Abstract
Heparan sulphate and dermatan sulphate have both antithrombotic and anticoagulant properties. These are, however, significantly weaker than those of a comparable amount of standard pig mucosal heparin. Antithrombotic and anticoagulant effects of glycosaminoglycans depend on their ability to catalyse the inhibition of thrombin and/or to inhibit the activation of prothrombin. Since heparan sulphate and dermatan sulphate are less sulphated than unfractionated heparin, we investigated whether the decreased sulphation contributes to the lower antithrombotic and anticoagulant activities compared with standard heparin. To do this, we compared the anticoagulant activities of heparan sulphate and dermatan sulphate with those of their derivatives resulphated in vitro. The ratio of sulphate to carboxylate in these resulphated heparan sulphate and dermatan sulphate derivatives was approximately twice that of the parent compounds and similar to that of standard heparin. Anticoagulant effects were assessed by determining (a) the catalytic effects of each glycosaminoglycan on the inhibition of thrombin added to plasma, and (b) the ability of each glycosaminoglycan to inhibit the activation of 125I-prothrombin in plasma. The least sulphated glycosaminoglycans were least able to catalyse the inhibition of thrombin added to plasma and to inhibit the activation of prothrombin. Furthermore, increasing the degree of sulphation improved the catalytic effects of glycosaminoglycans on the inhibition of thrombin by heparin cofactor II in plasma. The degree of sulphation therefore appears to be an important functional property that contributes significantly to the anticoagulant effects of the two glycosaminoglycans.
Full text
PDF

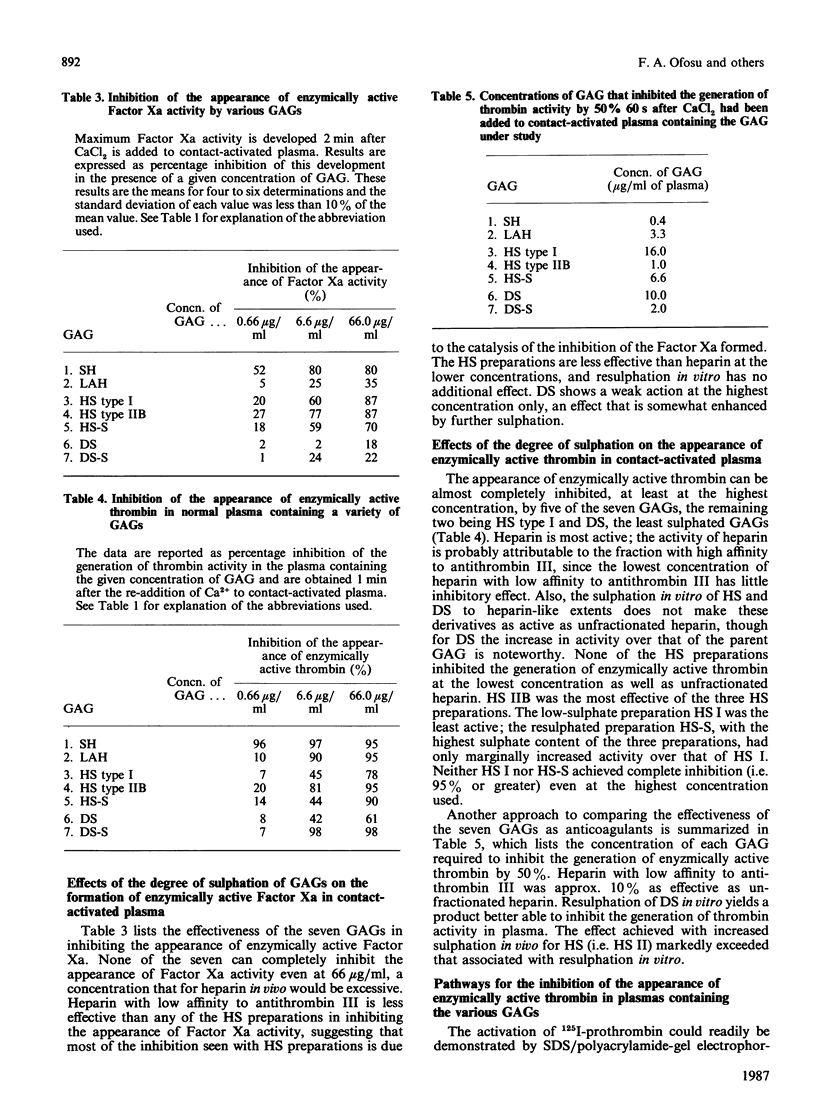
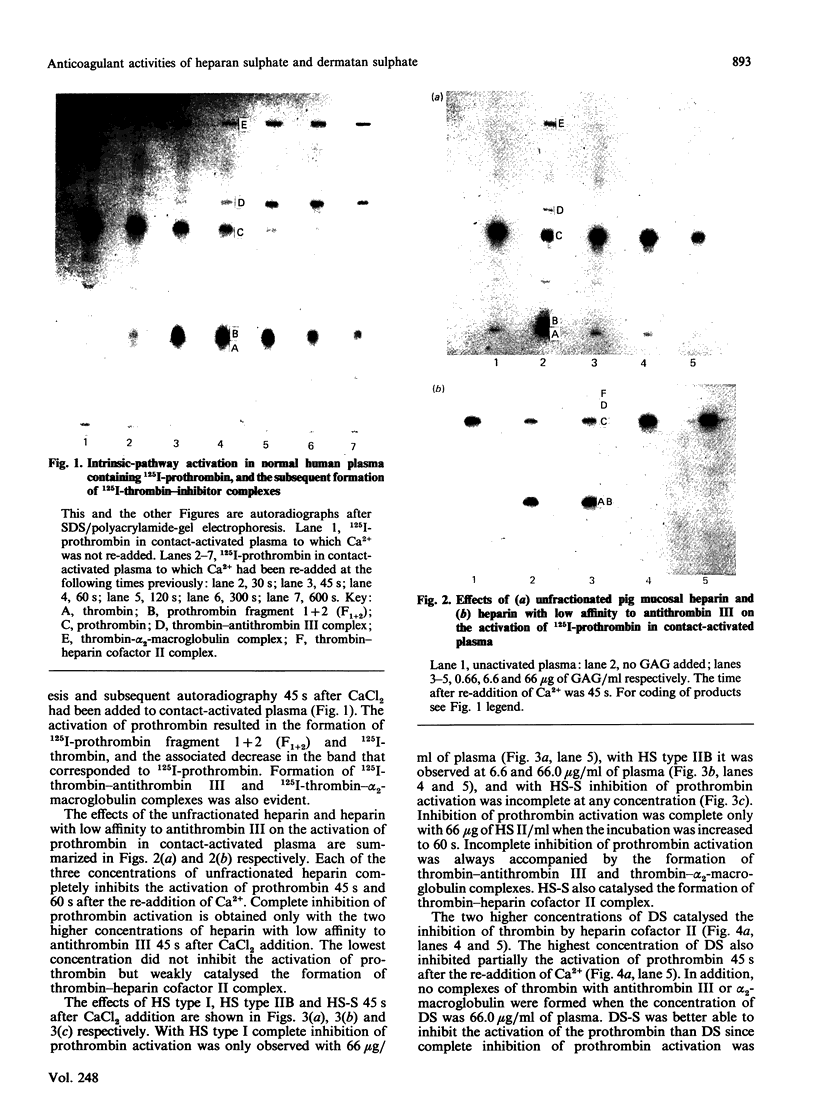
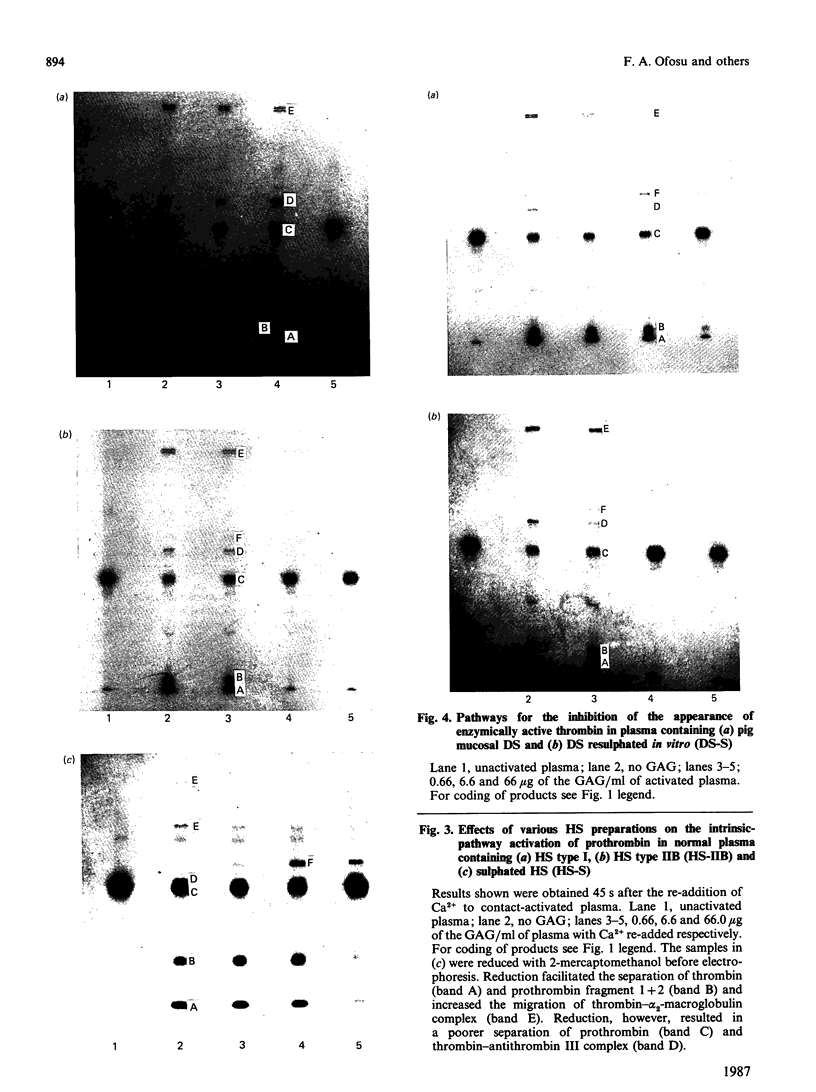


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bjornsson T. D., Nash P. V., Schaten R. The anticoagulant effect of chondroitin-4-sulfate. Thromb Res. 1982 Jul 1;27(1):15–21. doi: 10.1016/0049-3848(82)90273-0. [DOI] [PubMed] [Google Scholar]
- Buchanan M. R., Boneu B., Ofosu F., Hirsh J. The relative importance of thrombin inhibition and factor Xa inhibition to the antithrombotic effects of heparin. Blood. 1985 Jan;65(1):198–201. [PubMed] [Google Scholar]
- Casu B., Gennaro U. A conductimetric method for the determination of sulphate and carboxyl groups in heparin and other mucopolysaccharides. Carbohydr Res. 1975 Jan;39(1):168–176. doi: 10.1016/s0008-6215(00)82654-3. [DOI] [PubMed] [Google Scholar]
- Casu B., Johnson E. A., Mantovani M., Mulloy B., Oreste P., Pescador R., Prino G., Torri G., Zoppetti G. Correlation between structure, fat-clearing and anticoagulant properties of heparins and heparan sulphates. Arzneimittelforschung. 1983;33(1):135–142. [PubMed] [Google Scholar]
- Casu B. Structure and biological activity of heparin. Adv Carbohydr Chem Biochem. 1985;43:51–134. doi: 10.1016/s0065-2318(08)60067-0. [DOI] [PubMed] [Google Scholar]
- Cerskus A. L., Birchall K. J., Ofosu F. A., Hirsh J., Blajchman M. A. Effects of heparin fractions of different affinities to antithrombin III and thrombin on the inactivation of thrombin and factor Xa by antithrombin III. Can J Biochem Cell Biol. 1984 Oct;62(10):975–983. doi: 10.1139/o84-125. [DOI] [PubMed] [Google Scholar]
- Choay J., Petitou M., Lormeau J. C., Sinaÿ P., Casu B., Gatti G. Structure-activity relationship in heparin: a synthetic pentasaccharide with high affinity for antithrombin III and eliciting high anti-factor Xa activity. Biochem Biophys Res Commun. 1983 Oct 31;116(2):492–499. doi: 10.1016/0006-291x(83)90550-8. [DOI] [PubMed] [Google Scholar]
- GROSSMAN B. J., DORFMAN A. In vitro comparison of the antithrombic action of heparin and chondroitinsulfuric acid-B. Pediatrics. 1957 Sep;20(3):506–514. [PubMed] [Google Scholar]
- Hopwood J., Hök M., Linker A., Lindahl U. Anticoagulant activity of heparin: isolation of antithrombin-binding sites. FEBS Lett. 1976 Oct 15;69(1):51–54. doi: 10.1016/0014-5793(76)80651-5. [DOI] [PubMed] [Google Scholar]
- Hök M., Kjellén L., Johansson S. Cell-surface glycosaminoglycans. Annu Rev Biochem. 1984;53:847–869. doi: 10.1146/annurev.bi.53.070184.004215. [DOI] [PubMed] [Google Scholar]
- Izuka K., Murata K. Inhibitory effects of human aortic and venous acid glycosaminoglycans on thrombus formation. Atherosclerosis. 1972 Sep-Oct;16(2):217–224. doi: 10.1016/0021-9150(72)90055-x. [DOI] [PubMed] [Google Scholar]
- Jacobsson I., Lindahl U., Jensen J. W., Rodén L., Prihar H., Feingold D. S. Biosynthesis of heparin. Substrate specificity of heparosan N-sulfate D-glucuronosyl 5-epimerase. J Biol Chem. 1984 Jan 25;259(2):1056–1063. [PubMed] [Google Scholar]
- Johnson E. A. Characterisation and separation of sulphated glycosaminoglycuronans. Pharmacol Res Commun. 1982 Apr;14(4):289–320. doi: 10.1016/s0031-6989(82)80100-8. [DOI] [PubMed] [Google Scholar]
- Johnson E. A. Heparan sulphates from porcine intestinal mucosa. Preparation and physicochemical properties. Thromb Res. 1984 Sep 1;35(5):583–588. doi: 10.1016/0049-3848(84)90290-1. [DOI] [PubMed] [Google Scholar]
- Johnson E. A., Paterson M. S. Lead precipitation: an aid to separation of dermatan and mucokeratan sulfates from glycosaminoglycan mixtures. Anal Biochem. 1986 Oct;158(1):111–116. doi: 10.1016/0003-2697(86)90597-x. [DOI] [PubMed] [Google Scholar]
- KIRK J. E. Anticoagulant activity of human arterial mucopolysaccharides. Nature. 1959 Aug 1;184(Suppl 6):369–370. doi: 10.1038/184369a0. [DOI] [PubMed] [Google Scholar]
- Lasker S. E. The heterogeneity of heparins. Fed Proc. 1977 Jan;36(1):92–97. [PubMed] [Google Scholar]
- Lindahl U., Hök M. Glycosaminoglycans and their binding to biological macromolecules. Annu Rev Biochem. 1978;47:385–417. doi: 10.1146/annurev.bi.47.070178.002125. [DOI] [PubMed] [Google Scholar]
- Marciniak E. Factor-Xa inactivation by antithrombin. 3. Evidence for biological stabilization of factor Xa by factor V-phospholipid complex. Br J Haematol. 1973 Mar;24(3):391–400. doi: 10.1111/j.1365-2141.1973.tb01662.x. [DOI] [PubMed] [Google Scholar]
- Modi G. J., Blajchman M. A., Ofosu F. A. The isolation of prothrombin, Factor IX and Factor X from human Factor IX concentrates. Thromb Res. 1984 Dec 15;36(6):537–547. doi: 10.1016/0049-3848(84)90193-2. [DOI] [PubMed] [Google Scholar]
- Morrison L. M., Rucker P. G., Ershoff B. H. Prolongation of thrombus-formation time in rabbits given chondroitin sulfate A. J Atheroscler Res. 1968 Mar-Apr;8(2):319–327. doi: 10.1016/s0368-1319(68)80066-3. [DOI] [PubMed] [Google Scholar]
- Murata K., Nakazawa K., Hamai A. Distribution of acidic glycosaminoglycans in the intima, media and adventitia of bovine aorta and their anticoagulant properties. Atherosclerosis. 1975 Jan-Feb;21(1):93–103. doi: 10.1016/0021-9150(75)90096-9. [DOI] [PubMed] [Google Scholar]
- Ofosu F. A., Blajchman M. A., Modi G. J., Smith L. M., Buchanan M. R., Hirsh J. The importance of thrombin inhibition for the expression of the anticoagulant activities of heparin, dermatan sulphate, low molecular weight heparin and pentosan polysulphate. Br J Haematol. 1985 Aug;60(4):695–704. doi: 10.1111/j.1365-2141.1985.tb07474.x. [DOI] [PubMed] [Google Scholar]
- Ofosu F. A., Blajchman M. A., Modi G., Cerskus A. L., Hirsh J. Activation of factor X and prothrombin in antithrombin-III depleted plasma: the effects of heparin. 1981 Aug 15-Sep 1Thromb Res. 23(4-5):331–345. doi: 10.1016/0049-3848(81)90194-8. [DOI] [PubMed] [Google Scholar]
- Ofosu F. A., Cerskus A. L., Hirsh J., Smith L. M., Modi G. J., Blajchman M. A. The inhibition of the anticoagulant activity of heparin by platelets, brain phospholipids, and tissue factor. Br J Haematol. 1984 Jun;57(2):229–238. [PubMed] [Google Scholar]
- Ofosu F. A., Modi G. J., Hirsh J., Buchanan M. R., Blajchman M. A. Mechanisms for inhibition of the generation of thrombin activity by sulfated polysaccharides. Ann N Y Acad Sci. 1986;485:41–55. doi: 10.1111/j.1749-6632.1986.tb34566.x. [DOI] [PubMed] [Google Scholar]
- Ofosu F. A., Modi G. J., Smith L. M., Cerskus A. L., Hirsh J., Blajchman M. A. Heparan sulfate and dermatan sulfate inhibit the generation of thrombin activity in plasma by complementary pathways. Blood. 1984 Sep;64(3):742–747. [PubMed] [Google Scholar]
- Ofosu F. A., Modi G., Cerskus A. L., Hirsh J., Blajchman M. A. Heparin with low affinity to antithrombin III inhibits the activation of prothrombin in normal plasma. Thromb Res. 1982 Nov 15;28(4):487–497. doi: 10.1016/0049-3848(82)90165-7. [DOI] [PubMed] [Google Scholar]
- Ofosu F. A., Sie P., Modi G. J., Fernandez F., Buchanan M. R., Blajchman M. A., Boneu B., Hirsh J. The inhibition of thrombin-dependent positive-feedback reactions is critical to the expression of the anticoagulant effect of heparin. Biochem J. 1987 Apr 15;243(2):579–588. doi: 10.1042/bj2430579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg R. D., Armand G., Lam L. Structure-function relationships of heparin species. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3065–3069. doi: 10.1073/pnas.75.7.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. P., Merton R. E., Barrowcliffe T. W., Thunberg L., Lindahl U. Effects of heparin oligosaccharides with high affinity for antithrombin III in experimental venous thrombosis. Thromb Haemost. 1982 Jun 28;47(3):244–248. [PubMed] [Google Scholar]
- Tollefsen D. M., Blank M. K. Detection of a new heparin-dependent inhibitor of thrombin in human plasma. J Clin Invest. 1981 Sep;68(3):589–596. doi: 10.1172/JCI110292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh P. N., Biggs R., Gagnatelli G. Platelet antiheparin activity. Assay based on factor-Xa inactivation by heparin and antifactor Xa. Br J Haematol. 1974 Mar;26(3):405–419. doi: 10.1111/j.1365-2141.1974.tb00482.x. [DOI] [PubMed] [Google Scholar]