were determined by an RNA/DNA calculator (Pharmacia Biotech Company, Cambridge, UK), then adjusted to the same level by 0.1 % sterile diethyl pyrocarbonate (DEPC). According to the measured concentrations, the volume of total RNA for reverse transcription was confirmed. Concentrations of cDNA in each group were then regulated. Relative expression contents of CAT, Mn/SOD, and GPx mRNA were analyzed by 2−ΔΔCt using the RT-PCR method.
Table 1.
Primers used for quantitative real-time PCR
| Target gene (rattus norvegicus) | Gen bank accession no. | Primer | Sequence(5′-3′) | PCR fragment length (bp) |
|---|---|---|---|---|
| Mn/SOD | NM | Forward | ACCGAGGAGAAGTACCACGA | 245 |
| 001098153.1 | Reverse | CCTGAACCTTGGACTCCCAC | ||
| CAT | NM | Forward | GGTAACTGGGACCTTGTGGG | 204 |
| 012520.2 | Reverse | TCCATCTGGAATCCCTCGGT | ||
| GPx | NM | Forward | ACAGTATGTCTGCTGCTCGG | 143 |
| 030826.3 | Reverse | GAGGGACGCGACATTCTCAA | ||
| β-actin | NM | Forward | GTGGATCAGCAAGCAGGAGT | 96 |
| 031144.3 | Reverse | AGGGTGTAAAACGCAGCTCA |
The computational formula was as follows:
Statistics
The data are expressed as the mean ± SEM (standard error mean). Comparison between groups was made by one-way analysis of variance (ANOVA) followed by an appropriate post hoc test to analyze the difference. The statistical significances were achieved when p < 0.05 (*or # p < 0.05, **or ## p < 0.01).
Results
Effects of Baicalin and Aβ on Learning and Memory Deficit
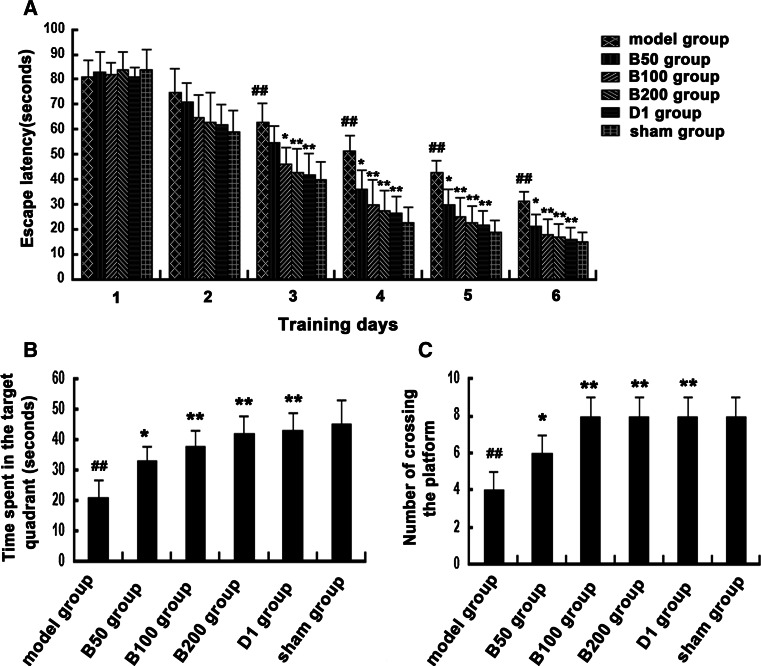
To evaluate whether baicalin could improve cognitive impairment caused by Aβ injection, morris water maze test was performed. As shown in Fig. 2a, compared to that of the sham group, the escape latency of model group was significantly longer on training days 3–6. 50 mg/kg baicalin was found to be effective in decreasing the escape latency on training days 4–6, while 100 and 200 mg/kg baicalin were more effective on training days 3–6.
Fig. 2.
Effects of baicalin and Aβ on learning and memory deficit. Rats (n = 6 in each group) were treated as described in the text. The Morris water maze test was performed. a The escape latencies during the training trial sessions. b The time spent in the target quadrant during the probe trial. c The number of rats crossing the platform during the probe trial. # p < 0.05 or ## p < 0.01 versus sham group; *p < 0.05 or **p < 0.01 versus model group
The results of the probe trial are presented in Fig. 2b, c. Rats treated with baicalin spent significantly more time in the target quadrant than the Aβ-injected rats. The number of Aβ-injected rats crossing the platform remarkably decreased compared with the normal rats, while baicalin treatment significantly improved the number of rats crossing the platform. These results suggested that baicalin had beneficial effects on learning and memory deficit of rats caused by Aβ.
Effects of Baicalin and Aβ on Hippocampus Injury
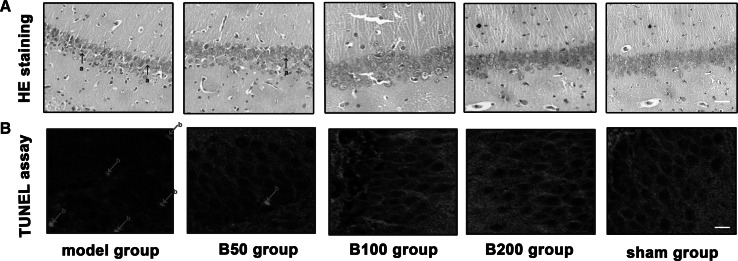
Histological examination of hippocampus injury was performed. As shown in Fig. 3a, compared to the sham group, the Aβ-injected rats displayed pathological features of nucleoli ambiguity, circumscription (between nuclei and cytoplasm), obfuscation, and cytoplasm diffusion. However, treatment with baicalin ameliorated Aβ-induced alterations and kept the pathological lesions close to normal range.
Fig. 3.
Effects of baicalin and Aβ on histological changes and apoptosis in rat hippocampus section. Rats (n = 6 in each group) were treated as described in the text. a Histological examination was performed by H&E staining (white bar stands for 40 μm), damaged neuron is marked with arrow (a). b Apoptosis examination was performed by TUNEL assay (white bar stands for 10 μm), TUNEL-positive cell is marked with arrow (b). All experiments were repeated three times and one is presented
To explore the protective effects of baicalin on Aβ-induced apoptosis, apoptosis was detected by TUNEL assay. As shown in Fig. 3b, from the result of this assay, it is evident that Aβ exposure significantly increased the number of TUNEL-positive cells. Treatment with baicalin showed an inhibitory effect on cell apoptosis.
Effects of Baicalin and Aβ on MDA Generation
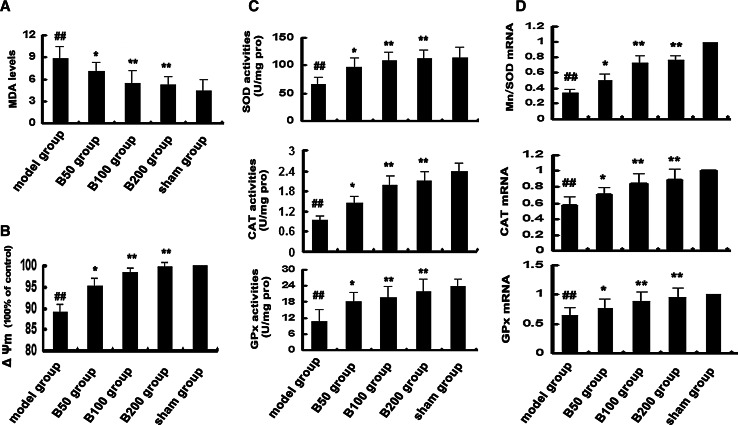
MDA is an end product of lipid peroxidation, and its level could reflect the extent of oxidative stress in an organism. As shown in Fig. 4a, compared to the sham group, rats that exposed to Aβ showed significant increase of MDA content. However, baicalin treatment effectively decreased Aβ-induced MDA generation.
Fig. 4.
Effects of baicalin and Aβ on oxidative stress in rat hippocampus. Rats (n = 6 in each group) were treated as described in the text. a The level of MDA was detected. b Mitochondrial membrane potential was measured. c The activities of total SOD, CAT, and GPx were detected. d The mRNA expressions of Mn/SOD, CAT, and GPx were detected by Q-PCR, and the ratio to β-actin was calculated, respectively. All experiments were repeated three times. # p < 0.05 or ## p < 0.01 versus sham group; *p < 0.05 or **p < 0.01 versus model group
Effects of Baicalin and Aβ on Antioxidant Enzymes
SOD, CAT, and GPx are three predominant antioxidant enzymes protecting cells against free radicals and subsequent oxidative damage. The effects of baicalin on the activities of SOD, CAT, and GPx against Aβ toxicity were analyzed (Fig. 4c). The results suggested that Aβ exposure suppressed the activities of the three antioxidant enzymes; nevertheless, baicalin treatment was found to be effective to increase their activities against Aβ toxicity.
In addition, the mRNA levels of CAT, Mn/SOD, and GPx were measured by Q-PCR to determine whether baicalin has effects on their gene expressions (Fig. 4d). Compared to the sham group, there was a significant decrease in the mRNA levels of CAT, Mn/SOD, and GPx in the model group. However, their gene expressions significantly increased in baicalin-treated groups, respectively.
Effects of Baicalin and Aβ on Mitochondrial Membrane Potential
Alteration of mitochondrial membrane potential is an important indicator of cytotoxicity, which could be induced by Aβ exposure. To study whether baicalin may attenuate cell death by stabilizing Aβ-induced mitochondrial dysfunction, the mitochondrial membrane potential (ΔΨm) was measured. As shown in Fig. 4b, Aβ exposure caused the depolarization of the mitochondrial membrane potential (89 % loss of ΔΨm compared to control), which were significantly reversed when rats were treated with baicalin.
Effects of Baicalin and Aβ on Bax/Bcl-2 Ratio
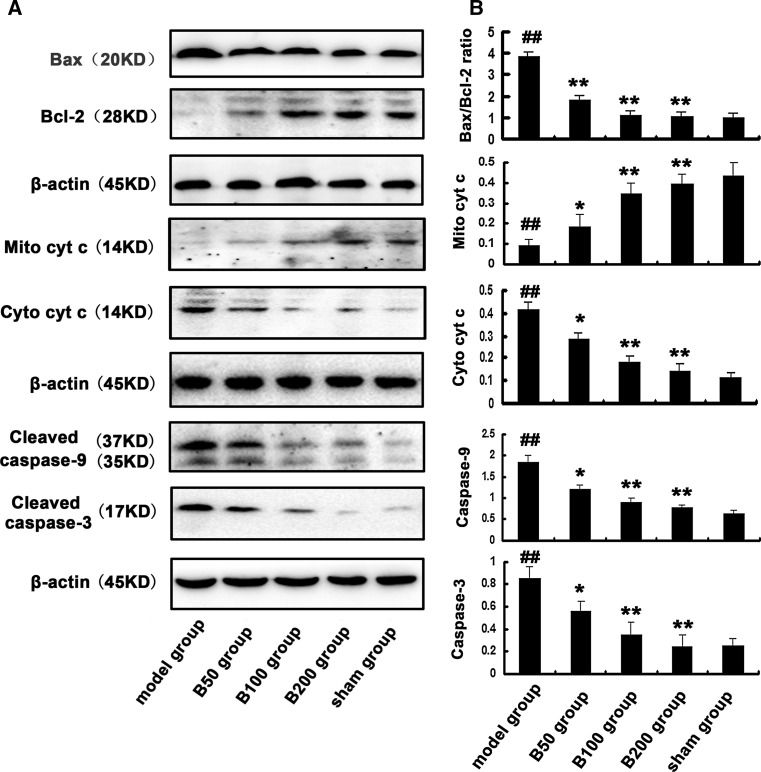
To investigate the effects of Aβ and baicalin on Bax and Bcl-2, two important regulators of mitochondria-dependent apoptotic pathway, Western blot was performed. As shown in Fig. 5, Aβ exposure significantly increased the ratio of Bax/Bcl-2 compared to the control. However, the Aβ-induced increase of Bax/Bcl-2 ratio was attenuated in rats treated with baicalin.
Fig. 5.
Effects of baicalin and Aβ on Bax/Bcl-2 ratio, cytochrome c release, and caspases activation in rat hippocampus. Rats (n = 6 in each group) were treated as described in the text. a The expression of Bax, Bcl-2, mitochondrial cytochrome c (Mito cyt c), cytosolic cytochrome c (Cyto cyt c), and cleaved caspase-9 and 3 were measured by Western blot, b and the ratio to β-actin was calculated. All the experiments were repeated three times. # p < 0.05 or ## p < 0.01 versus sham group; *p < 0.05 or **p < 0.01 versus model group
Effects of Baicalin and Aβ on Cytochrome c Release and Caspases Activation
Cytochrome c, caspase-9, and caspase-3 are known biomarkers of oxidative stress-induced cell death which is mediated via mitochondria-dependent apoptotic pathway. Their protein levels were detected by Western blot (Fig. 5). Compared to the sham group, the expression of cytosolic cytochrome c, cleaved caspase-9, and caspase-3 increased significantly in the model group. However, baicalin could inhibit the release of cytochrome c and activation of caspases induced by Aβ exposure in the baicalin treatment groups.
Effects of Baicalin and Aβ on Nuclear Levels of Nrf2
Nrf2 has been recognized as a key transcription factor against oxidative damage.
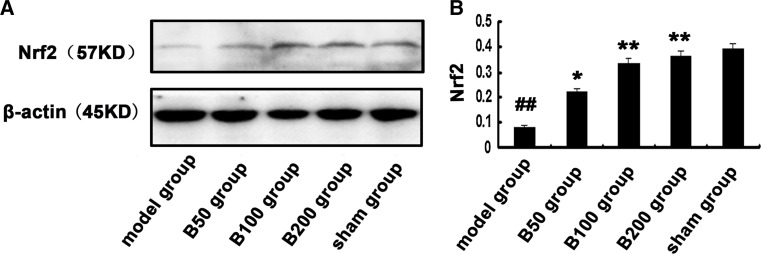
We investigated whether baicalin has effects on Nrf2. The protein level of Nrf2 in nuclear proteins was detected by Western blot. As shown in Fig. 6, Aβ exposure significantly decreased nuclear expression of Nrf2 compared to the control. However, baicalin treatment effectively suppressed the inhibitory effects of Aβ on Nrf2.
Fig. 6.
Effects of baicalin and Aβ on nuclear levels of Nrf2. Rats (n = 6 in each group) were treated as described in the text. a The nuclear levels of Nrf2 were measured by Western blot, b and the ratio to β-actin was calculated. All the experiments were repeated three times. # p < 0.05 or ## p < 0.01 versus sham group; *p < 0.05 or **p < 0.01 versus model group
Discussion
Synthetic Aβ (Aβ1–40 or Aβ1–42), which is analogous to peptides found in neuritic plaques in AD patients (Cetin and Dincer 2007), was commonly used in most injection AD models. Central administration of Aβ caused cholinergic dysfunction (Olariu et al. 2001), neuronal apoptosis (Ruan et al. 2010), oxidative stress (Bagheri et al. 2011), neuro-inflammation (Wang et al. 2012), and learning and memory deficits in rats (Cioanca et al. 2013), especially the intracranial injection of Aβ1–42 (Eftekharzadeh et al. 2012; Jhoo et al. 2004; Srivareerat et al. 2011). In this study, we assessed the therapeutic efficacy of baicalin, a natural phytochemical, in an Aβ1–42-intracranial injection rat model. We have examined the protective effect and molecular mechanism of baicalin against Aβ-induced cognitive deficit, oxidative stress, and neuron apoptosis caused by Aβ.
In the present study, after a 21-day treatment, learning and memory deficit was improved in baicalin-treated groups, as assessed by the morris water maze test. In addition, the number of damaged neuronal cells in the hippocampus was reduced in baicalin-treated groups, as indicated by histological analysis. Furthermore, the amount of apoptosis neurons in the hippocampus significantly decreased in the baicalin-treated groups, as shown by TUNEL assay. These results were consistent with the previous in vitro study (Yin et al. 2011), confirming that baicalin has protective effects against Aβ-induced cell death in vivo.
Numerous evidences indicate that oxidative stress is involved in Aβ-induced neuronal apoptosis and death (Butterfield et al. 2013). Aβ could induce overproduction of intracellular ROS, which causes peroxidation of protein and lipid in neurons, and ultimately can proceed to cell death if unchecked (Haass and Selkoe 2007). MDA is an end product of lipid peroxidation, and the level of MDA usually reflects the extent of oxidative stress and indirectly reflects the extent of injury. SOD, CAT, and GPx are important physiological antioxidants against free radicals and subsequent lipid peroxidation, which can eliminate excessive ROS (Kaur et al. 2008; Ataie et al. 2010). Previous studies have found that baicalin could reduce ROS induced by H2O2 or Aβ in cultured neuron (Zheng et al. 2014; Yin et al. 2011). However, whether baicalin could inhibit Aβ-induced oxidative stress in vivo remains unknown. The present study suggested that baicalin was effective in preventing oxidative stress triggered by Aβ. Aβ exposure induced oxidative stress, as shown by increased MDA generation. Moreover, decreased mRNA expression and activities of SOD, CAT, and GPx were observed, indicating that intracellular anti-oxidative capabilities were reduced by Aβ. However, these Aβ-induced changes were prevented when rats were treated with baicalin. These results indicated that baicalin elevated free-radical scavenging activities, which could exert a beneficial effect on oxidative stress caused by Aβ.
It is well known that oxidative stress-induced DNA damage could trigger cell apoptosis by inducing mitochondrial permeability transition pore (MPTP) (Kubli and Gustafsson 2012). MPTP is considered the “point of no return” for apoptotic cell death, which is able to switch cells to apoptotic death via oxidative stress-responsive signaling cascades (Keeble and Gilmore 2007). In addition, MPTP also can be directly induced by increased Bax/Bcl2 ratio, leading to the release of cytochrome c along with other proapoptotic proteins (Jacotot et al. 1999). Cytochrome c combines with dATP and Apaf-1, also with an inactive initiator procaspase-9, and forms an oligomeric complex called the apoptosome (Li et al. 1997). This apoptosome leads to the activation of procaspase-9, which then triggers a cascade of caspase-3 resulting in the morphologic and biochemical changes associated with apoptosis (Earnshaw et al. 1999). We detected significant increased Bax/Bcl-2 ratio, mitochondrial membrane potential decrease, cytochrome c release, and activation of caspase-9 and caspase-3 in the model group compared to the sham group, indicating that Aβ activated the mitochondrial apoptotic pathway. However, these alterations were all attenuated by baicalin treatment. These results were partly consistent with the anti-apoptotic effects of baicalin in previous in vitro study (Zheng et al. 2014), suggesting that baicalin is able to inhibit Aβ-induced activation of mitochondrial apoptotic pathway.
Nrf2 has been demonstrated to be a key transcription factor that regulates the induction of antioxidant genes (Vomhof-Dekrey and Picklo 2012). Under stress conditions, Nrf2 translocates into the nucleus where it binds DNA at a consensus sequence and elicits the antioxidant response by the induction of a battery of gene products, including antioxidant and phase II detoxification enzymes (Kensler et al. 2007; Li and Kong 2009). Activation of Nrf2 was found to protect cells against Aβ toxicity (Karkkainen et al. 2014; Hur et al. 2013). In this study, Aβ significantly inhibited Nrf2 translocation, as revealed by the decrease in the nuclear Nrf2 level, whereas treatment with baicalin strongly suppressed the inhibitory effects of Aβ on Nrf2. It seems that baicalin protects against oxidative stress via induction of the Nrf2 pathway.
Considering the involvement of Aβ-induced oxidative stress in the etiology and pathology of AD, antioxidant therapy that scavenges excessive ROS through the induction of endogenous antioxidant enzymes is thought to be one of the promising approaches to preventive interventions for AD (Park 2010). Many studies highlighted natural phytochemicals derived from medicinal herbs and foods as potential candidates which can protect neurons against various toxic compounds and exert beneficial effects on neuron. The present investigation provides evidences supporting the protective effects of baicalin against Aβ toxicity in vivo. Baicalin effectively improved Aβ-induced learning and memory deficit, hippocampus injury, and neuron apoptosis. Our study provides evidence supporting the clinical application of baicalin in treatment to Alzheimer’s disease.
Acknowledgments
Conflict of interest
The authors declare that there are no conflicts of interest.
Footnotes
Ding Haitao and Wang Haitao have contributed equally to the study.
References
- Ataie A, Sabetkasaei M, Haghparast A, Moghaddam AH, Kazeminejad B (2010) Neuroprotective effects of the polyphenolic antioxidant agent, Curcumin, against homocysteine-induced cognitive impairment and oxidative stress in the rat. Pharmacol Biochem Behav 96:378–385 [DOI] [PubMed] [Google Scholar]
- Bagheri M, Joghataei MT, Mohseni S, Roghani M (2011) Genistein ameliorates learning and memory deficits in amyloid β(1-40) rat model of Alzheimer’s disease. Neurobiol Learn Mem 95:270–276 [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Swomley AM, Sultana R (2013) Amyloid β-peptide (1-42)-induced oxidative stress in Alzheimer disease: importance in disease pathogenesis and progression. Antioxid Redox Signal 19:823–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Li G, Wang YF, Fan ZK, Yu DS, Wang ZD, Bi YL (2010) Neuroprotective effect of baicalin on compression spinal cord injury in rats. Brain Res 1357:115–123 [DOI] [PubMed] [Google Scholar]
- Cao Y, Mao X, Sun C, Zheng P, Gao J, Wang X, Min D, Sun H, Xie N, Cai J (2011) Baicalin attenuates global cerebral ischemia/reperfusion injury in gerbils via anti-oxidative and anti-apoptotic pathways. Brain Res Bull 85:396–402 [DOI] [PubMed] [Google Scholar]
- Cappai R, Barnham KJ (2008) Delineating the mechanism of Alzheimer’s disease Aβ peptide neurotoxicity. Neurochem Res 33:526–532 [DOI] [PubMed] [Google Scholar]
- Cetin F, Dincer S (2007) The effect of intrahippocampal β amyloid (1-42) peptide injection on oxidant and antioxidant status in rat brain. Ann NY Acad Sci 1100:510–517 [DOI] [PubMed] [Google Scholar]
- Chen DL, Zhang P, Lin L, Shuai O, Zhang HM, Liu SH, Wang JY (2013) Protective effect of Bajijiasu against β-amyloid-induced neurotoxicity in pc12 cells. Cell Mol Neurobiol 33:837–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng F, Lu Y, Zhong X, Song W, Wang X, Sun X, Qin J, Guo S, Wang Q (2013) Baicalin’s therapeutic time window of neuroprotection during transient focal cerebral ischemia and its antioxidative effects in vitro and in vivo. Evid Based Complement Alternat Med 2013:120261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cioanca O, Hritcu L, Mihasan M, Hancianu M (2013) Cognitive-enhancing and antioxidant activities of inhaled coriander volatile oil in amyloid β(1-42) rat model of Alzheimer’s disease. Physiol Behav 120:193–202 [DOI] [PubMed] [Google Scholar]
- Earnshaw WC, Martins LM, Kaufmann SH (1999) Mammalian caspases: structure, activation, substrates, and functions during apoptosis. Annu Rev Biochem 68:383–424 [DOI] [PubMed] [Google Scholar]
- Eftekharzadeh B, Ramin M, Khodagholi F, Moradi S, Tabrizian K, Sharif R, Azami K, Beyer C, Sharifzadeh M (2012) Inhibition of PKA attenuates memory deficits induced by β-amyloid (1-42), and decreases oxidative stress and NF-κB transcription factors. Behav Brain Res 226:301–308 [DOI] [PubMed] [Google Scholar]
- Haass C, Selkoe DJ (2007) Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid β-peptide. Nat Rev Mol Cell Biol 8:101–112 [DOI] [PubMed] [Google Scholar]
- Han M, Liu Y, Tan Q, Zhang B, Wang W, Liu J, Zhang XJ, Wang YY, Zhang JM (2011) Therapeutic efficacy of stemazole in a beta-amyloid injection rat model of Alzheimer’s disease. Eur J Pharmacol 657:104–110 [DOI] [PubMed] [Google Scholar]
- Hawkes CA, Ng V, Mclaurin J (2009) Small molecule inhibitors of Aβ-Aggregation and neurotoxicity. Drug Dev Res 70:111–124 [Google Scholar]
- Hur J, Pak SC, Koo BS, Jeon S (2013) Borneol alleviates oxidative stress via upregulation of Nrf2 and Bcl-2 in SH-SY5Y cells. Pharm Biol 51:30–35 [DOI] [PubMed] [Google Scholar]
- Jacotot E, Costantini P, Laboureau E, Zamzami N, Susin SA, Kroemer G (1999) Mitochondrial membrane permeabilization during the apoptotic process. Ann NY Acad Sci 887:18–30 [DOI] [PubMed] [Google Scholar]
- Jhoo JH, Kim HC, Nabeshima T, Yamada K, Shin EJ, Jhoo WK, Kim W, Kang KS, Jo SA, Woo JI (2004) β-amyloid (1-42)-induced learning and memory deficits in mice: involvement of oxidative burdens in the hippocampus and cerebral cortex. Behav Brain Res 155:185–196 [DOI] [PubMed] [Google Scholar]
- Karkkainen V, Pomeshchik Y, Savchenko E, Dhungana H, Kurronen A, Lehtonen S, Naumenko N, Tavi P, Levonen AL, Yamamoto M, Malm T, Magga J, Kanninen KM, Koistinaho J (2014) Nrf2 regulates neurogenesis and protects neural progenitor cells against Aβ toxicity. Stem Cells [DOI] [PubMed]
- Kaur R, Arora S, Singh B (2008) Antioxidant activity of the phenol rich fractions of leaves of Chukrasia tabularis A. Juss. Bioresour Technol 99:7692–7698 [DOI] [PubMed] [Google Scholar]
- Keeble JA, Gilmore AP (2007) Apoptosis commitment—translating survival signals into decisions on mitochondria. Cell Res 17:976–984 [DOI] [PubMed] [Google Scholar]
- Kensler TW, Wakabayashi N, Biswal S (2007) Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol 47:89–116 [DOI] [PubMed] [Google Scholar]
- Kubli DA, Gustafsson AB (2012) Mitochondria and mitophagy: the yin and yang of cell death control. Circ Res 111:1208–1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauren J, Gimbel DA, Nygaard HB, Gilbert JW, Strittmatter SM (2009) Cellular prion protein mediates impairment of synaptic plasticity by amyloid-beta oligomers. Nature 457:1128–1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Fernandez EJ, Good TA (2007) Role of aggregation conditions in structure, stability, and toxicity of intermediates in the Aβ fibril formation pathway. Protein Sci 16:723–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B, Sur B, Shim I, Lee H, Hahm DH (2014) Baicalin improves chronic corticosterone-induced learning and memory deficits via the enhancement of impaired hippocampal brain-derived neurotrophic factor and cAMP response element-binding protein expression in the rat. J Nat Med 68:132–143 [DOI] [PubMed] [Google Scholar]
- Li W, Kong AN (2009) Molecular mechanisms of Nrf2-mediated antioxidant response. Mol Carcinog 48:91–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X (1997) Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 91:479–489 [DOI] [PubMed] [Google Scholar]
- Liu YF, Gao F, Li XW, Jia RH, Meng XD, Zhao R, Jing YY, Wang Y, Jiang W (2012) The anticonvulsant and neuroprotective effects of baicalin on pilocarpine-induced epileptic model in rats. Neurochem Res 37:1670–1680 [DOI] [PubMed] [Google Scholar]
- Mebane-Sims I (2009) 2009 Alzheimer’s disease facts and figures. Alzheimer’s Dement 5:234–270 [DOI] [PubMed] [Google Scholar]
- Olariu A, Tran MH, Yamada K, Mizuno M, Hefco V, Nabeshima T (2001) Memory deficits and increased emotionality induced by β-amyloid(25-35) are correlated with the reduced acetylcholine release and altered phorbol dibutyrate binding in the hippocampus. J Neural Transm 108:1065–1079 [DOI] [PubMed] [Google Scholar]
- Park SY (2010) Potential therapeutic agents against Alzheimer’s disease from natural sources. Arch Pharm Res 33:1589–1609 [DOI] [PubMed] [Google Scholar]
- Qin L, Zhang J, Qin M (2013) Protective effect of cyanidin 3-O-glucoside on β-amyloid peptide-induced cognitive impairment in rats. Neurosci Lett 534:285–288 [DOI] [PubMed] [Google Scholar]
- Ruan CJ, Zhang L, Chen DH, Li Z, Du GH, Sun L (2010) Effects of trans-2,4-dimethoxystibene against the neurotoxicity induced by Aβ(25-35) both in vitro and in vivo. Neurosci Res 67:209–214 [DOI] [PubMed] [Google Scholar]
- Sabbagh MN (2009) Drug development for Alzheimer’s disease: where are we now and where are we headed? Am J Geriatr Pharmacother 7:167–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe DJ (1991) Amyloid protein and Alzheimer’s disease. Sci Am 265:68–71, 74–66, 78 [DOI] [PubMed]
- Seo JS, Kim TK, Leem YH, Lee KW, Park SK, Baek IS, Kim KS, Im GJ, Lee SM, Park YH, Han PL (2009) SK-PC-B70M confers anti-oxidant activity and reduces Aβ levels in the brain of Tg2576 mice. Brain Res 1261:100–108 [DOI] [PubMed] [Google Scholar]
- Song Y, Chen X, Wang LY, Gao W, Zhu MJ (2013) Rho kinase inhibitor fasudil protects against beta-amyloid-induced hippocampal neurodegeneration in rats. CNS Neurosci Ther 19:603–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivareerat M, Tran TT, Salim S, Aleisa AM, Alkadhi KA (2011) Chronic nicotine restores normal Aβ levels and prevents short-term memory and E-LTP impairment in Aβ rat model of Alzheimer’s disease. Neurobiol Aging 32:834–844 [DOI] [PubMed] [Google Scholar]
- Tarrago T, Kichik N, Claasen B, Prades R, Teixido M, Giralt E (2008) Baicalin, a prodrug able to reach the CNS, is a prolyl oligopeptidase inhibitor. Bioorg Med Chem 16:7516–7524 [DOI] [PubMed] [Google Scholar]
- Tusi SK, Khalaj L, Ashabi G, Kiaei M, Khodagholi F (2011) Alginate oligosaccharide protects against endoplasmic reticulum- and mitochondrial-mediated apoptotic cell death and oxidative stress. Biomaterials 32:5438–5458 [DOI] [PubMed] [Google Scholar]
- Vomhof-Dekrey EE, Picklo MJ Sr (2012) The Nrf2-antioxidant response element pathway: a target for regulating energy metabolism. J Nutr Biochem 23:1201–1206 [DOI] [PubMed] [Google Scholar]
- Wang C, Yang XM, Zhuo YY, Zhou H, Lin HB, Cheng YF, Xu JP, Zhang HT (2012) The phosphodiesterase-4 inhibitor rolipram reverses Aβ-induced cognitive impairment and neuroinflammatory and apoptotic responses in rats. Int J Neuropsychopharmacol 15:749–766 [DOI] [PubMed] [Google Scholar]
- Xue X, Qu XJ, Yang Y, Sheng XH, Cheng F, Jiang EN, Wang JH, Bu W, Liu ZP (2010) Baicalin attenuates focal cerebral ischemic reperfusion injury through inhibition of nuclear factor kappaB p65 activation. Biochem Biophys Res Commun 403:398–404 [DOI] [PubMed] [Google Scholar]
- Yankner BA (1989) Amyloid and Alzheimer’s disease—cause or effect? Neurobiol Aging 10:470–471 discussion 477–478 [DOI] [PubMed] [Google Scholar]
- Yankner BA, Dawes LR, Fisher S, Villa-Komaroff L, Oster-Granite ML, Neve RL (1989) Neurotoxicity of a fragment of the amyloid precursor associated with Alzheimer’s disease. Science 245:417–420 [DOI] [PubMed] [Google Scholar]
- Yin F, Liu J, Ji X, Wang Y, Zidichouski J, Zhang J (2011) Baicalin prevents the production of hydrogen peroxide and oxidative stress induced by Aβ aggregation in SH-SY5Y cells. Neurosci Lett 492:76–79 [DOI] [PubMed] [Google Scholar]
- Zheng WX, Wang F, Cao XL, Pan HY, Liu XY, Hu XM, Sun YY (2014) Baicalin protects PC-12 cells from oxidative stress induced by hydrogen peroxide via anti-apoptotic effects. Brain Inj 28:227–234 [DOI] [PubMed] [Google Scholar]
- Zhou QB, Duan CZ, Jia Q, Liu P, Li LY (2014) Baicalin attenuates focal cerebral ischemic reperfusion injury by inhibition of protease-activated receptor-1 and apoptosis. Chin J Integr Med 20:116–122 [DOI] [PubMed] [Google Scholar]