Abstract
The epidemic and experimental studies have confirmed that the obesity induced by high-fat diet not only caused neuronal insulin resistance, but also induced brain mitochondrial dysfunction as well as learning impairment in mice. Naringin has been reported to posses biological functions which are beneficial to human cognitions, but its protective effects on HFD-induced cognitive deficits and underlying mechanisms have not been well characterized. In the present study Male C57BL/6 J mice were fed either a control or high-fat diet for 20 weeks and then randomized into four groups treated with their respective diets including control diet, control diet + naringin, high-fat diet (HFD), and high-fat diet + naringin (HFDN). The behavioral performance was assessed by using novel object recognition test and Morris water maze test. Hippocampal mitochondrial parameters were analyzed. Then the protein levels of insulin signaling pathway and the AMP-activated protein kinase (AMPK) in the hippocampus were detected by Western blot method. Our results showed that oral administration of naringin significantly improved the learning and memory abilities as evidenced by increasing recognition index by 52.5 % in the novel object recognition test and inducing a 1.05-fold increase in the crossing-target number in the probe test, and ameliorated mitochondrial dysfunction in mice caused by HFD consumption. Moreover, naringin significantly enhanced insulin signaling pathway as indicated by a 34.5 % increase in the expression levels of IRS-1, a 47.8 % decrease in the p-IRS-1, a 1.43-fold increase in the p-Akt, and a 1.89-fold increase in the p-GSK-3β in the hippocampus of the HFDN mice versus HFD mice. Furthermore, the AMPK activity significantly increased in the naringin-treated (100 mg kg−1 d−1) group. These findings suggest that an enhancement in insulin signaling and a decrease in mitochondrial dysfunction through the activation of AMPK may be one of the mechanisms that naringin improves cognitive functions in HFD-induced obese mice.
Electronic supplementary material
The online version of this article (doi:10.1007/s10571-015-0201-y) contains supplementary material, which is available to authorized users.
Keywords: Naringin, High-fat diet, Cognitive deficits, Insulin signaling, Mitochondrial dysfunction, AMPK
Introduction
Obesity is often caused by and is correlated with consumption of diets that are high in fat. Recently, accumulating evidence from epidemiological (Francis and Stevenson 2013; Nilsson and Nilsson 2009; Sellbom and Gunstad 2012) and experimental studies (Kanoski and Davidson 2011) has demonstrated that diets rich in fat can induce brain dysfunction and deficits in memory. It has been shown that long-term consumption of a high-fat diet (HFD) in animal models caused neuronal insulin resistance (Arnold et al. 2014; Clegg et al. 2011; Pratchayasakul et al. 2011), indicated by the impairment of neuronal insulin receptor function, which is correlated with cognitive decline (Craft 2005; Greenwood and Winocur 2005; Moloney et al. 2010; Schubert et al. 2004).
In the central nervous system, brain mitochondria play an important role in energy-demanding neurotransmission and in controlling calcium homeostasis, which are important mechanisms for the learning and memory process. Disruption of electron transport chains can lead to decreased ATP with increased reactive oxygen species (ROS) production (Johannsen and Ravussin 2009; Rabol et al. 2006). Previous studies also have demonstrated the correlation between brain mitochondrial dysfunction and neurodegenerative disease (Reddy et al. 2012). Insulin resistance has been shown to deregulate both glucose and lipid metabolisms and decrease the activity of mitochondrial oxidative phosphorylation (Mohlig et al. 2004; Taddeo et al. 2014; Warren et al. 2014). Recent studies indicated that the consumption of a HFD leads to neuronal insulin resistance, also increases brain ROS production and disruptes the mitochondrial membrane potential (MMP) (Pintana et al. 2012; Pipatpiboon et al. 2013, 2012). Those findings suggest that IR dysfunction may correlate with the dysfunction of mitochondria.
Naringin, a citrus flavonoid, possesses antioxidant, anti-inflammatory, anti-apoptosis (Kim et al. 2009), and anti-carcinogenic effects (So et al. 1996). Naringin has been shown to have protective effects on mitochondria (Gaur et al. 2009; Huang et al. 2013; Rajadurai and Prince 2007; Ramesh and Alshatwi 2013) and slow down the progression of degenerative diseases. It has been reported that naringin administration attenuates mitochondrial dysfunction and improves behavioral alterations and cognitive impairments in 3-nitropropionic acid-induced Huntington models (Kumar and Kumar 2010), aluminum (Prakash et al. 2013) and d-galactose (Kumar et al. 2010) induced learning and memory impairment models. Our recent studies reported that naringin might attenuate brain energy metabolism disturbances and improve cognitive deficits in amyloid-induced Alzheimer’s disease mouse model (Wang et al. 2012). Recently, naringin has been shown to enhance insulin signaling and ameliorate metabolic syndrome in mice fed with a high-fat diet (Pu et al. 2012). In this current study, we investigated the effect of long-term naringin consumption on the improvement of memory loss and explored the underlying mechanisms in the HFD-induced obese mice. Based on these considerations, the enhancement of insulin signaling and the improvement of mitochondrial dysfunction in the mouse brain may be involved in the mechanisms by which naringin affects HFD-induced cognitive damage.
Materials and Methods
Animals
C57BL/6 mice were housed individually in plastic rodent cages and maintained on a 12 h light/dark cycle with ad libitum access to conventional standard rodent chow and water, with the constant temperature (23 ± 1 °C) and relative humidity (65 %). Protocols were conducted according to the University Policies on the Use and Care of Animals and were approved by the Institutional Animal Experiment Committee of Henan University of Science and Technology, China.
Group and Treatment
Sixty 4-week-old male mice were randomized into four groups and fed for 20 weeks with either control diet (CD) or high-fat diet (HFD) chow (Dyets Inc., Bethlehem, PA, USA). Mice were dosed with 100 mg/kg of naringin daily in either CD or HFD chow. The dose of naringin was selected based on other experimental studies (Pu et al. 2012; Wang et al. 2012, 2013). Mice body weight and food intake were weekly measured. Following behavioral assessment, animals were deeply anesthetized with isoflurane and sacrificed by decapitation after fasting for at least 5 h. Their plasma was collected for further analysis.
Behavioral Tests
Novel Object Recognition Test
The test procedure consisted of three sessions: habituation, training, and retention. Each mouse was habituated to the box (30 × 30 × 35 cm), with 10 min of exploration in the absence of objects for 3 days (habituation session). During the training session, two objects were placed at the back corner of the box. A mouse was then placed in the box and the total time spent exploring the two objects (blue wooden cubes of side 3 cm) was recorded for 10 min. During the retention session, the mice were placed back in the same box 24 h after the training session, in which one of the familiar objects used during the training was replaced with a novel object (a yellow wooden cylinder of diameter 3 cm and height 3 cm). The animals were then allowed to explore freely for 5 min, the exploration time for the familiar (T F) or the new object (T N) during the test phase was recorded. The exploration time for the familiar (T F) or the new object (T N) during the test phase was videotaped and analyzed using the Noldus Ethovision XT software (Noldus Information Technology, Wageningen, The Netherlands). Memory was defined by the recognition index (RI) for the novel object as the following formula: RI = T N/(T N + T F) × 100 %. To control for odor cues, the OF arena and the objects were thoroughly cleaned with 10 % odorless soap, dried, and ventilated for a few minutes between mice (Bevins and Besheer 2006; Takamura et al. 2011).
Morris Water Maze
Spatial learning and memory was tested using the Morris water maze, performed 1 day after the end of novel object recognition test. The protocol for the Morris water maze test was modified from previously reported methods (Laczo et al. 2009; Liang et al. 1994). Briefly, the apparatus included a pool with a diameter of 100 cm that was filled with opaque water at approximately 22 ± 1 °C. An escape platform (15 cm in diameter) was placed 0.5 cm below the water surface. Geometric objects with contrasting colors were set at the remote ends of the water tank as references. Room temperature was constant, and the lighting was even throughout the room. Spatial memory is assessed by recording the latency time for the animal to escape from the water onto a submerged escape platform during the learning phase. The mice were subjected to four trials per day for 5 consecutive days. The mice were allowed to stay on the platform for 15 s before and after each trial. The time that it took for an animal to reach the platform (latency period) was recorded. Twenty-four hours after the learning phase, the mice were underwent the probe test and swam freely in the water tank without the platform for 60 s, and during the probe test, the time and the crossing number of the original platform were recorded and used to assess the spacial memory ability of mice. Monitoring was performed with a video tracking system (Noldus Ltd, Ethovision XT, Holland).
Body Weight and Biochemical Analysis
Body weight was measured every week. After 20 weeks on a high-fat diet, a glucose tolerance test was done by intra-peritoneal glucose injection (2 g/kg) following a 6 h food withdrawal. Immediately before and at 15, 30, 60, 90, and 120 min after glucose administration, blood glucose levels were measured with an Ascensia Elite glucose meter (Bayer Corporation, Mishawaka, IN, USA). For glucose/insulin measurements, blood samples were taken by tail vein puncture as described (Miller et al. 2005). For insulin measurements, whole blood was centrifuged at 16,000×g for 7 min to pellet blood cells. The plasma was transferred to a fresh tube and placed on dry ice, after which it was stored at −70 °C. Serum insulin levels were measured with the appropriate enzyme-linked immunosorbent assay kits (ALPCO Diagnostics, Windham, NH, USA). Plasma levels of non-esterified fatty acids were measured using a non-esterified fatty acids assay C kit (Wako Chemicals, Richmond, VA, USA) according to the manufacturer’s instructions. Total cholesterol was measured directly in whole blood using a Cardiochek meter (PTS, Indianapolis, IL, USA).
Preparation of Isolated Mitochondria
After behavioural tests, the mice were euthanatized with isoflurane and brain mitochondria were isolated, performed using a standard procedure (Dragicevic et al. 2010). First, brains were quickly removed and placed on ice. The hippocampus of mice (n = 6) was carefully dissected following anatomical guidelines and placed in a glass Dounce homogenizer containing five times the volume of isolation buffer (215 mM mannitol, 75 mM sucrose, 0.1 % BSA, 1 mM EGTA, 20 mM HEPES (Na+), pH 7.2). Following homogenisation, a low-speed spin (1300 g for 5 min) to remove unbroken cells and nuclei was performed. The supernatant was carefully placed in fresh tubes, topped off with isolation buffer and spun down again at 13,000×g for 10 min. The supernatant was discarded and the resultant mitochondrial pellets were suspended in 500 μl of isolation buffer with 1 mM EGTA (215 mM mannitol, 75 mM sucrose, 0.1 % BSA, 20 mM HEPES (Na+), pH 7.2) and 0.1 % digitonin (in DMSO) was added to the pellets to disrupt the synaptosomes. After 5 min, samples were brought to a final volume of 2 ml using isolation buffer containing 1 mM EGTA and centrifuged at 13,000×g for 15 min. Next the pellets were resuspended in isolation buffer without EGTA (75 mM sucrose, 215 mM mannitol, 0.1 % BSA, and 20 mM HEPES with the pH adjusted to 7.2 using KOH) and was centrifuged at 10,000×g for 10 min. The final mitochondrial pellet was suspended in isolation buffer without EGTA to yield a final protein concentration of approximately 10 mg/ml and immediately stored on ice. To normalize the results, the protein concentrations were determined with all the samples on the same microwell plate using a BCA protein assay kit. We performed the experiment twice (n = 2) with 6 control diet mice, 6 naringin-treated control diet mice, 6 naringin-treated HFD mice, and 6 control HFD mice.
Membrane Potential Measurements on Isolated Mitochondria
A 200 μM stock solution of JC-1 (5,5,6,6-tetrachloro-1,1,3,3-tetraethylbenzimidazolylcarbocyanine iodide) was made using DMSO as the solvent. The assay buffer contained mitochondrial isolation buffer with the addition of 5 mM pyruvate and 5 mM malate. One hundred fifty microliter of assay buffer and 20 μl (1.2 mg/ml final concentration) of mitochondria were added to the wells of a 96-well black, clear bottom microplate (Corning) followed by the addition of 1 μM JC-1 and mixed gently. The microplate was covered with aluminum foil and left at room temperature for 20 min before reading. Fluorescence (excitation 530/25 nm, emission 590/35 nm) was then measured.
Reactive oxygen Species Production from Isolated Mitochondria
Mitochondrial ROS production was measured following incubation of isolated mitochondria with 25 μM 2,7-dichlorodihydrofluorescein diacetate for 20 min and then the DCF fluorescence (excitation filter 485/20 nm, emission filter 528/20 nm) was read as previously described (Brown et al. 2004). In short, 100 μg (0.8 mg/ml final concentration) of isolated mitochondria were added to 120 μl of KCl-based respiration buffer with 5 mM pyruvate and 2.5 mM malate added as respiratory substrates and 25 μM 2,7-dichlorodihydrofluorescein diacetate. Mitochondrial ROS production in the presence of oligomycin (to increase ROS production) or FCCP (to decrease ROS production) were performed to ensure measurement values were within the range of the indicator.
ATP Level Determination in Isolated Mitochondria
ATP was determined luminometrically using ATP bioluminescence assay kit (Sigma, St. Louis, MO, USA) according to the provided protocol. Mitochondrial samples were assayed for ATP content using the ATP dependence of the light emitting luciferase-catalyzed oxidation of luciferin. ATP concentration was calculated according to a standard curve and related to protein content.
Western Blot Analysis
Following behavioral assessment, animals were deeply anesthetized with isoflurane and sacrificed by decapitation. The hippocampus (n = 4) was directly homogenized in RIPA buffer containing 0.1 % PMSF and 0.1 % protease inhibitor cocktail (Sigma, MO, USA). The lysates were centrifuged at 14,000×g for 30 min at 4 °C and the supernatant was used for protein analyses. The protein concentration in supernatants was determined using the BCA method. Equal amounts of soluble protein were separated by SDS-PAGE and transferred onto a nitrocellulose membrane (Immobilon NC; Millipore, Molsheim, France). Immunoblotting was performed with antibodies specific for phospho-AMPK (Ser172, 1:1000), AMPK (1:1000), p-IRS-1(Ser 612, 1:1000), IRS-1(1:1000), p-AKT (Ser473, 1:1000), AKT (1:1000), p-GSK3β (Ser9,1:1000), and GSK3β (1:1000) (Cell Signaling Technology). Primary antibodies were visualized using anti-rabbit HRP-conjugated secondary antibodies (Santa Cruz Biotechnology, Inc.) and a chemiluminescent detection system (Western blotting Luminal Reagent; Santa Cruz Biotechnology, Inc.). Variations in sample loading were normalized relative to GAPDH.
Statistical Analysis
All data were expressed as the mean ± SEM. For the Morris water maze tests, escape latency in the hidden platform trial were analzsed with two-way ANOVA of repeated measures, while one-way ANOVA was conducted on the data obtained from the probe trial. The recognition index in the novel object recognition test was analyzed by one-way ANOVA, followed by LSD (equal variances assumed) or Dunnett’sT3 (equal variances not assumed) for a post hoc test between groups. Histological assays and mitochondrial data were analyzed by one-way ANOVA, followed by LSD. All analyses were performed with SPSS statistical package (version 13.0 for Windows, SPSS Inc., USA). Differences were considered significant at a p value <0.05.
Results
Behavioral Test
Naringin Ameliorates Recognition Memory of HFD-Induced Obese Mice in Novel Object Recognition
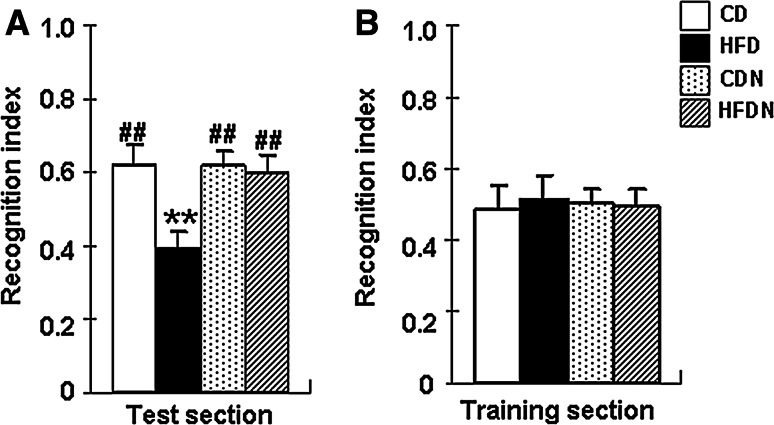
To evaluate cognitive function, a novel object recognition test was carried out in CD mice, CDN mice, HFD mice, and HFDN mice. In the test section, there was a significant overall group difference in the recognition index (F (3, 56) = 29.42, p < 0.01) among the four groups. Compared with CD mice, the recognition index (p < 0.01) was significantly reduced in HFD mice. Naringin (100 mg kg−1 d−1) markedly increased the recognition index by 52.5 % in the HFDN versus HFD group (Fig. 1a). There was no significant difference in recognition index between CD mice and CDN mice (p > 0.05). In addition, there was no significant difference in the recognition index (Fig. 1b) in training session between the four groups of mice (p > 0.05).
Fig. 1.
Effect of naringin on the recognition memory in HFD-induced obese mice detected by a novel object recognition test. The recognition index in the test section (a) and training section (b) of mice on a control diet (CD) and treated with 100 mg/kg/d naringin (CDN), high-fat diet (HFD), high-fat diet and treated with 100 mg/kg/d naringin (HFDN) were measured. Values are presented as mean ± S.E.M. The analysis was performed using one-way ANOVA with a LSD post hoc test between groups. (n = 15, **p < 0.01 vs. CD mice; ## p < 0.01 vs. HFD mice)
Naringin Improves the Learning and Memory of HFD-Induced Obese Mice in the Morris Water Maze
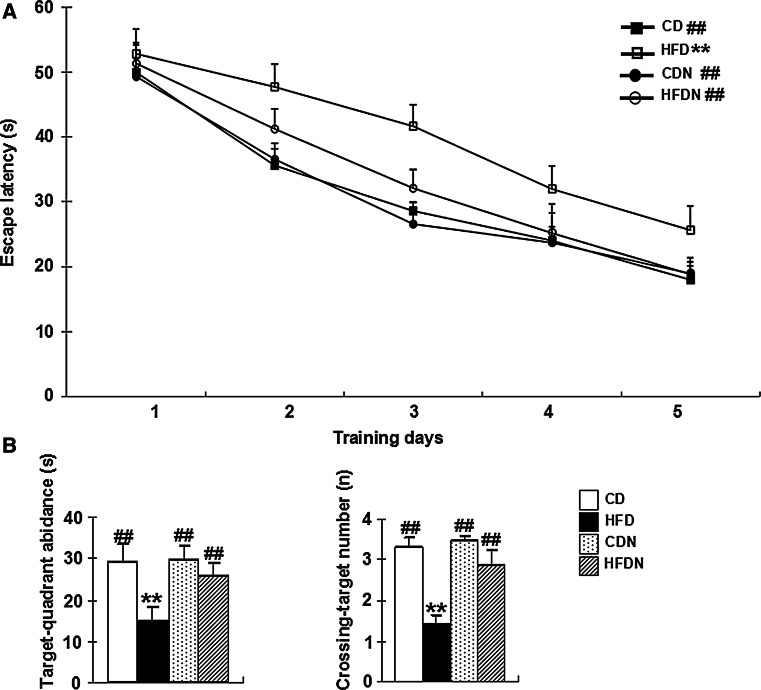
To assess spatial reference learning and memory function, all mice underwent testing in the Morris water maze after 20 weeks administration. Spatial learning was assessed in the hidden platform task in all mice. As shown in Fig. 2a, there was a significant overall group difference in escape latency among the four groups (group effect: F (3, 56) = 15.95, p < 0.01; training day effect: F (4, 224) = 106.10, p < 0.01; group × training day interaction: F (12, 224) = 0.99, p > 0.05). The latency to finding the submerged platform decreased every day, but the escape latency in HFD mice was significantly longer than that of the CD group (p < 0.01). The HFDN mice showed decreased escape latencies (p < 0.01) compared to the HFD mice (Fig. 2a). Note that all the mice had the same level of performance at the start of the experiment (no significant individual effects was observed in the first five trials on day 1).
Fig. 2.
Effect of naringin on learning and memory in HFD-induced obese mice using the Morris water maze. Escape latency during 5 days of hidden platform tests (a) and the target-quadrant abidance and the crossing-target number in the probe test (b) were tabulated. All data are presented as mean ± S.E.M. (n = 15, **p < 0.01 vs. CD mice; ## p < 0.01 vs. HFD mice)
In the probe test, the time spent in target quadrant and the crossing-target number were measured for 60 s on the 6th day after the last acquisition test. As shown in Fig. 2b, there was a significant overall group difference in the time spent in target quadrant (F (3, 56) = 25.43, p < 0.05) and crossing-target number (F (3, 56) = 16.96, p < 0.05) amongst the four groups. The HFD mice showed an obvious 49.1 % decrease in the time spent in target quadrant and a 50.0 % decrease in crossing-target number compared to the CD controls. Compared to the HFD mice, the time spent in target quadrant and the crossing-target number of the HFDN mice were significantly increased by 72.9 % and 1.05-fold, respectively. There was no significant difference in the time spent in target quadrant and the crossing-target number between CD mice and CDN mice (p > 0.05). In addition, there was no significant difference in swimming speed and path length in the probe test between the four groups of mice (Fig. S1).
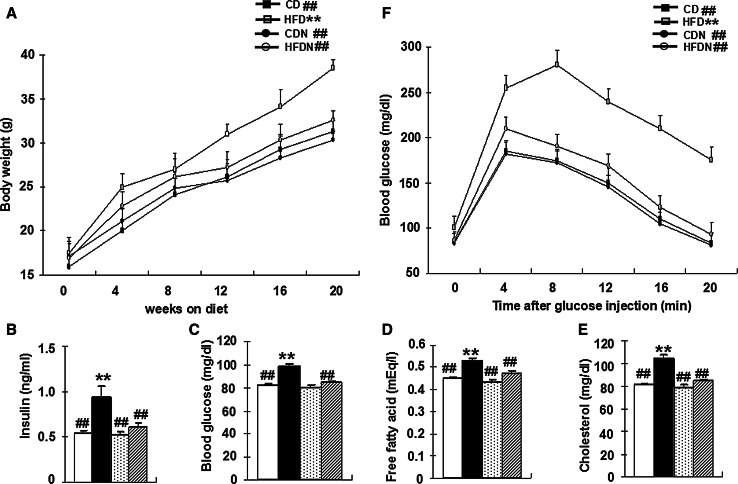
Naringin Prevents Obesity and Restores Abnormal Glucose, Fatty Acid and Cholesterol Metabolism to Near-Normal Levels in HFD-Induced Obese Mice
A substantial amount of evidence has demonstrated that obesity that can induce excess lipid accumulation, abnormalities in intracellular energy fluxes and nutrient availability, might be a chronic stimulus for insulin resistance (Ozcan et al. 2004). On the basis of these observations, we further examined body weight, the levels of blood insulin, glucose, free fatty acids and cholesterol and glucose tolerance in each group. Our data showed that after 20 weeks of a high-fat diet, mice exhibited significant differences in body weight (F (3, 56) = 20.51, p < 0.01) (Fig. 3a). Moreover, feeding a high-fat diet to mice for 20 weeks caused significantly increased levels of blood insulin (F (3, 56) = 29.45, p < 0.01) (Fig. 3b), glucose (F (3, 56) = 24.01, p < 0.01) (Fig. 3c), free fatty acids (F (3, 56) = 15.24, p < 0.01) (Fig. 3d), and cholesterol (F (3, 56) = 47.92, p < 0.01) (Fig. 3e). A glucose tolerance test was done for each group to investigate systemic insulin sensitivity. Exposure to a high-fat diet resulted in significant glucose intolerance in mice. After 20 weeks of a high-fat diet, mice showed significantly higher glucose levels on glucose challenge than CD group (p < 0.01) (Fig. 3f). Hence, a high-fat diet predisposed mice to peripheral insulin resistance and type-2 diabetes. However, naringin markedly reversed the metabolic changes in the HFDN versus HFD group (p < 0.01). As shown in the Fig. S2, there was no significant difference in food intake between the four groups of mice (p > 0.05).
Fig. 3.
Naringin prevents obesity and restores abnormal glucose, fatty acid and cholesterol metabolism in HFD-induced obese mice. Total body weight (a), blood insulin, glucose, free fatty acids, and cholesterol levels after 6-h daytime food withdrawal (b), Glucose tolerance tests (c) were performed. All data are presented as mean ± S.E.M. (n = 15, **p < 0.01 vs. CD mice; ## p < 0.01 vs. HFD mice)
Naringin Alleviates Mitochondrial Dysfunction in HFD-Induced Obese Mice
Decreased MMP, impaired ATP syntheses and enhanced ROS production are usually a direct consequence of mitochondrial dysfunction and might contribute additionally to mitochondrial damage. The HFD mice have obvious mitochondrial defects, exhibiting a 70 % decrease in MMP (p < 0.01), a 63.6 % decrease in ATP contents (p < 0.01) and a 2.58-fold increase in ROS production (p < 0.01) of hippocampal mitochondria when compared with CD control mice. Naringin (100 mg kg−1 d−1) can significantly attenuate mitochondrial damage as indicated by increasing MMP, ATP levels and decreasing ROS production by 1.89-fold, 1.25-fold, and 66.2 %, respectively, in the hippocampal mitochondria of the HFDN group versus HFD group (Fig. 4).
Fig. 4.
Analysis of isolated hippocampus mitochondrial function from HFD-induced obese mice treated with naringin. MMP (a), ROS Production (b), and ATP levels (c) were examined. All data are presented as mean ± S.E.M. (n = 6, **p < 0.01 vs. CD mice; ## p < 0.01 vs. HFD mice)
Naringin Attenuates Impaired Insulin Signaling in HFD-Induced Obese Mice
In order to explore the effect of naringin on brain insulin signaling, the expression levels of IRS-1 and the phosphorylation of IRS-1, Akt/GSK-3β were investigated. The levels of the IRS-1 (p < 0.05), p-Akt (p < 0.01), and p-GSK-3β (p < 0.01) were significantly decreased and the level of the p-IRS-1 (p < 0.01) were significantly increased in HFD mice compared to the CD group. Naringin (100 mg kg−1 d−1) significantly attenuated insulin signaling impairments as evidenced by a 34.5 % increase in the expression levels of IRS-1 (p < 0.05), a 47.8 % decrease in the p-IRS-1 (p < 0.01), a 1.43-fold increase in the p-Akt (p < 0.01), and a 1.89-fold increase (p < 0.01) in the p-GSK-3β in the hippocampus of the HFDN mice versus HFD mice (Fig. 5).
Fig. 5.
Naringin improves insulin signaling dysfunctions by increasing the expression levels of insulin receptor substrate 1(IRS-1) and the phosphorylation of IRS-1, Akt/GSK-3β in the hippocampus of HFD-induced obese mice. a The relative levels of IRS-1, p-IRS-1, p-AKT, and p-GSK-3β were detected by Western blotting from hippocampus tissues of CD mice, HFD mice, CDN mice, and HFDN mice, and a representative experiment was shown. b The quantitative analysis of IRS-1, p-IRS-1 p-AKT, and p-GSK-3β using GAPDH, IRS-1, AKT, and GSK-3β as normalization, respectively. All data are presented as mean ± S.E.M. (n = 4, *p < 0.05, **p < 0.01 vs. CD mice; # p < 0.05, ## p < 0.01 vs. HFD mice)
Naringin Increases AMPK Activity in HFD-Induced Obese Mice
The AMP-activated protein kinase (AMPK) is a proline-directed ser/thr kinase that associates with insulin signaling and mitochondrial function. As phosphorylated AMPK Thr172 (pAMPK172) is a marker of AMPK activation; total AMPK and pAMPK172 were measured by Western blot to evaluate the activation of AMPK. The levels of pAMPK172 were significantly decreased in HFD mice compared to the CD group. The HFDN mice exhibited a 1.44-fold increase in the levels of pAMPK172 compared to the HFD mice (Fig. 6a, b).
Fig. 6.
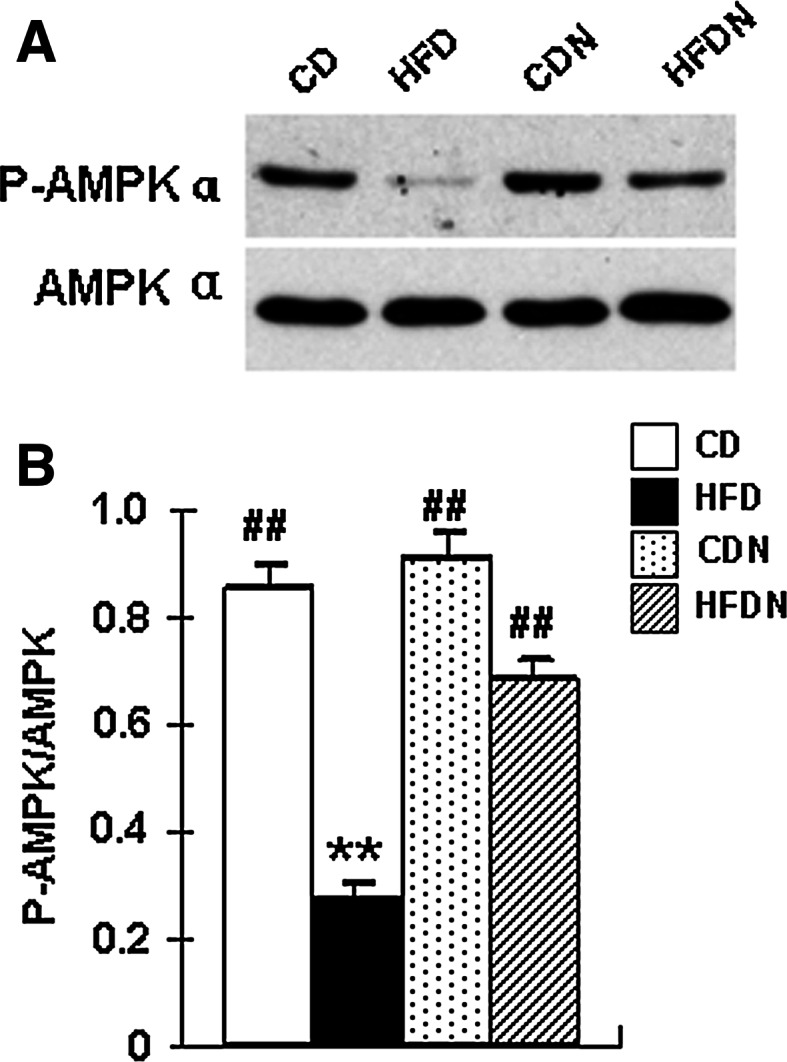
Naringin increases AMPK activity in the hippocampus of HFD-induced obese mice. Protein lysates were prepared from the hippocampus tissues of CD mice, HFD mice, CDN mice, and HFDN mice. The abundance of phosphorylated AMPKα and the expression of total AMPK were determined by Western blotting (a). The average blot densitometry of three independent experiments is shown (b). All data are presented as mean ± S.E.M. (n = 4, **p < 0.01 vs. CD mice; ## p < 0.01 vs. HFD mice)
Discussion
In the present study, we found naringin ameliorated cognitive deficits, as indicated by enhancing learning and memory (i.e., increasing recognition index by 52.5 % in the novel object recognition test and inducing a 1.05-fold increase in the crossing-target number in the probe test) in mice caused by HFD consumption. The results demonstrated that naringin improved insulin signaling impairment, attenuated mitochondrial dysfunction in mitochondria isolated from the hippocampus, and increased the activity of AMPK in HFD-induced obese mice.
Accumulating evidence indicates that neuronal insulin resistance is the underlying mechanism in a HFD-induced cognitive impairments. Insulin signaling enhances neurite outgrowth and synapse formation, regulates expression and localization of GABA (Wan et al. 1997), NMDA (Christie et al. 1999; Skeberdis et al. 2001), and AMPA (Huang et al. 2004; Wang and Linden 2000) receptors, and plays a critical role in synaptic plasticity (Chiu et al. 2008; van der Heide et al. 2005) and cognitive function (Benedict et al. 2007; Kern et al. 2001; Kleinridders et al. 2014; Schulingkamp et al. 2000). Consistent with previous studies (Arnold et al. 2014; McNeilly et al. 2011; Stranahan et al. 2008), our results also demonstrate defective insulin signaling, as indicating by the decreased expression levels of insulin receptor substrate 1(IRS-1) and the phosphorylation of Akt/GSK-3β, the increased expression level of IRS-1 serine phosphorylation, and impaired learning and memory in HFD-induced obesity mice. AMPK, a key energy modulator, plays a role in the regulation of insulin resistance. Activated AMPK can enhance insulin sensitivity by directly phosphorylating IRS1 (Jakobsen et al. 2001), by phosphorylating the mTOR upstream inhibitor TSC2 (Inoki et al. 2003), or by suppressing endoplasmic reticulum stress response (Dong et al. 2010; Li et al. 2014). Conversely, the inhibition or decrease of AMPK activity promotes insulin resistance in cell lines (Kraegen et al. 2006; Saha et al. 2010), animal models (Buhl et al. 2002; Harmel et al. 2014), and obesity associated patients (Gauthier et al. 2011; Kola et al. 2008; Xu et al. 2012). In addition, a line of agents has been shown to improve insulin resistance through increasing AMPK activation (Zhou et al. 2001). Moreover, AMPK has been reported to act as a modulator of long-term potentiation (LTP) (Potter et al. 2010), and is required for the memory formation (Dash et al. 2006). In this present study, naringin can restore decreased AMPK activity (Fig. 6) and attenuate insulin signaling dysfunction (Fig. 5) that are observed in HFD-induced obese mice to that which are observed in CD mice. Naringin also improved learning and memory deficits (Figs. 1, 2). The amelioration of insulin resistance and impaired insulin signaling in CNS is an effective way of preventing or reversing the cognitive deficits and attenuating the atrophy that is observed in obesity (Baker et al. 2011; Craft et al. 2012; Pintana et al. 2012; Pipatpiboon et al. 2012). The positive effect of naringin on cognition might profit from enhancing the insulin sensitivity and attenuating insulin signaling dysfunction which may be through increasing the activity of AMPK.
Our results indicate that naringin can significantly ameliorate mitochondrial dysfunction caused by 20-week HFD consumption by restoration of mitochondrial MMP, ROS, and ATP levels in mitochondria (Fig. 4). AMPK has been implicated in the regulation of mitochondrial function. Activation of AMPK, or overexpression of constitutively activated AMPK, can enhance mitochondrial biogenesis and improve mitochondrial function in vitro (Choi et al. 2010; Dong et al. 2013; Shin et al. 2009) and in vivo (Moran et al. 2013; Ng et al. 2012). Conversely, the inhibition of AMPK leads to an increase in ROS production, a decrease in mitochondrial biogenesis and thus worsens mitochondrial dysfunction (Finocchietto et al. 2011; Wang and Brautigan 2013). Mitochondrial dysfunction and increased ROS generation can lead to insulin resistance (Yano et al. 2011). The reduction of ROS production and keeping ROS production at a low level has been shown to improve insulin signaling (Loh et al. 2009). Therefore, the effects of naringin in reducing brain mitochondrial dysfunction through activating AMPK may be one of the reasons that naringin improves neuronal insulin sensitivity in the brain.
In conclusion, naringin reverses HFD-induced cognitive deficits through the improvement of insulin resistance via enhancing insulin signaling and the attenuation of mitochondrial dysfunction in the mouse brain. The regulation of AMPK activity may be involved in the mechanisms by which naringin affects HFD phenotypes. Naringin could be recommended as a possible candidate for the prevention and therapy of cognitive deficits in type-2 diabetes mellitus and Alzheimer disease.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Fig. S1. Swimming speed and path length in the probe test of Morris water maze. The swimming speed (A) and path length in the probe test (B) of mice on a control diet (CD) and treated with 100 mg/kg/day naringin (CDN), high-fat diet (HFD), high-fat diet and treated with 100 mg/kg/day naringin (HFDN) were tabulated. All data are presented as mean ± S.E.M. (n = 15). Supplementary material 1 (DOCX 355 kb)
Fig. S2. Effects of naringin on food intake in HFD-induced obese mice. Food intake of mice during treatment was measured. All data are presented as mean ± S.E.M. (n = 15). Supplementary material 2 (DOCX 221 kb)
Acknowledgments
The present work was supported by National Natural Science Foundation of China (U1304806 and U1304809), the China Scholarship Council (File No. 201408410296), and the Scientific Research Fund of Henan University of Science and Technology (NO. 09001664).
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Statement
All applicable international, national, and institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were approved by the Animal Ethics Committee of Henan University of Science and Technology in China and in accordance with the ethical standards of the institution. Informed consent was obtained from all individual participants included in the study.
Abbreviations
- AD
Alzheimer’s disease
- AMPK
AMP-activated protein kinase
- GSK3β
Glycogen synthase kinase-3β
- HFD
High-fat diet
- IRS-1
Insulin receptor substrate 1
- MMP
Mitochondrial membrane potential
- ROS
Reactive oxygen species
References
- Arnold SE, Lucki I, Brookshire BR, Carlson GC, Browne CA, Kazi H et al (2014) High fat diet produces brain insulin resistance, synaptodendritic abnormalities and altered behavior in mice. Neurobiol Dis 67:79–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker LD, Cross DJ, Minoshima S, Belongia D, Watson GS, Craft S (2011) Insulin resistance and Alzheimer-like reductions in regional cerebral glucose metabolism for cognitively normal adults with prediabetes or early type 2 diabetes. Arch Neurol 68:51–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict C, Hallschmid M, Schultes B, Born J, Kern W (2007) Intranasal insulin to improve memory function in humans. Neuroendocrinology 86:136–142 [DOI] [PubMed] [Google Scholar]
- Bevins RA, Besheer J (2006) Object recognition in rats and mice: a one-trial non-matching-to-sample learning task to study ‘recognition memory’. Nat Protoc 1:1306–1311 [DOI] [PubMed] [Google Scholar]
- Brown MR, Geddes JW, Sullivan PG (2004) Brain region-specific, age-related, alterations in mitochondrial responses to elevated calcium. J Bioenerg Biomembr 36:401–406 [DOI] [PubMed] [Google Scholar]
- Buhl ES, Jessen N, Pold R, Ledet T, Flyvbjerg A, Pedersen SB, Pedersen O, Schmitz O, Lund S (2002) Long-term AICAR administration reduces metabolic disturbances and lowers blood pressure in rats displaying features of the insulin resistance syndrome. Diabetes 51:2199–2206 [DOI] [PubMed] [Google Scholar]
- Chiu SL, Chen CM, Cline HT (2008) Insulin receptor signaling regulates synapse number, dendritic plasticity, and circuit function in vivo. Neuron 58:708–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SH, Kim YW, Kim SG (2010) AMPK-mediated GSK3beta inhibition by isoliquiritigenin contributes to protecting mitochondria against iron-catalyzed oxidative stress. Biochem Pharmacol 79:1352–1362 [DOI] [PubMed] [Google Scholar]
- Christie JM, Wenthold RJ, Monaghan DT (1999) Insulin causes a transient tyrosine phosphorylation of NR2A and NR2B NMDA receptor subunits in rat hippocampus. J Neurochem 72:1523–1528 [DOI] [PubMed] [Google Scholar]
- Clegg DJ, Gotoh K, Kemp C, Wortman MD, Benoit SC, Brown LM, D’Alessio D, Tso P, Seeley RJ, Woods SC (2011) Consumption of a high-fat diet induces central insulin resistance independent of adiposity. Physiol Behav 103:10–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft S (2005) Insulin resistance syndrome and Alzheimer’s disease: age- and obesity-related effects on memory, amyloid, and inflammation. Neurobiol Aging 26(Suppl 1):65–69 [DOI] [PubMed] [Google Scholar]
- Craft S, Baker LD, Montine TJ, Minoshima S, Watson GS, Claxton A et al (2012) Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: a pilot clinical trial. Arch Neurol 69:29–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash PK, Orsi SA, Moore AN (2006) Spatial memory formation and memory-enhancing effect of glucose involves activation of the tuberous sclerosis complex-Mammalian target of rapamycin pathway. J Neurosci 26:8048–8056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Zhang M, Wang S, Liang B, Zhao Z, Liu C, Wu M, Choi HC, Lyons TJ, Zou MH (2010) Activation of AMP-activated protein kinase inhibits oxidized LDL-triggered endoplasmic reticulum stress in vivo. Diabetes 59:1386–1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong GZ, Jang EJ, Kang SH, Cho IJ, Park SD, Kim SC, Kim YW (2013) Red ginseng abrogates oxidative stress via mitochondria protection mediated by LKB1-AMPK pathway. BMC Complement Altern Med 13:64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragicevic N, Mamcarz M, Zhu Y, Buzzeo R, Tan J, Arendash GW, Bradshaw PC (2010) Mitochondrial amyloid-beta levels are associated with the extent of mitochondrial dysfunction in different brain regions and the degree of cognitive impairment in Alzheimer’s transgenic mice. J Alzheimers Dis 20(Suppl 2):S535–S550 [DOI] [PubMed] [Google Scholar]
- Finocchietto PV, Holod S, Barreyro F, Peralta JG, Alippe Y, Giovambattista A, Carreras MC, Poderoso JJ (2011) Defective leptin-AMP-dependent kinase pathway induces nitric oxide release and contributes to mitochondrial dysfunction and obesity in ob/ob mice. Antioxid Redox Signal 15:2395–2406 [DOI] [PubMed] [Google Scholar]
- Francis H, Stevenson R (2013) The longer-term impacts of Western diet on human cognition and the brain. Appetite 63:119–128 [DOI] [PubMed] [Google Scholar]
- Gaur V, Aggarwal A, Kumar A (2009) Protective effect of naringin against ischemic reperfusion cerebral injury: possible neurobehavioral, biochemical and cellular alterations in rat brain. Eur J Pharmacol 616:147–154 [DOI] [PubMed] [Google Scholar]
- Gauthier MS, O’Brien EL, Bigornia S, Mott M, Cacicedo JM, Xu XJ, Gokce N, Apovian C, Ruderman N (2011) Decreased AMP-activated protein kinase activity is associated with increased inflammation in visceral adipose tissue and with whole-body insulin resistance in morbidly obese humans. Biochem Biophys Res Commun 404:382–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood CE, Winocur G (2005) High-fat diets, insulin resistance and declining cognitive function. Neurobiol Aging 26(Suppl 1):42–45 [DOI] [PubMed] [Google Scholar]
- Harmel E, Grenier E, Bendjoudi Ouadda A, El Chebly M, Ziv E, Beaulieu JF, Sane A, Spahis S, Laville M, Levy E (2014) AMPK in the small intestine in normal and pathophysiological conditions. Endocrinology 155:873–888 [DOI] [PubMed] [Google Scholar]
- Huang CC, Lee CC, Hsu KS (2004) An investigation into signal transduction mechanisms involved in insulin-induced long-term depression in the CA1 region of the hippocampus. J Neurochem 89:217–231 [DOI] [PubMed] [Google Scholar]
- Huang H, Wu K, You Q, Huang R, Li S (2013) Naringin inhibits high glucose-induced cardiomyocyte apoptosis by attenuating mitochondrial dysfunction and modulating the activation of the p38 signaling pathway. Int J Mol Med 32:396–402 [DOI] [PubMed] [Google Scholar]
- Inoki K, Zhu T, Guan KL (2003) TSC2 mediates cellular energy response to control cell growth and survival. Cell 115:577–590 [DOI] [PubMed] [Google Scholar]
- Jakobsen SN, Hardie DG, Morrice N, Tornqvist HE (2001) 5′-AMP-activated protein kinase phosphorylates IRS-1 on Ser-789 in mouse C2C12 myotubes in response to 5-aminoimidazole-4-carboxamide riboside. J Biol Chem 276:46912–46916 [DOI] [PubMed] [Google Scholar]
- Johannsen DL, Ravussin E (2009) The role of mitochondria in health and disease. Curr Opin Pharmacol 9:780–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanoski SE, Davidson TL (2011) Western diet consumption and cognitive impairment: links to hippocampal dysfunction and obesity. Physiol Behav 103:59–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern W, Peters A, Fruehwald-Schultes B, Deininger E, Born J, Fehm HL (2001) Improving influence of insulin on cognitive functions in humans. Neuroendocrinology 74:270–280 [DOI] [PubMed] [Google Scholar]
- Kim HJ, Song JY, Park HJ, Park HK, Yun DH, Chung JH (2009) Naringin protects against rotenone-induced apoptosis in human neuroblastoma SH-SY5Y cells. Korean J Physiol Pharmacol 13:281–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinridders A, Ferris HA, Cai W, Kahn CR (2014) Insulin action in brain regulates systemic metabolism and brain function. Diabetes 63:2232–2243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kola B, Christ-Crain M, Lolli F, Arnaldi G, Giacchetti G, Boscaro M, Grossman AB, Korbonits M (2008) Changes in adenosine 5′-monophosphate-activated protein kinase as a mechanism of visceral obesity in Cushing’s syndrome. J Clin Endocrinol Metab 93:4969–4973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraegen EW, Saha AK, Preston E, Wilks D, Hoy AJ, Cooney GJ, Ruderman NB (2006) Increased malonyl-CoA and diacylglycerol content and reduced AMPK activity accompany insulin resistance induced by glucose infusion in muscle and liver of rats. Am J Physiol Endocrinol Metab 290:E471–E479 [DOI] [PubMed] [Google Scholar]
- Kumar P, Kumar A (2010) Protective effect of hesperidin and naringin against 3-nitropropionic acid induced Huntington’s like symptoms in rats: possible role of nitric oxide. Behav Brain Res 206:38–46 [DOI] [PubMed] [Google Scholar]
- Kumar A, Prakash A, Dogra S (2010) Naringin alleviates cognitive impairment, mitochondrial dysfunction and oxidative stress induced by d-galactose in mice. Food Chem Toxicol 48:626–632 [DOI] [PubMed] [Google Scholar]
- Laczo J, Vlcek K, Vyhnalek M, Vajnerova O, Ort M, Holmerova I, Tolar M, Andel R, Bojar M, Hort J (2009) Spatial navigation testing discriminates two types of amnestic mild cognitive impairment. Behav Brain Res 202:252–259 [DOI] [PubMed] [Google Scholar]
- Li H, Min Q, Ouyang C, Lee J, He C, Zou MH, Xie Z (2014) AMPK activation prevents excess nutrient-induced hepatic lipid accumulation by inhibiting mTORC1 signaling and endoplasmic reticulum stress response. Biochim Biophys Acta 1842:1844–1854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang KC, Hon W, Tyan YM, Liao WL (1994) Involvement of hippocampal NMDA and AMPA receptors in acquisition, formation and retrieval of spatial memory in the Morris water maze. Chin J Physiol 37:201–212 [PubMed] [Google Scholar]
- Loh K, Deng H, Fukushima A, Cai X, Boivin B, Galic S et al (2009) Reactive oxygen species enhance insulin sensitivity. Cell Metab 10:260–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeilly AD, Williamson R, Sutherland C, Balfour DJ, Stewart CA (2011) High fat feeding promotes simultaneous decline in insulin sensitivity and cognitive performance in a delayed matching and non-matching to position task. Behav Brain Res 217:134–141 [DOI] [PubMed] [Google Scholar]
- Miller RA, Buehner G, Chang Y, Harper JM, Sigler R, Smith-Wheelock M (2005) Methionine-deficient diet extends mouse lifespan, slows immune and lens aging, alters glucose, T4, IGF-I and insulin levels, and increases hepatocyte MIF levels and stress resistance. Aging Cell 4:119–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohlig M, Isken F, Ristow M (2004) Impaired mitochondrial activity and insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med 350:2419–2421. author reply 2419-2421 [PubMed] [Google Scholar]
- Moloney AM, Griffin RJ, Timmons S, O’Connor R, Ravid R, O’Neill C (2010) Defects in IGF-1 receptor, insulin receptor and IRS-1/2 in Alzheimer’s disease indicate possible resistance to IGF-1 and insulin signalling. Neurobiol Aging 31:224–243 [DOI] [PubMed] [Google Scholar]
- Moran C, Sanz-Rodriguez A, Jimenez-Pacheco A, Martinez-Villareal J, McKiernan RC, Jimenez-Mateos EM et al (2013) Bmf upregulation through the AMP-activated protein kinase pathway may protect the brain from seizure-induced cell death. Cell Death Dis 4:e606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng CH, Guan MS, Koh C, Ouyang X, Yu F, Tan EK, O’Neill SP, Zhang X, Chung J, Lim KL (2012) AMP kinase activation mitigates dopaminergic dysfunction and mitochondrial abnormalities in Drosophila models of Parkinson’s disease. J Neurosci 32:14311–14317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson LG, Nilsson E (2009) Overweight and cognition. Scand J Psychol 50:660–667 [DOI] [PubMed] [Google Scholar]
- Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Gorgun C, Glimcher LH, Hotamisligil GS (2004) Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 306:457–461 [DOI] [PubMed] [Google Scholar]
- Pintana H, Apaijai N, Pratchayasakul W, Chattipakorn N, Chattipakorn SC (2012) Effects of metformin on learning and memory behaviors and brain mitochondrial functions in high fat diet induced insulin resistant rats. Life Sci 91:409–414 [DOI] [PubMed] [Google Scholar]
- Pipatpiboon N, Pratchayasakul W, Chattipakorn N, Chattipakorn SC (2012) PPARgamma agonist improves neuronal insulin receptor function in hippocampus and brain mitochondria function in rats with insulin resistance induced by long term high-fat diets. Endocrinology 153:329–338 [DOI] [PubMed] [Google Scholar]
- Pipatpiboon N, Pintana H, Pratchayasakul W, Chattipakorn N, Chattipakorn SC (2013) DPP4-inhibitor improves neuronal insulin receptor function, brain mitochondrial function and cognitive function in rats with insulin resistance induced by high-fat diet consumption. Eur J Neurosci 37:839–849 [DOI] [PubMed] [Google Scholar]
- Potter WB, O’Riordan KJ, Barnett D, Osting SM, Wagoner M, Burger C, Roopra A (2010) Metabolic regulation of neuronal plasticity by the energy sensor AMPK. PLoS ONE 5:e8996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash A, Shur B, Kumar A (2013) Naringin protects memory impairment and mitochondrial oxidative damage against aluminum-induced neurotoxicity in rats. Int J Neurosci 123:636–645 [DOI] [PubMed] [Google Scholar]
- Pratchayasakul W, Kerdphoo S, Petsophonsakul P, Pongchaidecha A, Chattipakorn N, Chattipakorn SC (2011) Effects of high-fat diet on insulin receptor function in rat hippocampus and the level of neuronal corticosterone. Life Sci 88:619–627 [DOI] [PubMed] [Google Scholar]
- Pu P, Gao DM, Mohamed S, Chen J, Zhang J, Zhou XY, Zhou NJ, Xie J, Jiang H (2012) Naringin ameliorates metabolic syndrome by activating AMP-activated protein kinase in mice fed a high-fat diet. Arch Biochem Biophys 518:61–70 [DOI] [PubMed] [Google Scholar]
- Rabol R, Boushel R, Dela F (2006) Mitochondrial oxidative function and type 2 diabetes. Appl Physiol Nutr Metab 31:675–683 [DOI] [PubMed] [Google Scholar]
- Rajadurai M, Prince PS (2007) Preventive effect of naringin on cardiac mitochondrial enzymes during isoproterenol-induced myocardial infarction in rats: a transmission electron microscopic study. J Biochem Mol Toxicol 21:354–361 [DOI] [PubMed] [Google Scholar]
- Ramesh E, Alshatwi AA (2013) Naringin induces death receptor and mitochondria-mediated apoptosis in human cervical cancer (SiHa) cells. Food Chem Toxicol 51:97–105 [DOI] [PubMed] [Google Scholar]
- Reddy PH, Tripathi R, Troung Q, Tirumala K, Reddy TP, Anekonda V et al (2012) Abnormal mitochondrial dynamics and synaptic degeneration as early events in Alzheimer’s disease: implications to mitochondria-targeted antioxidant therapeutics. Biochim Biophys Acta 1822:639–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha AK, Xu XJ, Lawson E, Deoliveira R, Brandon AE, Kraegen EW, Ruderman NB (2010) Downregulation of AMPK accompanies leucine- and glucose-induced increases in protein synthesis and insulin resistance in rat skeletal muscle. Diabetes 59:2426–2434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert M, Gautam D, Surjo D, Ueki K, Baudler S, Schubert D et al (2004) Role for neuronal insulin resistance in neurodegenerative diseases. Proc Natl Acad Sci USA 101:3100–3105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulingkamp RJ, Pagano TC, Hung D, Raffa RB (2000) Insulin receptors and insulin action in the brain: review and clinical implications. Neurosci Biobehav Rev 24:855–872 [DOI] [PubMed] [Google Scholar]
- Sellbom KS, Gunstad J (2012) Cognitive function and decline in obesity. J Alzheimers Dis 30(Suppl 2):S89–S95 [DOI] [PubMed] [Google Scholar]
- Shin SM, Cho IJ, Kim SG (2009) Resveratrol protects mitochondria against oxidative stress through AMP-activated protein kinase-mediated glycogen synthase kinase-3beta inhibition downstream of poly(ADP-ribose)polymerase-LKB1 pathway. Mol Pharmacol 76:884–895 [DOI] [PubMed] [Google Scholar]
- Skeberdis VA, Lan J, Zheng X, Zukin RS, Bennett MV (2001) Insulin promotes rapid delivery of N-methyl-d-aspartate receptors to the cell surface by exocytosis. Proc Natl Acad Sci USA 98:3561–3566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- So FV, Guthrie N, Chambers AF, Moussa M, Carroll KK (1996) Inhibition of human breast cancer cell proliferation and delay of mammary tumorigenesis by flavonoids and citrus juices. Nutr Cancer 26:167–181 [DOI] [PubMed] [Google Scholar]
- Stranahan AM, Norman ED, Lee K, Cutler RG, Telljohann RS, Egan JM, Mattson MP (2008) Diet-induced insulin resistance impairs hippocampal synaptic plasticity and cognition in middle-aged rats. Hippocampus 18:1085–1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taddeo EP, Laker RC, Breen DS, Akhtar YN, Kenwood BM, Liao JA et al (2014) Opening of the mitochondrial permeability transition pore links mitochondrial dysfunction to insulin resistance in skeletal muscle. Mol Metab 3:124–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamura A, Okamoto Y, Kawarabayashi T, Yokoseki T, Shibata M, Mouri A et al (2011) Extracellular and intraneuronal HMW-AbetaOs represent a molecular basis of memory loss in Alzheimer’s disease model mouse. Mol Neurodegener 6:20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Heide LP, Kamal A, Artola A, Gispen WH, Ramakers GM (2005) Insulin modulates hippocampal activity-dependent synaptic plasticity in a N-methyl-d-aspartate receptor and phosphatidyl-inositol-3-kinase-dependent manner. J Neurochem 94:1158–1166 [DOI] [PubMed] [Google Scholar]
- Wan Q, Xiong ZG, Man HY, Ackerley CA, Braunton J, Lu WY, Becker LE, MacDonald JF, Wang YT (1997) Recruitment of functional GABA(A) receptors to postsynaptic domains by insulin. Nature 388:686–690 [DOI] [PubMed] [Google Scholar]
- Wang L, Brautigan DL (2013) Alpha-SNAP inhibits AMPK signaling to reduce mitochondrial biogenesis and dephosphorylates Thr172 in AMPKalpha in vitro. Nat Commun 4:1559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YT, Linden DJ (2000) Expression of cerebellar long-term depression requires postsynaptic clathrin-mediated endocytosis. Neuron 25:635–647 [DOI] [PubMed] [Google Scholar]
- Wang D, Gao K, Li X, Shen X, Zhang X, Ma C, Qin C, Zhang L (2012) Long-term naringin consumption reverses a glucose uptake defect and improves cognitive deficits in a mouse model of Alzheimer’s disease. Pharmacol Biochem Behav 102:13–20 [DOI] [PubMed] [Google Scholar]
- Wang DM, Yang YJ, Zhang L, Zhang X, Guan FF, Zhang LF (2013) Naringin enhances CaMKII activity and improves long-term memory in a mouse model of Alzheimer’s disease. Int J Mol Sci 14:5576–5586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren BE, Lou PH, Lucchinetti E, Zhang L, Clanachan AS, Affolter A, Hersberger M, Zaugg M, Lemieux H (2014) Early mitochondrial dysfunction in glycolytic muscle, but not oxidative muscle, of the fructose-fed insulin-resistant rat. Am J Physiol Endocrinol Metab 306:E658–E667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu XJ, Gauthier MS, Hess DT, Apovian CM, Cacicedo JM, Gokce N, Farb M, Valentine RJ, Ruderman NB (2012) Insulin sensitive and resistant obesity in humans: AMPK activity, oxidative stress, and depot-specific changes in gene expression in adipose tissue. J Lipid Res 53:792–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano M, Watanabe K, Yamamoto T, Ikeda K, Senokuchi T, Lu M et al (2011) Mitochondrial dysfunction and increased reactive oxygen species impair insulin secretion in sphingomyelin synthase 1-null mice. J Biol Chem 286:3992–4002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J et al (2001) Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 108:1167–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Swimming speed and path length in the probe test of Morris water maze. The swimming speed (A) and path length in the probe test (B) of mice on a control diet (CD) and treated with 100 mg/kg/day naringin (CDN), high-fat diet (HFD), high-fat diet and treated with 100 mg/kg/day naringin (HFDN) were tabulated. All data are presented as mean ± S.E.M. (n = 15). Supplementary material 1 (DOCX 355 kb)
Fig. S2. Effects of naringin on food intake in HFD-induced obese mice. Food intake of mice during treatment was measured. All data are presented as mean ± S.E.M. (n = 15). Supplementary material 2 (DOCX 221 kb)