Abstract
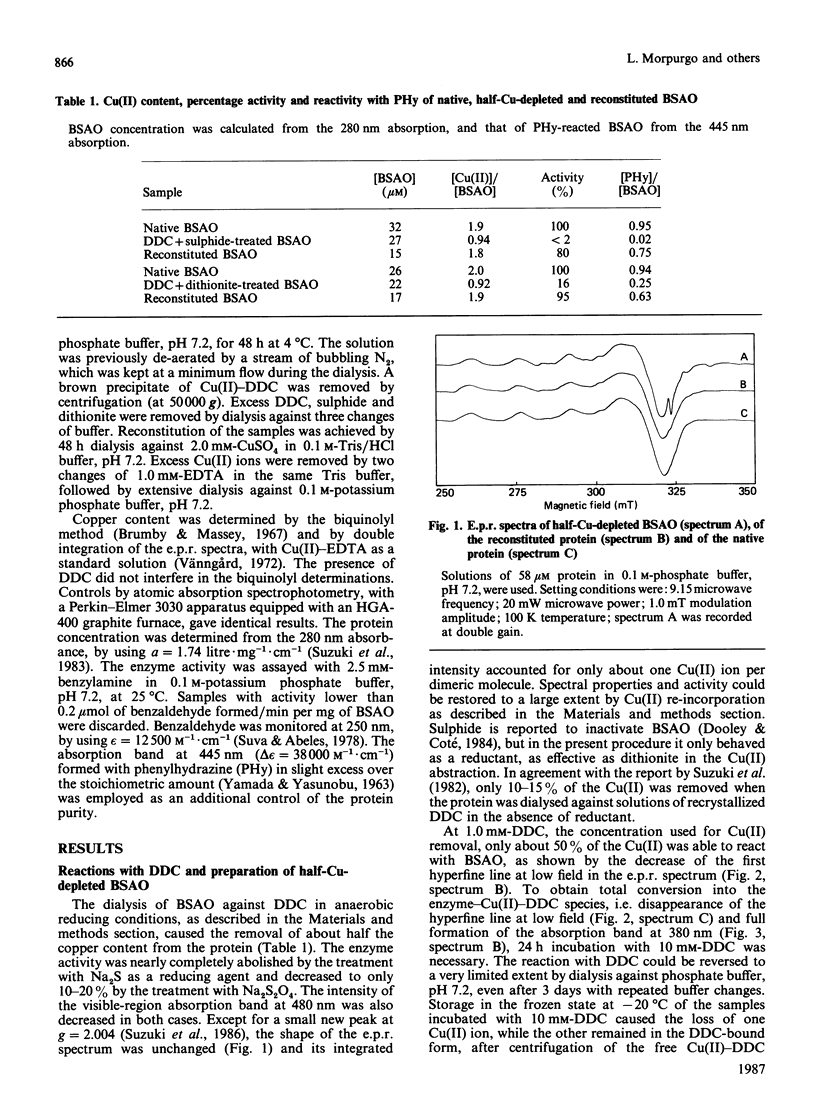
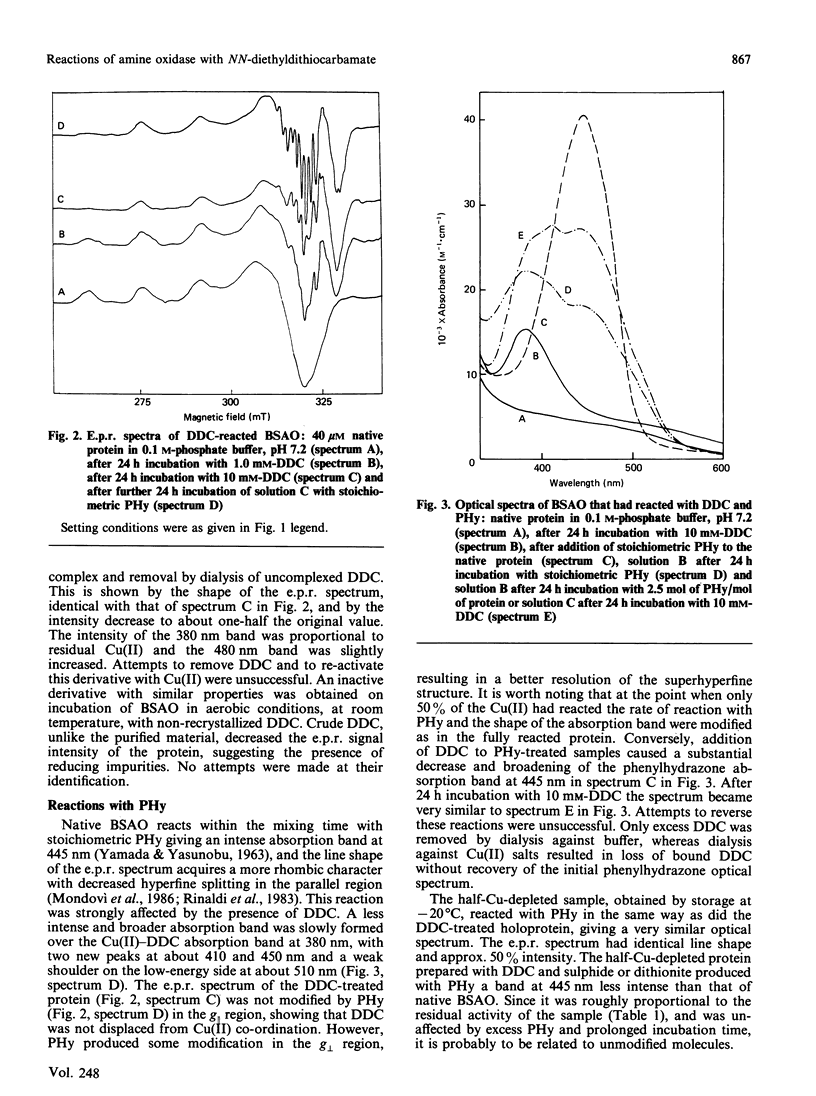
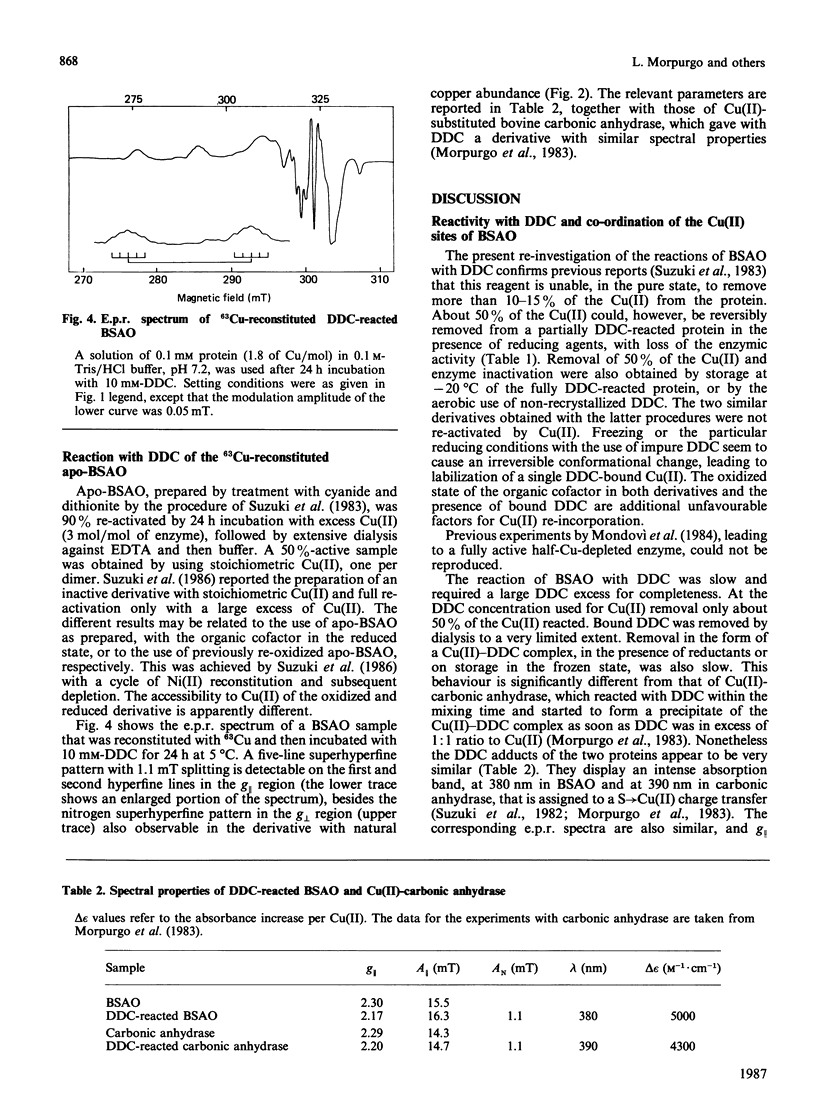
NN-Diethyldithiocarbamate (DDC) was able to bind, at 1.0 mM concentration, only about 50% the Cu(II) ions of bovine plasma amine oxidase. Under reducing conditions, this Cu(II) was removed with inactivation of the enzyme. Up to 90% activity could be recovered by treatment with excess Cu(II). The organic cofactor, sensitive to carbonyl reagents, was reduced in the half-Cu-depleted protein and no longer bound phenylhydrazine. The fully reacted protein, in the presence of 10 mM-DDC, lost 50% Cu(II) upon storage at -20 degrees C, but in this case the residual Cu(II) was in the DDC-bound form and the cofactor was in the oxidized state, as it could still bind phenylhydrazine. In the presence of DDC, the rate of reaction with phenylhydrazine was always low, even at 50% DDC saturation, and all derivatives showed identical modifications of the optical and e.p.r. spectra with respect to the phenylhydrazone of the native protein. It is concluded that the two Cu(II) ions are not equivalent, that removal of a single Cu(II) is sufficient to inhibit the re-oxidation of the organic cofactor, and that both Cu(II) ions are in some way involved in the reaction with phenylhydrazine. After reaction with DDC, the optical and e.p.r. spectra of 63Cu(II)-amine oxidase and of 63Cu(II)-carbonic anhydrase [Morpurgo, Desideri, Rigo, Viglino & Rotilio (1983) Biochim. Biophys. Acta 746, 168-175] are very similar and show distorted equatorial co-ordination to Cu(II) of two sulphur atoms and two magnetically equivalent nitrogen atoms.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker G. J., Knowles P. F., Pandeya K. B., Rayner J. B. Electron nuclear double-resonance (ENDOR) spectroscopy of amine oxidase from pig plasma. Biochem J. 1986 Jul 15;237(2):609–612. doi: 10.1042/bj2370609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker R., Boden N., Cayley G., Charlton S. C., Henson R., Holmes M. C., Kelly I. D., Knowles P. F. Properties of cupric ions in benzylamine oxidase from pig plasma as studied by magnetic-resonance and kinetic methods. Biochem J. 1979 Jan 1;177(1):289–302. doi: 10.1042/bj1770289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooley D. M., Coté C. E. Inactivation of beef plasma amine oxidase by sulfide. J Biol Chem. 1984 Mar 10;259(5):2923–2926. [PubMed] [Google Scholar]
- Knowles P. F., Pandeya K. B., Rius F. X., Spencer C. M., Moog R. S., McGuirl M. A., Dooley D. M. The organic cofactor in plasma amine oxidase: evidence for pyrroloquinoline quinone and against pyridoxal phosphate. Biochem J. 1987 Jan 15;241(2):603–608. doi: 10.1042/bj2410603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobenstein-Verbeek C. L., Jongejan J. A., Frank J., Duine J. A. Bovine serum amine oxidase: a mammalian enzyme having covalently bound PQQ as prosthetic group. FEBS Lett. 1984 May 21;170(2):305–309. doi: 10.1016/0014-5793(84)81333-2. [DOI] [PubMed] [Google Scholar]
- Moog R. S., McGuirl M. A., Cote C. E., Dooley D. M. Evidence for methoxatin (pyrroloquinolinequinone) as the cofactor in bovine plasma amine oxidase from resonance Raman spectroscopy. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8435–8439. doi: 10.1073/pnas.83.22.8435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morpurgo L., Desideri A., Rigo A., Viglino P., Rotilio G. Reaction of N,N-diethyldithiocarbamate and other bidentate ligands with Zn, Co and Cu bovine carbonic anhydrases. Inhibition of the enzyme activity and evidence for stable ternary enzyme-metal-ligand complexes. Biochim Biophys Acta. 1983 Aug 16;746(3):168–175. doi: 10.1016/0167-4838(83)90071-7. [DOI] [PubMed] [Google Scholar]
- Rinaldi A., Floris G., Sabatini S., Finazzi-Agrò A., Giartosio A., Rotilio G., Mondovì B. Reaction of beef plasma and lentil seedlings Cu-amine oxidases with phenylhydrazine. Biochem Biophys Res Commun. 1983 Sep 30;115(3):841–848. doi: 10.1016/s0006-291x(83)80011-4. [DOI] [PubMed] [Google Scholar]
- Suva R. H., Abeles R. H. Studies on the mechanism of action of plasma amine oxidase. Biochemistry. 1978 Aug 22;17(17):3538–3545. doi: 10.1021/bi00610a018. [DOI] [PubMed] [Google Scholar]
- Suzuki S., Sakurai T., Nakahara A., Manabe T., Okuyama T. Effect of metal substitution on the chromophore of bovine serum amine oxidase. Biochemistry. 1983 Mar 29;22(7):1630–1635. doi: 10.1021/bi00276a016. [DOI] [PubMed] [Google Scholar]
- Suzuki S., Sakurai T., Nakahara A. Roles of the two copper ions in bovine serum amine oxidase. Biochemistry. 1986 Jan 28;25(2):339–341. doi: 10.1021/bi00350a009. [DOI] [PubMed] [Google Scholar]
- Turini P., Sabatini S., Befani O., Chimenti F., Casanova C., Riccio P. L., Mondovì B. Purification of bovine plasma amine oxidase. Anal Biochem. 1982 Sep 15;125(2):294–298. doi: 10.1016/0003-2697(82)90009-4. [DOI] [PubMed] [Google Scholar]
- Williams T. J., Falk M. C. Spatial relationship between the copper and carbonyl cofactors in the active site of pig plasma amine oxidase. J Biol Chem. 1986 Dec 5;261(34):15949–15954. [PubMed] [Google Scholar]
- YAMADA H., YASUNOBU K. T. MONOAMINE OXIDASE. IV. NATURE OF THE SECOND PROSTHETIC GROUP OF PLASMA MONOAMINE OXIDASE. J Biol Chem. 1963 Aug;238:2669–2675. [PubMed] [Google Scholar]



