Abstract
Growing evidence indicates that microRNAs (miRNAs) are important mediators of brain development and neurite growth. However, the affected signaling mechanisms are not clearly clarified. In the present study, we confirm that miR-29c is expressed during mice brain development and increases neurite outgrowth via decreasing PTEN expression. We first screen the picked-out miR-29c up-regulated in PC12 cells induced by nerve growth factor (NGF). In silico analysis of possible miR-29c targets, VEGFA, MAPK3, PDGFB, and PTEN mRNA are proposed as relatively likely putative binding sites for miR-29c. Subsequently, we detect that miR-29c is involved in brain development and has a negative relationship with the expression of PTEN. Then, using luciferase reporter assay,we demonstrate that miR-29c could directly target to the 3′-UTR of PTEN mRNA and result in down-expression of PTEN. By infecting PC12 cells with lentiviral pLKO-miR-29c or control, we also find that increasing levels of miR-29c markedly increase Akt phosphorylation level, and thus, promote neurite outgrowth of PC12 cells. Together, our results identify that miR-29c is required for mice brain development and modulates neurite outgrowth in PC12 cells via targeting PTEN and has a promising therapeutic target for neural disease.
Keywords: miRNA, Brain development, Neurite outgrowth, miR-29c, PTEN, Akt
Introduction
MicroRNAs (miRNAs) are a class of small non-coding RNA molecules (~22 nt), whose main function is repressing translation or inducing mRNA cleavage by binding to the 3′-untranslated region (UTR) of target gene mRNA (Bartel 2009), and thereby, inhibiting the translation from mRNA to protein (Fan et al. 2014). In recent years, many diseases including almost all types of cancers have been connected to aberrant expression of miRNAs and the importance of miRNAs in cancer etiology is clear (Chang et al. 2007; Esquela-Kerscher et al. 2006). However, its signaling mechanisms in the nervous system are not clearly clarified. Growing evidences support that miRNAs can modulate cellular differentiation and migration by regulating a wide range of target mRNAs. Reconstitution of miR-29b/c in RMS in mice inhibits tumor growth and stimulates differentiation by targeting YY1 transcription factor (Wang et al. 2008). The down-regulation of miR-29c in nasopharyngeal carcinomas might contribute to the metastatic tumor invasion through reduced regulation of extracellular matrix targets or related proteins (Sengupta et al. 2008).
MiR-29c acts as one of the miR-29 family members that also include the closely related miR-29a and miR-29b. Mature miR-29s are highly conserved in human, mouse, and rat. Mature miR-29s share identical sequences at nucleotide positions 2–7, the seed region that plays a key role in determining which protein-coding genes a microRNA would target (Kriegel et al. 2012). In the development of hepatocellular carcinoma (HCC), miR-29a is involved in the regulation of migration of hepatoma cells mediated by Hepatitis B virus X protein (HBx) through modulation of Akt phosphorylation (Kong et al. 2011). In diabetic neuropathy, down-regulation of miR-29b was associated with higher apoptosis rate and more serious axonal swelling via inhibiting axon-generation genes and stimulating neurodegenerative genes. Restoration of miR-29b by mimic experiment could reverse the above neuropathy through abolishing Smad3 activation (Zhang et al. 2014).
In this study, we found that miR-29c expression levels increased during NGF-induced differentiation of PC12 cells, and several predicted nerve growth-associated genes, such as VEGFA, MAPK3, PDGFB, and PTEN, decreased during this process. Among them, we demonstrated that PTEN is a direct target gene of miR-29c. Our further study confirms that miR-29c is involved in brain development and has a negative relationship with the expression of PTEN. Alteration of miR-29c expression with stable miR-29c expression PC12 cells could promote neurite outgrowth by modulating PTEN expression, increasing Akt phosphorylation levels.
Materials and Methods
Cell Culture and Induction of Cell Differentiation
PC12 cells (purchased from the Type Culture Collection of the Chinese Academy of Sciences, Shanghai, China.) were grown in high-glucose (25 mM) DMEM/F12 (Hyclone, Logan, UT) with 10 % fetal bovine serum (Hyclone, Logan, UT) and Penicillin–Streptomycin Solution (50U/ml penicillin, 50 μg/ml Streptomycin, Hyclone).
To induce the PC12 cells to differentiate, cells were plated onto 6-well tissue culture plates at a relatively low density (2 × 103 cells cm−2) in DMEM/F12 (Hyclone, Logan, UT) medium with 5 % FBS. After plating cells for 24 h, a concentrated stock of NGF (human recombinant NGF, Sigma, Missouri, USA) was then added to above culture medium till final concentration of 50 ng/ml. Cells exposed to vehicle alone (5 % FBS culture medium) were used as controls.
Animal
Balb/c mice from embryonic 15 days to 4 week were provided by Suzhou University experimental center and maintained in SPF level housing [temperature at 21 ± 2 °C, humidity 30–35 %, 12/12-h dark/light cycle, with free access to food and water]. All experiment procedures were in accordance with guidelines of animal research from Science and technology department of China, and have been approved by ethic committee of animal research in Suzhou University.
Target Prediction
Several freeware programs available on the internet are commonly used to search for potential miRNA–mRNA binding. We used three such items of software, TargetScan (http://www.targetscan.org/), PicTar (http://pictar.mdc-berlin.de), and microT (http://www.targetscan.org/). There are all three identified VEGFA, MAPK3, PDGFB, and PTEN having relatively likely putative binding site for miR-29c.
Real-Time PCR (miRNA)
Total RNA was extracted from mice brain or 5 × 106 cells using Trizol (Life Technologies, USA) according to the manufacturer’s manual. We used Poly(A) tailing kit (NEW ENGLAND Biolabs, USA) for miRNAs detection. The ingredients 1 μg total RNA was mixed with 2 μl Poly(A) Buffer(10 ×), 2 μl ATP (10 nM), 0.5μlE. coli Poly(A) Polymerase I (E-PAP). The total volume was adjusted to the 20 μl with RNase–free ddH2O. Single-stranded cDNA was obtained from RNA by reverse transcriptase (TAKARA, China). The ingredients 0.5 μg total Poly(A) tailing RNA was mixed with 2 μl M-MLV buffer (5×), 0.5μldNTP mixture (10 mM), 0.25 μlRNase inhibitor(40 U/μl), 0.25μlRTase M-MLV (RNase H-) (200 U/μl), and 1 μl adaptor(dT)15(50 μM). The total volume was adjusted to the 20 μl with RNase–free ddH2O. Reverse transcription process was performed at 42 °C for 60 min, followed by an inactivation reaction at 70 °C for 15 min. The PCR mixture contains 10 μl qPCR Master Mix (2×) (Bio-Rad, USA), and 1 μl cDNA. The total volume was increased to the 20 μl with ddH2O. The Primer powder has been fixed to the bottom of the 96-well plate. Each well was added with 20 μl of PCR mixture. RT-PCR was performed using a CFX96™ Real-Time Instrument (Bio-Rad, USA). Real-Time PCR (RT-PCR) was conducted using the iQ™ SYBR Green qPCR Supermix (Bio-Rad, USA) by using the following primers (Tabal 1). Thermal cycle parameters were 95 °C for 5 min, 40 cycles at 95 °C for 15 s, 60 °C for 15 s, and 72 °C for 20 s, and 65–95 °C drawing dissociation curve. The expression of each gene was defined from the threshold cycle (Ct), and melting temperatures (T m) were recorded. Using the ΔΔCt method analyzes relative changes in gene expression. Each test was independently repeated four times (n = 4).
Real-Time PCR (mRNA)
Total RNA (1 μg aliquots) was converted to cDNA with reverse transcriptase kit (TAKARA, China). The resultant cDNA was diluted 1:5. The quantitative PCR procedures were carried out according to the manufacturer’s protocol and Real-time PCR SYBR Green qPCR Supermix (Bio-Rad) for rat/mice GAPDH, PTEN (Applied Sangon Biotech, China) were used. The CFX96™ Real-Time Instrument was used for amplification and detection. The relative expression was calculated using the comparative CT method. Relative mRNA levels were normalized to endogenous GAPDH mRNA expression for each sample (as previous description). The CT value was obtained from the amplification plot with the aid of SDS software.
Western Blotting
Western blotting analysis was performed to detect PTEN, p-Akt, NF-200, and GAP-43 protein expression in mice brain and PC12 cell lines. The cultured cells were rinsed with cold PBS before being treated with RIPA lysis buffer at 4 °C for 10 min. Then, the mixture was centrifuged under 4 °C at 12,000 r/min for 15 min. The supernatant was removed, and the protein concentration was measured with BCA method. 40 μg of protein was loaded, and was separated by 10 % SDS-PAGE and transferred to the PVDF membrane. The membrane was blocked by 5 % non-fat milk powder for 1 h at room temperature before the 4 °C overnight incubation with primary antibodies anti-PTEN, anti-p-Akt, anti-Akt, anti-NF-200, and anti-GAP-43 (Cell Signal technology, USA) at 1:1,000, followed with a goat-anti-rabbit IgG-HRP secondary antibody (Thermo Pierce, USA). Protein levels were normalized to β-actin, using a mouse monoclonal anti-GAPDH antibody (Thermo Pierce, USA).
Transfection of miRNA Mimics and Inhibitors
In order to determine the biological effects of each individual miRNAs on neurite outgrowth, we performed functional analyses for injury-induced miRNAs. Gain-of-function experiments were performed with Pre-miRNA Precursor Molecules (Biomics Biotechnologies, China), which are also called miRNA mimics. With transfection reagent, these small, chemically modified double-stranded RNA molecules can be introduced into cells and be taken up into the RNA-induced silencing complex (RISC), mimicking endogenous mature miRNAs activity. Loss-of-function analyses were performed with Anti-miRNA inhibitors. The miRNA inhibitors are chemically modified, single-stranded nucleic acids designed to specifically bind to complementary miRNAs. The binding between endogenous miRNA and miRNA inhibitors down-regulates endogenous miRNAs activity.
All miRNA mimics and miRNA inhibitors were obtained from Biomics. Transient transfections of PC12 cells were performed using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s protocol.
Expression Plasmids
To obtain the miR-29c expressing lentiviral vector pLKO-miR-29c, a region of about 60 bp containing the miR-29c DNA sequences were obtained from Sangon Biotech with Forward: ccggtgaccgatttctcctggtgttcctcgaggaacaccaggagaaatcggtcatttttg; Reverse:aattcaaaaatgaccgatttctcctggtgttcctcgaggaacaccaggagaaatcggtca primers and cloned into EcoRI and Age I sites of the lentiviral pLKO.1-puro-vector, purchased by Addgene (Plasmid 8453).The pLKO.1-puro-sh-GFP was used as control with the following primers: forward: ccgggcaagctgaccctgaagttcatctcgagatga-acttcagggtcacgttgctttttg; reverse: aattcaaaaagcaagctgaccctgaagttcatctcgagatgaactcagggtcacgttgc.
Cell Transfection and Lentivirus Infection
PC12 cells were, respectively, infected by lentiviral pLKO-miR-29c vector (PC12-pLKO- miR-29c cells) and lentiviral pLKO-sh-GFP vector (PC12-pLKO-sh-GFP cells) by using polybrene (8 μg ml−1) to medium the membrane surface charge. After that, PC12-pLKO-miR-29c cells and PC12-pLKO-sh-GFP cells were cultured with puromycin (8 μg ml−1).
Luciferase Reporter Assay
miRNA response elements (MREs) or the whole 3′-UTRs of the target genes were cloned into the psiCHECK-2 vector (Promega, USA) between the XhoI and NotI sites immediately 3′ downstream of the Renilla luciferase gene. The top (sense) and bottom (anti-sense) strands of each MRE were designed to contain XhoI and NotI sites, respectively. After synthesis, these were annealed and ligated into the psiCheck-2 vector. A 789-bp segment containing the miR-29c MRE in the 3′-UTRs of PTEN were synthesized as mini-genes with or without seven mismatches (AGCACCA was mutated to ATTATTA) in the seed region of miR-29c MREs and sub-cloned into the psiCHECK-2 vector. 80 ng of each psiCHECK-2 construct was co-transfected with 100nM 29c mimic into HEK-293T cells in a 24-well plate using Lipofectamine 2000 (Invitrogen). After 48 h, the cell extract was obtained, and firefly and Renilla luciferase activities were measured with the dual-luciferase reporter system (Promega, USA) according to the manufacturer’s instructions.
Immunocytochemistry
PC12-pLKO-miR-29c cells and PC12-pLKO-sh-GFP cells at their third culture passage were seeded into the 24-well plate (1 × 104 cells/well), respectively, and cultured as described previously. After 72 h neural induction culture, the medium was removed; cells were washed three times with PBS. Subsequently, the PC12-pLKO-miR-29c cells and PC12-pLKO-sh-GFP cells were fixed with 4 % paraformaldehyde for 2 h at room temperature. After washing three times with PBS, the cells were permeabilized with 0.3 % Triton X-100 in PBS for 10 min at room temperature. Then, the cells were washed three times with PBS and blocked in 5 % normal goat serum for 1 h at RT. PC12- pLKO-miR-29c cells and PC12-pLKO-sh-GFP cells were subsequently incubated for 12 h with the primary antibodies at 4 °C. Primary antibodies were Rabbit anti-GAP 43 (1:200, no: BA0878, BOSTER, China), Mouse anti-neurofilament 200KD (NF200 KD 1:200, no: ab77745, Abcam, UK). After incubation with primary antibody, the cells were washed three times with PBS and incubated with a corresponding secondary antibody for 1 h at RT. Secondary antibodies were Cy3-labeled goat-anti Rabbit-IgG antibody and Cy3-labeled goat-anti Mouse-IgG antibody (1:200, no: BA1032, BOSTER, China). Cell nuclei were stained with Hoechst 33342 (1:1,000, no: H3570, Life Technologies, USA). The control group is standard PC12 cells in culture without neural induction culture. Stained cells were observed using an inverted fluorescence microscope (Leica, Germany).
Statistical Analysis
A Student’s t test was used to determine the significance of differences between the treated samples and the controls where values resulted from quantitative Real-time PCR, or permutation of target prediction. Statistical analysis was performed using Microsoft Excel.
Results
miR-29c Up-Regulated and PTEN Down-Regulated During PC12 Cell Differentiation Induced by NGF
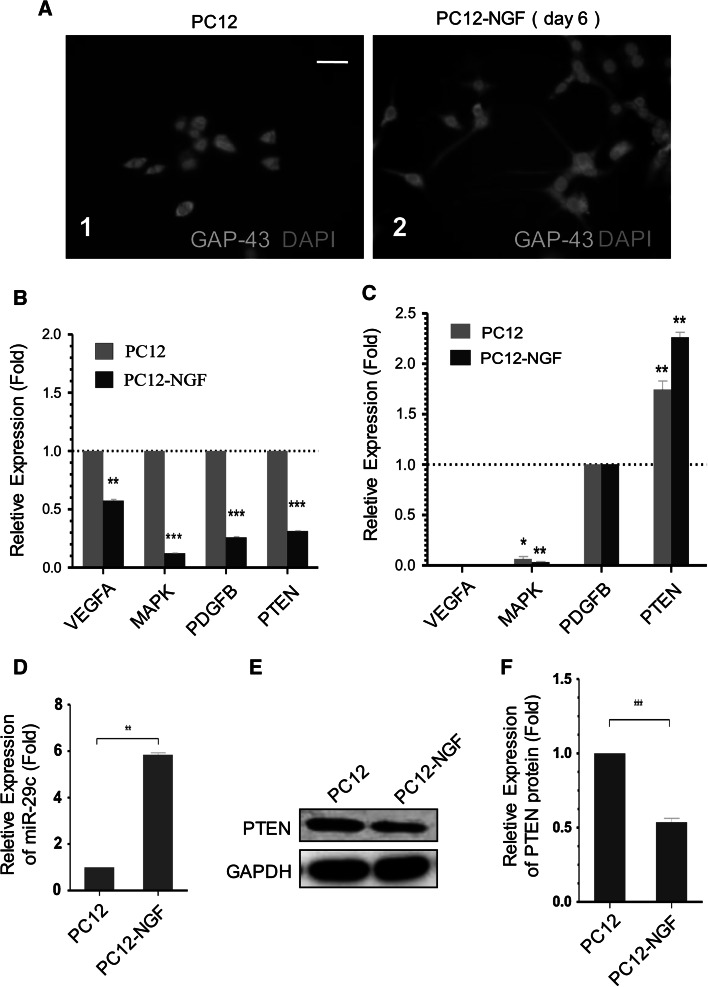
It is demonstrated that NGF, epidermal growth factor (EGF), fibroblast growth factor (FGF), or KCl can induce PC12 cell differentiation and neurite formation, and that NGF is able to lead to a significant neurite outgrowth (Pollock et al. 1990). NGF could induce the cell differentiation, leading to the smaller cell bodies, neurites formation, and proliferation decrease. In the absence of NGF, the cells are relatively rounded and have little visible neuritis (Fig. 1a). When PC12 cells were cultured on 6-well plates in DMEM/F12 containing 5 % FBS and 50 ng/ml NGF, neuritis outgrowth was observed 2 days after induction and achieved the maximal extension in 6 days (Fig. 1a).
Fig. 1.
miR-29c up-regulated and PTEN down-regulated during PC12 Cell differentiation induced by NGF. a Morphological changes of PC12 cells after NGF treatment. Representative immunofluorescence with anti-GAP43 for PC12 cells cultured without NGF (1) displays little visible neuritis. PC12 cells were treated with 50ng/ml NGF for 6 days (2). As shown in the figure, both neuritis length and the number of neuritis-presenting cells gradually increased under the effect of NGF (Scale bar = 50μm). b, c The VEGFA, MAPK3, PDGFB, and PTEN expression level changes during NGF-induced differentiation of PC12 cells. Mean ± SD (n = 4). ***P < 0.001. **P < 0.01. *P < 0.05. d The miR-29c expression level changes during NGF-induced differentiation of PC12 cells. Mean ± SD (n = 4). **P < 0.01. e, f The PTEN expression at protein level between the two kinds of cells and GAPDH served as an internal control. Mean ± SD (n = 4). ***P < 0.001
Prediction of downstream-genes for miR-29c was performed by using Targetscan (http://www.targetscan.org). We applied Targetscan to search the most probable genes against the sequences of miR-29c in databases of rodent transcription. In these predicted genes, those related with neural outgrowth genes were selected as the putative genes for next study. The prediction results are not shown. Among these results, we choose VEGFA, MAPK3, PDGFB, and PTEN for further study. We thus detected the picked-out genes levels 2 days after the addition of 50 ng/ml NGF (Fig. 1b, c). The results indicated that all of these four genes expression levels decreased during NGF-induced differentiation of PC12 cells (Fig. 1b). Among them, previous studies have confirmed that PTEN plays important roles in neural development and differentiation (Maire et al. 2014; Zhang et al. 2012). So we choose PTEN for further study. Within 48 h after NGF treatment, relative mRNA and protein levels of PTEN decrease, approximately, 69 % and half, as compared to the undifferentiated PC12 cells (Fig. 1b, e, f).
Expression Levels of miR-29c and PTEN During Mice Brain Development
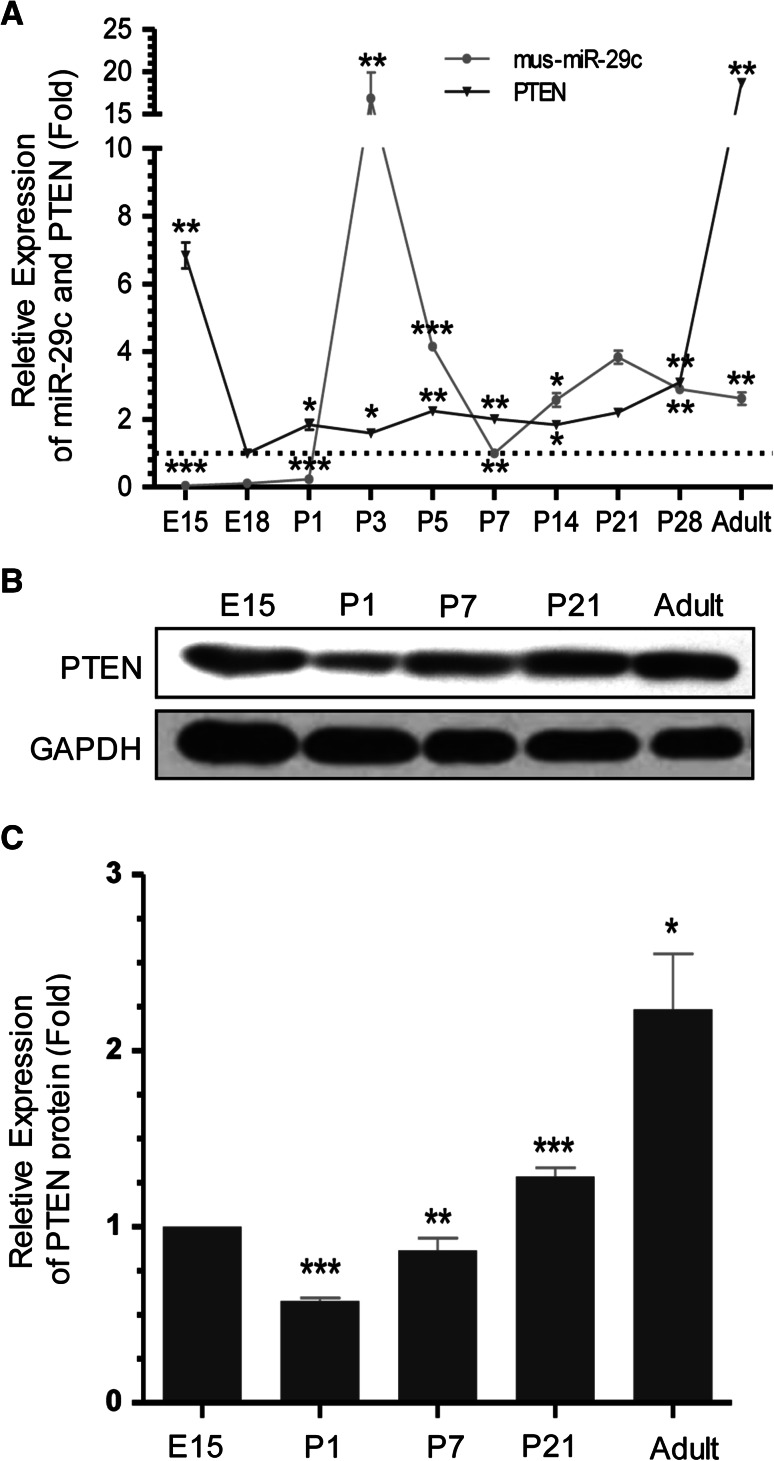
Since previous studies identified miR-29 family to play an important role in neuronal cell survival and muscle differentiation (Roshan et al. 2014; Wei et al. 2013), we wanted to determine if miR-29c was involved in brain development as well. The expression pattern of miR-29c in the central nervous system of mice was examined by RT-PCR and Western blotting. Figure 2a shows the mRNA expression levels of miR-29c and PTEN from embryonic 15 days to adult. MiR-29c was detected at a low expression level during gestation period. However, miR-29c was released after 3 days from birth and reduced at a stable level after that. Moreover, this phenomenon has a negative relationship with the expression of PTEN at mRNA levels. In the mice brain, miR-29c were increased developmentally, however, PTEN expression decreased on E18 in amount (Fig. 2a). After that, we examined the expression of PTEN using Western blot assay in the brain during mice brain development. Figure 2b, c show that the expression of PTEN is at a high expression level during gestation period. However, with the releasing of miR-29c after birth, the expression of PTEN reduces rapidly. After miR-29c reducing at a stable level, the PTEN expression increases at a stable level (Table 1).
Fig. 2.
Expression levels of miR-29c and PTEN during mice brain development. a Real-time PCR analysis shows relative expression of individual members of miR-29c and PTEN from embryonic 15 days to adult; Mean ± SD (n = 4). ***P < 0.001. **P < 0.01. *P < 0.05. b, c The PTEN expression at the protein level was just the same trend with the PTEN mRNA during the mice brain development, and GAPDH served as an internal control. Mean ± SD (n = 4). ***P < 0.001. **P < 0.01. *P < 0.05
Table 1.
The primers for real-time PCR
| Primer | Sequence (5′ to 3′) | Base (bp) |
|---|---|---|
| MAPK3 forward primer | GAGGTCGATGTCCGTGTCA | 19 |
| MAPK3 reverse primer | ATGCGATCTGGGGTTGTC | 18 |
| PDGFB forward primer | CTCCATCCGCTCCTTTGA | 18 |
| PDGFB reverse primer | TTCCGACTCGACTCCAGAAT | 20 |
| VEGFA forward primer | GCTGCTGTAACGATGAAG | 18 |
| VEGFA reverse primer | ATCTGCTGTGCTGTAGGA | 18 |
| PTEN forward primer | AAGGACGGACTGGTGTAA | 18 |
| PTEN reverse primer | CCTGAGTTGGAGGAGTAGAT | 20 |
| mus-GAPDH forward primer | GAAGGGTGGAGCCAAAAG | 18 |
| mus-GAPDH reverse primer | ACCAGTGGATGCAGGGAT | 18 |
| rno-GAPDH forward primer | GCAAGTTCAACGGCACAG | 18 |
| rno-GAPDH reverse primer | ACGCCAGTAGACTCCACGAC | 20 |
| mmu-miR-29c forward | TGACCGATTTCTCCTGGTGTTC | 22 |
| mmu-miR-29c reverse | GCGAGCACAGAATTAATACGAC | 22 |
| rno-miR-29c forward | TGACCGATTTCTCCTGGTGTTC | 22 |
| rno-miR-29c reverse | GCGAGCACAGAATTAATACGAC | 22 |
| u6 forward | CTCGCTTCGGCAGCACA | 17 |
| u6 reverse | GCGAGCACAGAATTAATACGAC | 22 |
Up-Regulation of miR-29c Reduces PTEN Expression in PC12 Cells
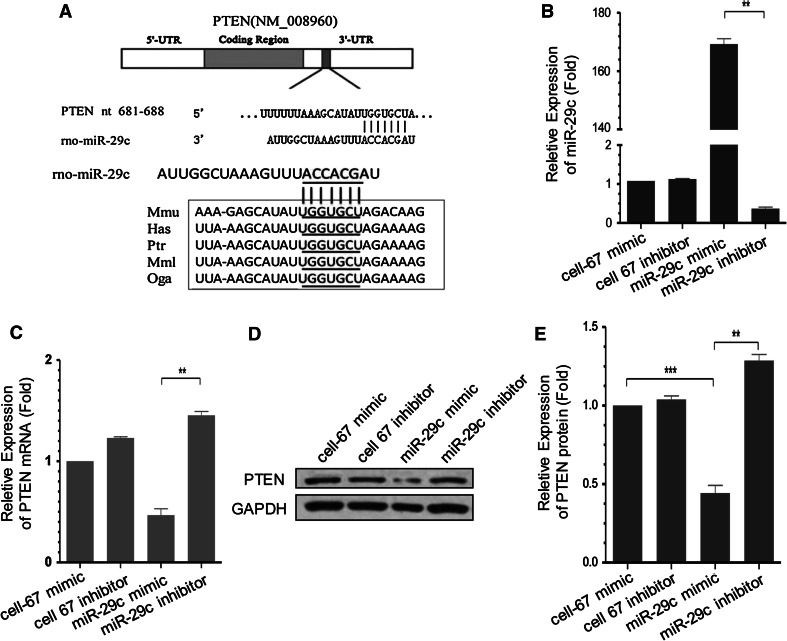
A sequence analysis indicated that the potential miR-29c targeting sequences are located at nt681-688 (Fig. 3a) and nt1741-1747 of the PTEN 3′-UTR, but just nt681-688 of nucleotide sequences are highly conserved across different species (Fig. 3a). The findings prompted us to further validate these target regions in the following functional assays.
Fig. 3.
Up-regulation of miR-29c reduces PTEN expression in PC12 Cells. a Schematic representation of the putative highly conserved miR-29c target sites in PTEN mRNA. The putative miR-29c target sequences at PTEN 3′UTR are highly conserved across different species. The underlined sites are predicted targets for miR-29c within the PTEN 3′UTR. b RT-PCR confirmed the increase of miR-29c level in PC12 cells after the transfection of miR-29c mimic. On the contrary, transfection of miR-29c inhibitor decreased the expression of miR-29c. Mean ± SD (n = 4). **P < 0.01. c miR-29c negatively regulated PTEN expression at mRNA level. Treatment of miR-29c mimics in PC12 cells culture significantly inhibited PTEN expression as compared with that of mimic control groups. On the contrary, suppression of miR-29c activity significantly enhanced the expression of PTEN mRNA. Mean ± SD (n = 4). **P < 0.01. d Western blot analysis of PTEN expression exhibited similar negative correlation of miR-29c and PTEN expression. Cells transfected with miR-29c mimics had decreased protein level of PTEN, whiles cells transfected with miR-29c inhibitors had an increased expression of PTEN. GAPDH was used as the loading control and was used to normalize densitometry values. e The quantification of densitometric levels of PTEN. Mean ± SD (n = 4). **P < 0.01. ***P < 0.001
First, we confirmed that miR-29c levels were altered by transfecting PC12 cells with miR-29c mimic using RT-PCR. MiR-29c mimic increased miR-29c levels 169-fold, while miR-29c inhibitor decreased miR-29c levels by 64 % compared to inhibitor control (Fig. 3b).
Since miRNAs can lead to reduced protein levels either by translational arrest or degradation of mRNA, we assessed levels of PTEN mRNA following transfection, and found that miR-29c mimic reduced PTEN mRNA levels to 54 % (Fig. 3c). On the contrary, miR-29c inhibitors significantly elevated the mRNA level of PTEN (Fig. 3c). These results demonstrated that miR-29c level is inversely correlated to PTEN expression at mRNA level in PC12 cells.
We further examined the expression of PTEN using Western blot assay in PC12 cells. Our data showed that inhibition of endogenous miR-29c by transfection with miR-29c inhibitor resulted in a significant increase of PTEN expression by 28 % (Fig. 3d, e), while miR-29c mimic reduced PTEN protein levels by 56 % compared with the mimic negative control group (Fig. 3d, e).
miR-29c Directly Down-Regulates PTEN Expression
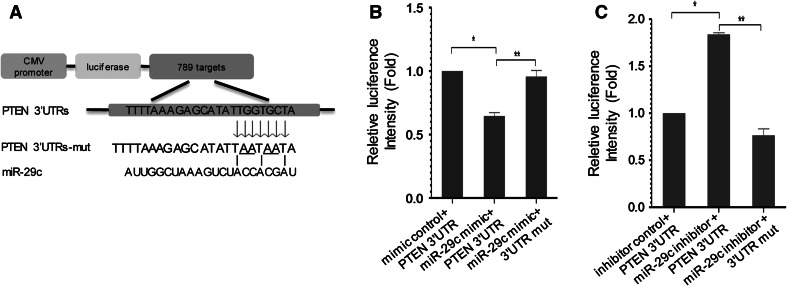
We searched the sequence in the TargetScan and found that the 3′-UTR of the rat PTEN gene contains one separate miR-29c-binding seed sequences that are conserved through evolution. To demonstrate the direct interaction between miR-29c and PTEN mRNA, we then constructed the luciferase reporter system containing the binding site (PTEN-3′-UTR-wt) or mutated site (PTEN-3′-UTR-mut) to downstream of the psiCHECK-2 luciferase reporter gene. This vector was co-transfected into 293T cells with miR-29c mimic or negative controls. Luciferase activity in miR-29c group was decreased markedly compared with negative controls by 36 % (Fig. 4b). MiR-29c mimic did not affect the luciferase activity in the psiCHECK-2-PTEN-mut vector (Fig. 4b). When blocking the expression of miR-29c with miR-29c inhibitor, we get increased luciferase intensity in 293T cells (Fig. 4c). The psiCHECK-2-PTEN-mut vector co-transfected with miR-29c inhibitor did not show changes in luciferase activity in 293T cells (Fig. 4c). These results support the bioinformatics prediction indicating the 3′-UTR of PTEN mRNA as a target for miR-29c.
Fig. 4.
miR-29c directly down-regulates PTEN expression. a Predict PTEN was potential targets for miR-29c and its validation. b, c Luciference reporter assay was performed to detect the effect of miR-29c and anti-sense miR-29c on the luciference intensity controlled by 3′UTR of PTEN. Mean ± SD (n = 4). **P < 0.01. *P < 0.05
The Effect of miR-29c on Axonal Outgrowth in PC12 Cells
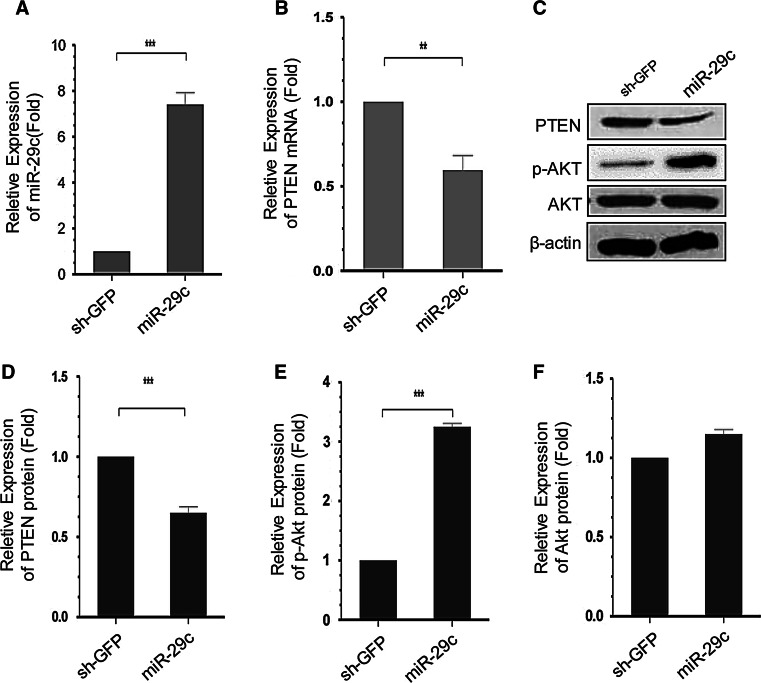
Since PTEN is reported to restrict axon branching, we further investigated the role of miR-29c in neurite growth. First, we generated two stable cell lines: PC12-pLKO-miR-29c and PC12-pLKO-sh-GFP. We then searched for changes in PTEN mRNA and protein levels by RT-PCR and Western blotting. Actually, introduction of miR-29c caused a significant increase of miR-29c expression by 7.4 times compared to control (Fig. 5a) and decreased PTEN mRNA and protein levels, respectively, 41 % (Fig. 5b) and 35 % (Fig. 5c, d). In PC12-pLKO-miR-29c cells, there was a significant decrease in PTEN expression accompanied by up-regulation of AKT phosphorylation 3.2 times compared with control (Fig. 5c, e, f).
Fig. 5.
The effect of miR-29c on PC12 cells. a RT-PCR confirmed the increase of miR-29c level in PC12 cells after transfected the lentivirus of miR-29c vectors. Mean ± SD (n = 4). ***P < 0.001. b miR-29c vectors negatively regulated PTEN expression at mRNA level. PC12-pLKO-miR-29c cells cultures significantly inhibited PTEN expression as compared with that of PC12-pLKO-sh-GFP cells. Mean ± SD (n = 4). **P < 0.01. c Western blot analysis of PTEN expression exhibited similar negative correlation of miR-29c and PTEN expression. PC12-pLKO-miR-29c cells had decreased protein level of PTEN and increased protein level of p-Akt, but did not change the total Akt level. GAPDH was used to normalize densitometry values. d, f The quantification of densitometric levels of PTEN, p-Akt, and Akt. Mean ± SD (n = 4). ***P < 0.001
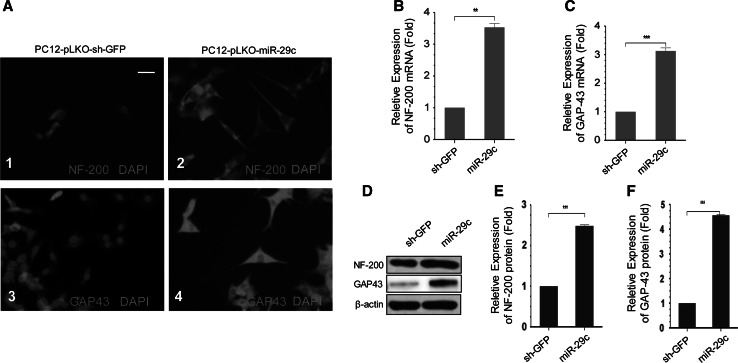
As a strong association between neurite outgrowth and expression of GAP-43 and NF-200 has been reported in previous studies (Benowitz et al. 1997; Lin et al. 2014), we next studied GAP-43 and NF-200 expression in PC12-sh-GFP cells and PC12-miR-29c cells. We observed significant increase in GAP-43 and NF-200 immunostaining caused in PC12-pLKO-miR-29c cells (Fig. 6a). GAP-43 mRNA level was further studied with RT-PCR. Figure 6b, c clearly demonstrated a significant increase in GAP-43 and NF-200 mRNA in PC12-pLKO-miR-29c cells, as compared to the PC12-pLKO-sh-GFP cells. To verify the RT-PCR result, Western blot analysis was performed. PC12-pLKO-miR-29c cells expressed NF-200 and GAP-43 protein at least twice and fourfold as much as PC12-pLKO-sh-GFP cells, respectively (Fig. 6d, f). It is consistent with immune fluorescent data demonstrating significant increase in axon outgrowth after over-expression of miR-29c.
Fig. 6.
The effect of miR-29c on axonal outgrowth. a Representative immunofluorescence with anti-GAP43 and anti-NF-200 for PC12-pLKO-miR-29c (2 and 4) and PC12-pLKO-sh-GFP cells (1 and 3) after 72 h culture. Mean ± SD (n = 4). Scale bar = 50 μm. b, c RT-PCR showed that PC12-pLKO-miR-29c expressed NF-200, GAP-43 higher than PC12-pLKO-sh-GFP cells after the 72 h culture. Mean ± SD (n = 4). **P < 0.01. ***P < 0.001. d Western blot analysis represents the protein bands on the gel for PC12-pLKO-miR-29c and PC12-pLKO-sh-GFP cells. There were significant differences in neural protein expression. e, f The quantification of densitometry levels of GAP-43 and NF-200. According to the quantification, the cells probably co-express some markers. Mean ± SD (n = 4). ***P < 0.001
Discussion
In the current study, we demonstrate the function of miR-29c/PTEN pathway on neurite outgrowth in PC12 cells and the existence of miR-29c/PTEN pathway during mice brain development. Our findings provide the first evidence that miR-29c has an important role in neurite outgrowth and mice brain development via down-regulating PTEN expression.
Emerging evidences indicate that miRNAs are important mediators of axonal regeneration and neurodevelopment (Hong et al. 2014; Sun et al. 2014). Since NGF is an important mediator leading to a significant neurite outgrowth in neurons, many studies explore the expression profile of miRNAs under NGF treatment, such as miR-17-92 cluster and miR-29 cluster (Montalban et al. 2014; Zhang et al. 2013). Since mature miR-29s share identical sequences at nucleotide positions 2–7, the seed region plays a key role in determining which protein-coding genes a miRNA would target. In this study, we find that miR-29c up-regulate by NGF-induced differentiation in PC12 cells. Previous studies show that miR-29a could regulate migration of hepatoma cells mediated by Hepatitis B virus X protein (HBx) through modulation of Akt phosphorylation (Kong et al. 2011). Moreover, Tumaneng et al. have already confirmed that miR-29c down-regulates PTEN protein by targeting the PTEN 3′UTR in the eventual increase of organ size. Further study showed that YAP mediated this process by binding the promoter region of miR-29c, particularly the region 150-300 bp upstream of the transcription start site that harbors a TEAD consensus site (Tumaneng et al. 2012). Our findings further confirm that miR-29c could promote neural outgrowth by down-regulating PTEN and increasing Akt phosphorylation for the first time.
Although neurite outgrowth is accompanied by altered transcriptions of more than 1,000 genes, recent studies demonstrate that miRNAs and its downstream participate in neuron development, proliferation, and differentiation. For example, miR-124 represses ROCK1 expression to promote neurite elongation through the activation of the PI3K/Akt signal pathway (Gu et al. 2014), while miR-26a promotes neurite outgrowth by repressing PTEN expression (Li et al. 2013). In this report, we exhibited that miR-29c could directly decrease the expression of PTEN, increase phosphorylation of Akt, active Akt pathway, and promote neurite outgrowth.
Previous studies have examined the effects of PTEN on neuronal differentiation. To date, the few studies have consistently found that PTEN deletions do not prevent neuronal differentiation but can alter neuronal morphology, proliferation, and migration (Izumi et al. 2011; Lachyankar et al. 2000). It is generally known that PTEN is important for central axon growth (Park et al. 2008). Loss of PTEN expression is associated with cerebellar dysplasia in human patients (Liaw et al. 1997), and conditional knockout mouse studies have also demonstrated dysplasia and migrational defects (Liaw et al. 1997; Backman et al. 2001). All in all, our results confirm that miR-29c could promote neurite outgrowth via targeting on PTEN. Although it is too early to speculate on the potential clinical use of miRNAs, it gives a promising research hint. The results of the present study could propose a possible scenario that over-expression of miR-29c affects neurite outgrowth by down-regulating PTEN and other miR-29c targets. However, the underlying mechanisms require further investigation.
Taken together, we showed that miR-29c could regulate neurite outgrowth and play important roles in mice brain development by targeting PTEN, a major inhibitor of axonal growth. We also noted that knockdown of PTEN by miR-29c might promote Akt phosphorylation to improve the outgrowth ability of neurite. It is suggested that miR-29c is an important regulator of neurite outgrowth via targeting PTEN and has a promising therapeutic target for neural disease.
Acknowledgments
This study was supported by Grants from the Changzhou Science & Technology Bureau (CJ20122027) and the National Science Foundation of China (8137218, 81471263).
Conflict of interest
We state that the manuscript, or parts of it, have not been and will not be submitted elsewhere for publication. Hongjun Zou, Ya Ding, Weifeng Shi, Xu Xu, Aihua Gong, Zhijian Zhang, Jinbo Liu have agreed to the submission of the final manuscript and declare no conflicts of interest.
Footnotes
An erratum to this article is available at http://dx.doi.org/10.1007/s10571-017-0493-1.
References
- Backman SA et al (2001) Deletion of pten in mouse brain causes seizures, ataxia and defects in soma size resembling Lhermitte-duclos disease. Nat Genet 29:396–403 [DOI] [PubMed] [Google Scholar]
- Bartel DP (2009) Micrornas: target recognition and regulatory functions. Cell 136:215–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz LI et al (1997) Gap-43: an intrinsic determinant of neuronal development and plasticity. Trends Neurosci 20:84–91 [DOI] [PubMed] [Google Scholar]
- Chang TC et al (2007) Micrornas in vertebrate physiology and human disease. Annu Rev Genomics Hum Genet 8:215–239 [DOI] [PubMed] [Google Scholar]
- Esquela-Kerscher A et al (2006) Oncomirs: micrornas with a role in cancer. Nat Rev Cancer 6:259–269 [DOI] [PubMed] [Google Scholar]
- Fan Y et al (2014) Down-regulation of mir-29c in human bladder cancer and the inhibition of proliferation in t24 cell via pi3k-akt pathway. Med Oncol 31:65 [DOI] [PubMed] [Google Scholar]
- Gu X et al (2014) Mir-124 represses rock1 expression to promote neurite elongation through activation of the pi3k/akt signal pathway. J Mol Neurosci 52:156–165 [DOI] [PubMed] [Google Scholar]
- Hong P et al (2014) Functional requirement of dicer1 and mir-17-5p in reactive astrocyte proliferation after spinal cord injury in the mouse. Glia 62:2044–2060 [DOI] [PubMed]
- Izumi B et al (2011) Microrna-223 expression in neutrophils in the early phase of secondary damage after spinal cord injury. Neurosci Lett 492:114–118 [DOI] [PubMed] [Google Scholar]
- Kong G et al (2011) Upregulated microrna-29a by hepatitis b virus × protein enhances hepatoma cell migration by targeting pten in cell culture model. PLoS One 6:e19518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegel AJ et al (2012) The mir-29 family: genomics, cell biology, and relevance to renal and cardiovascular injury. Physiol Genomics 44:237–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachyankar MB et al (2000) A role for nuclear pten in neuronal differentiation. J Neurosci 20:1404–1413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B et al (2013) Mir-26a promotes neurite outgrowth by repressing pten expression. Mol Med Rep 8:676–680 [DOI] [PubMed] [Google Scholar]
- Liaw D et al (1997) Germline mutations of the pten gene in cowden disease, an inherited breast and thyroid cancer syndrome. Nat Genet 16:64–67 [DOI] [PubMed] [Google Scholar]
- Lin W et al (2014) Bone marrow stromal cells promote neurite outgrowth of spinal motor neurons by means of neurotrophic factors in vitro. Neurol Sci 35:449–457 [DOI] [PubMed] [Google Scholar]
- Maire CL et al (2014) Pten loss in olig2 expressing neural progenitor cells and oligodendrocytes leads to interneuron dysplasia and leukodystrophy. Stem Cells 32:313–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montalban E et al (2014) Mir-21 is an ngf-modulated microrna that supports ngf signaling and regulates neuronal degeneration in pc12 cells. Neuromol Med 16:415–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KK et al (2008) Promoting axon regeneration in the adult cns by modulation of the pten/mtor pathway. Science 322:963–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock JD et al (1990) Differential effects of ngf, fgf, egf, camp, and dexamethasone on neurite outgrowth and sodium channel expression in pc12 cells. J Neurosci 10:2626–2637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roshan R et al (2014) Brain-specific knockdown of mir-29 results in neuronal cell death and ataxia in mice. RNA 20:1287–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta S et al (2008) Microrna 29c is down-regulated in nasopharyngeal carcinomas, up-regulating mrnas encoding extracellular matrix proteins. Proc Natl Acad Sci USA 105:5874–5878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun E et al (2014) Micrornas: small molecules with big roles in neurodevelopment and diseases. Exp Neurol. doi:10.1016/j.expneurol.2014.08.005 [DOI] [PubMed]
- Tumaneng K et al (2012) Yap mediates crosstalk between the hippo and pi(3)k-tor pathways by suppressing pten via mir-29. Nat Cell Biol 14:1322–1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H et al (2008) Nf-kappab-yy1-mir-29 regulatory circuitry in skeletal myogenesis and rhabdomyosarcoma. Cancer Cell 14:369–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W et al (2013) Mir-29 targets akt3 to reduce proliferation and facilitate differentiation of myoblasts in skeletal muscle development. Cell Death Dis 4:e668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X et al (2014) Mir-29b protects dorsal root ganglia neurons from diabetic rat. Cell Biochem Biophys 70:1105–1111 [DOI] [PubMed]
- Zhang Y et al (2012) Pten deletion enhances survival, neurite outgrowth and function of dopamine neuron grafts to mitopark mice. Brain 135:2736–2749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y et al (2013) The microrna-17-92 cluster enhances axonal outgrowth in embryonic cortical neurons. J Neurosci 33:6885–6894 [DOI] [PMC free article] [PubMed] [Google Scholar]