Abstract
The retroviral RNA genome is dimeric, consisting of two identical strands of RNA linked near their 5′ ends by a dimer linkage structure. Previously it was shown that human foamy virus (HFV) RNA transcribed in vitro contained three sites, designated SI, SII, and SIII, which contributed to the dimerization process (O. Erlwein, D. Cain, N. Fischer, A. Rethwilm, and M. O. McClure, Virology 229:251–258, 1997). To characterize these sites further, a series of mutants were designed and tested for their ability to dimerize in vitro. The primer binding site and a G tetrad in SI were dispensable for dimerization. However, a mutant that changed the 3′ end of SI migrated slower on nondenaturing gels than wild-type RNA dimers. The sequence composition of the SII palindrome, consisting of 10 nucleotides, proved to be critical for in vitro dimerization, since mutations within this sequence or replacement of the sequence with a different palindrome of equal length impaired in vitro dimerization. The length of the palindrome also seems to play an important role. A moderate extension to 12 nucleotides was tolerated, whereas an extension to 16 nucleotides or more impaired dimerization. When nucleotides flanking the palindrome were mutated in a random fashion, dimerization was unaffected. Changing the SIII sequence also led to decreased dimer formation, confirming its contribution to the dimerization process. Interesting mutants were cloned into the infectious molecular clone of HFV, HSRV-2, and were transfected into BHK-21 cells. Mutations in SII that reduced dimerization in vitro also abolished virus replication. In contrast, constructs containing mutations in SI and SIII replicated to some extent in cell culture after an initial drop in viral replication. Analysis of the SIM1 mutant revealed reversion to the wild type but with the insertion of an additional two nucleotides. Analysis of cell-free virions demonstrated that both replication-competent and replication-defective mutants packaged nucleic acid. Thus, efficient dimerization is a critical step for HFV to generate infectious virus, but HFV RNA dimerization is not a prerequisite for packaging.
Foamy viruses (spumaviruses) are a subfamily of the family Retroviridae. Although they have a similar genomic structure to other retroviruses and replication is characterized by reverse transcription and provirus integration (17, 53), the foamy virus life cycle is quite divergent from that of other retroviruses (reviewed in reference 38). For example, expression of the Pol protein differs in that it is expressed from its own spliced mRNA rather than as a Gag-Pol fusion protein (16, 61), both Gag and Env proteins are needed for particle release (2, 21, 50), and vectors based on foamy virus sequences need nucleotides in the pol open reading frame (ORF) in addition to nucleotides in the 5′ end for transduction (19, 28, 58). Atypically for retroviruses, but like hepadnaviruses, reverse transcription is a late event in the human foamy virus (HFV) life cycle, resulting in a considerable number of cell-free virions containing full-length infectious DNA (38, 42, 61).
The retroviral genome consists of two identical copies of RNA associated noncovalently at the 5′ ends by a dimer linkage sequence (DLS) (3, 32, 44). The mechanism of dimerization is still not fully understood, although two models have been suggested. The kissing-loop model, first proposed for human immunodeficiency virus type 1 (HIV-1), involves a palindromic sequence in a hairpin-loop structure called the dimer initiation sequence. It was proposed that the palindromic sequence initiates dimerization through a Watson-Crick base pairing to form an immature RNA dimer (27, 34, 49, 55). This mechanism has also been proposed for avian leukosis sarcoma virus (23), HIV-2 (12), simian immunodeficiency virus (12), and murine leukemia virus (MLV) (22, 26, 43) and may represent a common retroviral dimerization mechanism. Following particle release and protein maturation, the nucleocapsid protein is thought to mediate dimer maturation through conformational changes resulting in a more extensive and stable dimer (14, 20, 24).
Alternatively, purine-rich motifs or guanine stretches may be involved in dimerization through the formation of purine-base tetrads stabilized by monovalent cations (1, 41, 56). In fact, G-rich sequences are important for the dimerization of Moloney murine sarcoma virus RNA (39). For other retroviruses, however, mutation of the purine-rich motifs indicates that they are not essential for dimerization (4, 6, 30, 54).
Conservation of RNA dimerization suggests that the process is biologically important in the virus life cycle. This is supported by the fact that HIV-1 DLS mutations lead to replication defects in vivo, particularly in packaging (13, 36, 48). Moreover, the DLS is located in a region of the genome that also encodes important regulatory features, such as the primer binding site (PBS), the major splice donor, the gag start codon, and cis-acting sequences for packaging. Thus, RNA dimerization may play a role in reverse transcription, translation, and encapsidation of viral genomic RNA.
Dimerization was initially studied using short RNA sequences transcribed in vitro (8, 14, 51). More recently, the dimerization of HIV-1 has been studied in vivo (5, 27, 36, 48). While the kissing stem-loop is crucial for in vitro dimerization of both MLV and HIV-1, it is involved in, but not essential for, viral replication in vivo (5, 13, 22, 27, 48).
It was recently demonstrated that three sites (SI, SII, and SIII) are involved in the in vitro dimerization of HFV RNA (18). These sites were located within a 159-nucleotide RNA fragment at the 5′ end of the genome. SI overlaps the PBS, SII contains the palindromic sequence UCCCUAGGGA, and SIII flanks the start codon of the gag ORF. In the present study, mutations were introduced into SI, SII, and SIII and the mutated RNA was tested for its ability to dimerize in vitro. Interesting mutations were also introduced into the infectious molecular clone of HFV, HSRV-2 (52). Analysis of protein expression and virus titer revealed that mutations that reduced dimerization in vitro also inhibited virus spread in cell culture, indicating the importance of the dimerization process in the virus life cycle. When these mutants were analyzed for their nucleic acid content, it was found that they all could package the viral genome. These data confirm that the DLS plays an important role in the virus life cycle but indicate that at least in the case of HFV, dimerization is not a prerequisite for packaging.
MATERIALS AND METHODS
Cell lines.
The baby hamster kidney (BHK-21) cell line and its derivative, FAB (59), were cultured in Dulbecco's modified Eagle's minimal essential medium supplemented with 5% fetal calf serum, 25,000 U of penicillin per ml, and 250 μg of streptomycin per ml. FAB cells are BHK-21 cells containing a single integrated copy of the β-Gal gene under the control of the HFV long terminal repeat promoter. BHK/Bel-1 cells (7) were cultured in the same medium containing G418 to a final concentration of 0.5 μg/μl.
Plasmid construction.
All cloning was done using standard procedures. Plasmids for directing expression of the HFV dimerization region were constructed using the pSPT322-950 vector previously described (18), which was used to generate in vitro RNA transcripts. These mutants are depicted in Table 1. Several SI mutants (SIM1, SIM2, SIM3, SIM4) and SII mutants (SIIM1, SIIM2, SIIM3, SIIM4) were constructed by ligating annealed complementary oligonucleotides (0.5 nmol) containing the required mutations into pSPT322-950 digested with MunI (position 346 of the viral RNA) and StyI (position 398). SIM5 was constructed by deleting the sequences between MunI (position 342) and StyI (position 398), resulting in a 51-bp deletion. SIM6 was constructed by deleting the sequences between HpaI (position 360) from SIM3 and StyI (position 402), resulting in a 38-bp deletion. The mutant SIIM5 was constructed by digesting pSPT322-950 with StyI (position 398) and XhoI (position 946). A 5′ primer complementary to positions 398 to 430 of the proviral DNA but containing the nucleotide changes CCTCC to TTCTT (406 to 410) and a 3′ primer complementary to positions 946 to 910 (containing an XhoI site at the 3′ end) were used to amplify a 548-nucleotide fragment using standard PCR techniques (18). The resulting DNA fragment was inserted into the vector pSPT322-950 to create SIIM5. Constructing SIIM6 required a two-step cloning procedure. DNA was amplified from positions 406 to 946 and inserted into pSPT322-950 via EcoRI and SacI. The resulting PCR product was inserted into the pSPT322-950 vector via EcoR1 and Sac1 restriction sites. A second PCR fragment was amplified from positions 319 to 395 and joined to the intermediate construct via EcoRI and NaeI sites to create S11M6. Construction of SIIM7 required a two-step cloning procedure. First, sequences after the SII palindrome (positions 406 to 946) were PCR amplified, changing the palindrome into a BstBI site (TTCGAA), and the amplicon was inserted into pSPT322-950. Second, the sequences upstream of the palindrome (319 to 395) were PCR amplified. SIIM7 was then created by joining the two PCR products via the BstBI site. SIIM8, which changed the five nucleotides 3′ to the palindrome, was constructed by annealing oligonucleotides (positions 399 to 482) containing the mutated nucleotides and inserting them into the pSPT322-950 vector.
TABLE 1.
Illustration of the mutations in SI, SII, and SIII together with their dimerization (in vitro), viral replication, and RNA packaging (in cell culture) propertiesa
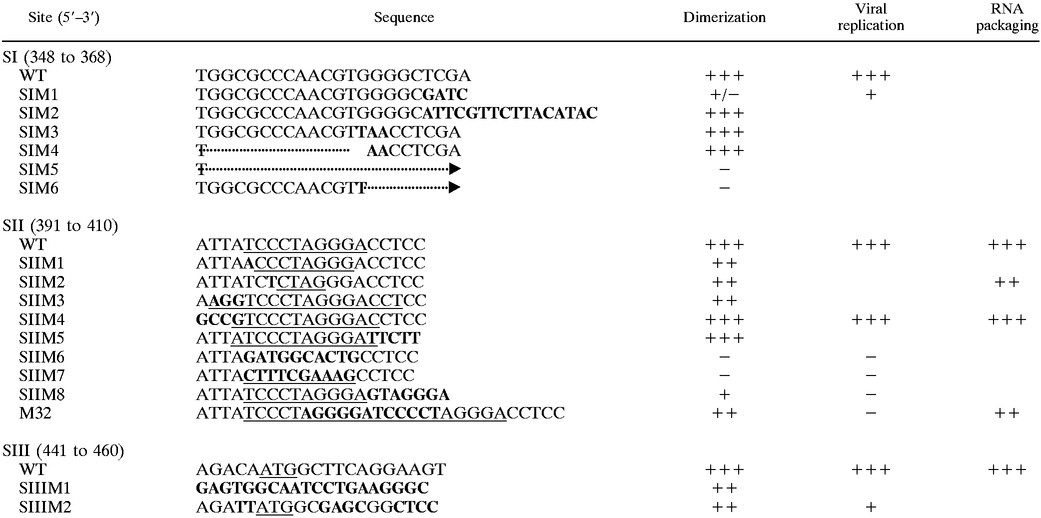 |
The nucleotide sequence of wild-type RNA for SI, SII, and SIII are shown with the corresponding mutant sequences. Nucleotides changed from the wild-type (WT) sequence are shown in bold. In the SI column the dotted line corresponds to deleted nucleotides. In the case of SIM5 and SIM6, the arrows indicate that the nucleotides have been deleted up to the second C in SII. In the SII column, palindromic sequences are underlined. The mutant SIIM8 could potentially form two palindromic structures, either involving 10 complementary nucleotides or a 12-nucleotide palindromic stem with 5 intervening nucleotides, as shown. In the SIII column, the gag start codon (ATG) is underlined. The relative abilities of the RNAs to dimerize in vitro, replicate, and package RNA in cell culture compared with the wild-type are indicated. +, 20 to 40%; ++, 40 to 70%; +++, over 70%; −, no dimerization, replication, or RNA packaging; +/−, dimerization ambiguous.
To construct SIIIM1, sequences from positions 461 to 946 were amplified by PCR and the resulting DNA fragment was inserted into pSPT322-950 via StyI and XhoI sites. Subsequently, annealed oligonucleotides spanning the sequences from positions 399 to 457 were included. To construct SIIIM2, annealed oligonucleotides spanning from positions 399 to 482 containing the desired changes were cloned into the vector pSPT322-950. The resulting constructs containing the mutations were confirmed by sequence analysis. For all constructs, DNA was digested with MboII to generate mutant RNA up to position 480 in order to test in vitro dimerization.
Transfer of mutations into pHSRV-2.
In the first instance, the pHSRV-2 mutants SIM1, SIIM2, SIIM4, SIIM6, and SIIM7 were produced by using pUC19 (Boehringer Mannheim) as an intermediate vector. A fragment of pHSRV-2 from the KpnI site (DNA position 2) to the PstI site (position 2973) was amplified using the Expand High Fidelity PCR system (Boehringer Mannheim) and was cloned into plasmid pUC19, giving pUC19/HSRV-2. This fragment contains the long terminal repeat, the leader sequence, and some gag sequence. Second, the mutated sequence was inserted into the pUC19/HSRV-2 vector via EcoRV and MfeI sites. Finally, the mutated HSRV-2 sequence was transferred from pUC19 into the wild-type pHSRV-2 via KpnI and SwaI (position 2867) sites. To construct pHSRV-2/SIIM8, a PCR-amplified sequence downstream of the SII palindrome (positions 1241 to 2891) was exchanged for the KpnI/SwaI fragment of pHSRV-2 to produce an intermediate construct. A PCR-amplified sequence upstream of the SII palindrome (positions 2 to 1233) containing an XcaI site at the 3′ end was then ligated into the intermediate vector via KpnI and SmaI sites. The mutant SIIIM2 was constructed by PCR amplifying a fragment of pHSRV-2 from position 1274 to position 2915. The PCR product was inserted as a KpnI/SwaI fragment into pHSRV-2, with the env sequences between NdeI and NheI deleted. Proviral sequences upstream of SIII from position 2 (KpnI site) to position 1271, incorporating an AseI site at the 3′ end, were PCR amplified and cloned into the pUC19 cleaved with KpnI and NdeI. Finally, the mutated sequences in SIII were inserted into pHSRV-2 as a KpnI/PacI fragment to generate SIIIM2. The resulting constructs containing the mutations were confirmed by sequence analysis.
Analysis of mutants in cell culture.
All transfections were carried out using the Calcium Phosphate Mammalian Transfection Kit (Promega) using a total of 10 μg of plasmid DNA per 2 × 105 BHK-21 or BHK/Bel-1 cells. Cultures were passaged on days 2, 7, and 10 posttransfection (p.t.), supernatant fluid (200 μl) was removed on days 2, 3, 5, 6, 7, 8, 9, 10, 13, and 14, and then the supernatant was stored at −70°C. Reverse transcriptase (RT) activity in the supernatant was assayed as described below. From supernatant (1.5 ml) taken on days 3, 6, and 14, virus titer was assayed by end-point dilution and by β-galactosidase staining of FAB cells (59). Infected cells were analyzed for viral protein expression on days 2, 7, and 14, as described below. On day 14, DNA was extracted from 106 cells to analyze the constructs by PCR, restriction enzyme digestion, and sequencing (ABI Prism dRhodamine dye terminator ready reaction kit; Perkin Elmer).
Analysis of the virions.
For RNase protection assays and Western blotting, 106 BHK/Bel-1 cells (7) were transfected in quadruplicate, each with 10 μg of the respective mutant DNA. Two days after transfection, the supernatant from the four flasks was pooled and concentrated using Centricon columns (Amersham) and virus particles were pelleted through a 20% sucrose cushion in an Optima TLX ultracentrifuge (Beckman) for 2 h at 50,000 rpm. The pellet was resuspended in 50 μl of H2O, and 10 μl was used for Western blotting to detect viral antigens by using human antiserum (a gift from A. Rethwilm, University of Dresden, Dresden, Germany). The nucleic acids were extracted from the remaining 40 μl using the total RNA isolation kit (Qiagen) according to the manufacturer's instructions. Gag-specific anti-sense RNA was generated by linearizing plasmid pSPT322-950 (18) with EcoRV and transcribing it with T7 polymerase in the presence of 32P (Amersham). This resulted in RNA which was complementary to gag ORF sequences 950 to 703 (protecting a fragment of 248 nucleotides) relative to the start site of transcription. A total of 70,000 cpm were used in the RNase protection assay (RPA) using the RPA kit II (Ambion), which was carried out according to the manufacturer's instructions.
RT assay.
RT activity was assayed by the colorimetric C-type RT assay (Cavidi Tech AB) (15, 40). Briefly, polyadenylic acid [poly(A)] covalently coupled to the wells of a 96-well microtiter plate constituted the template for the incorporation of the nucleoside analogue, 5′-bromodeoxyuridine triphosphate, with oligo(dT)22 as a primer. The incorporated 5′-bromodeoxyuridine monophosphate product was detected colorimetrically at 405 nM using alkaline phosphatase-conjugated anti-bromodeoxyuridine monoclonal antibody and p-nitrophenyl phosphate substrate after a 1-h incubation.
Analysis of virus proteins.
Lysates from transfected cells (106 cells per 40 μl) or from cell-free virus resuspended in protein-denaturing buffer (33) were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis on a 12% polyacrylamide gel and then were transferred to a nitrocellulose membrane (Amersham) for Western blot analysis. HFV proteins were detected using a 1:100 dilution of anti-HFV human serum and a 1:5,000 dilution of an anti-human immunoglobulin G-alkaline phosphatase conjugate (Serolab) and was developed in a solution containing 0.3 mg of nitroblue tetrazolium per ml, 0.15 mg of 5-bromo-4-chloro-3-indolylphosphate (BCIP), 100 mM Tris-HCl (pH 9.5), 100 mM NaCl, 5 mM MgCl2, and 1 mM CaCl2 for 5 to 30 min.
RESULTS
Role of SII in dimer formation.
Previously, the role of SII (positions 391 to 410 of the viral RNA) in dimer formation had been investigated in vitro by masking the site with cDNA oligonucleotides and by introducing mutations into the site (18). In this study further mutations were introduced into SII (Table 1) to determine the specific nucleotides involved. When tested for their dimerization properties in vitro, SIIM1, SIIM2, and SIIM3 resulted in significant reductions in dimer formation (Fig. 1a, SIIM1 lanes, SIIM2 lanes, and SIIM3 lanes) compared with the wild-type RNA (Fig. 1a, first two lanes). SIIM1 disrupts only the end nucleotide of the palindrome, SIIM2 disrupts most of the palindrome, and SIIM3 increases the size of the existing palindrome. The reduction in dimerization observed with these 3 mutants confirms the importance of the palindrome and suggests its size is also critical for dimerization in vitro. The result seen with SIIM3 is consistent with that seen for the previously reported M32 (18), which introduced an extended palindrome of 12 nucleotides.
FIG. 1.
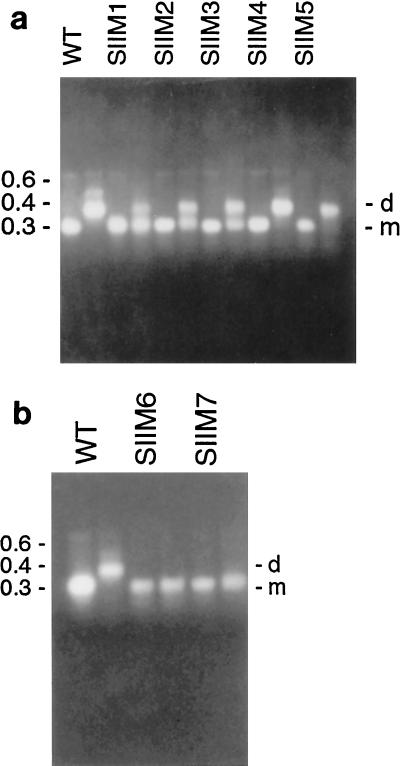
Mutations to sequences in and surrounding the palindrome in SII, as shown by agarose gel electrophoresis. (a) First two lanes, wild-type (WT) RNA, nucleotides 322 to 480; next two lanes, SIIM1; next two lanes, SIIM2; next two lanes, SIIM3; next two lanes, SIIM4; next two lanes, SIIM5. (b) First two lanes, wild-type RNA, nucleotides 322 to 480; next two lanes, SIIM6; next two lanes, SIIM7. The first lane of each pair contains denatured RNA, the second lane contains native RNA. The numbers to the left of the gels indicate nucleic acid sizes (in kilobases). d, dimer; m, monomer.
However, the mutants SIIM4 and SIIM5, which changed the nucleotides flanking the palindrome and increased the existing palindrome by two nucleotides, dimerized to the same extent as the wild-type RNA (Fig. 1a, SIIM4 lanes and SIIM5 lanes), indicating that the sequences immediately surrounding the palindrome are not important for in vitro dimerization. Moreover, a moderate increase of the palindrome by two nucleotides can be tolerated in vitro, in contrast to SIIM3, which increases the palindrome by six nucleotides and impairs dimerization.
Total disruption of the palindrome, which was caused by SIIM6, or formation of a new palindromic sequence, which was caused by SIIM7, inhibited dimer formation (Fig. 1b, SIIM6 lanes and SIIM7 lanes). In addition, SIIM8 greatly reduced dimerization, forming mainly monomers (not shown), possibly through destabilization of the dimeric structure. These results, together with the previously reported results for M32 and M43, demonstrate that the palindrome is the important sequence in SII, since disruption of this sequence results in a reduction in dimer formation. The size and nucleotide composition also appear to be important factors in dimerization. Extension of the palindrome by two nucleotides, whether by an A/U or a G/C, was tolerated (SIIM4 and SIIM5), while a longer nucleotide extension (SIIM3 and the previously reported M32 [18]) was not, indicating a limit to the size of a tolerated palindromic extension. No shortening of the palindrome was tolerated (SIIM1).
Role of SI and SIII in dimer formation.
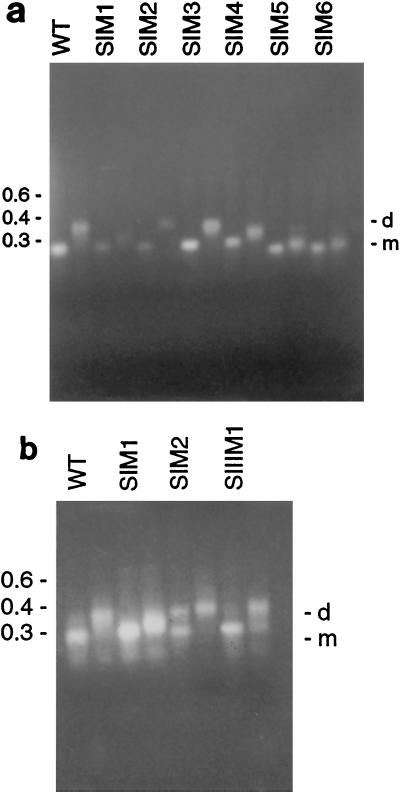
Since SI (positions 348 to 368 of the viral RNA) was shown to affect dimer formation, mutants were designed to investigate the specific nucleotides involved. The construct SIM1 was designed to change the last four nucleotides of SI, which were not part of the PBS. This construct produced an indeterminate band on the agarose gel, which was halfway between monomer and dimer (Fig. 2a, third and fourth lanes). The same result was obtained each time the dimer experiment was performed (more than four occasions).
FIG. 2.
Mutations in SI and SIII, as shown by agarose gel electrophoresis. (a) First two lanes, wild-type (WT) RNA, nucleotides 322 to 480; next two lanes, SIM1; next two lanes, SIM2; next two lanes, SIM3; next two lanes, SIM4; next two lanes, SIM5; next two lanes, SIM6. (b) First two lanes, wild-type RNA, nucleotides 322 to 480; next two lanes, SIM1; next two lanes, SIM2; next two lanes, SIIIM1. The first lane of each pair contains denatured RNA, the second lane contains native RNA. The numbers to the left of the gels indicate nucleic acid sizes (in kilobases). d, dimer; m, monomer.
Random nucleotides were introduced into the wild-type sequence from the end of the PBS in SI (position 365) up to position 380 (between SI and SII). The resulting HFV construct, SIM2, dimerized efficiently (Fig. 2a, fifth and sixth lanes), suggesting that the nucleotides at the mutated positions were not involved in HFV dimerization.
It has been proposed previously that G tetrads may play an essential role in retroviral genomic dimerization (1, 56). Since SI contained a G tetrad (positions 360 to 363), the mutant SIM3 was designed, replacing the G tetrad in the PBS with UAAC. This construct was able to dimerize to wild-type levels (Fig. 2a, seventh and eighth lanes), indicating that G tetrads are not involved in HFV dimerization in vitro.
SIM4 contained a deletion from the PBS up to and including the first nucleotide of the G tetrad (position 360), with the remaining three Gs changed to AAC, and dimerization was not impaired (Fig. 2a, 9th and 10th lanes). Since SIM4 left the last five nucleotides of SI unchanged, a construct was designed to change the last four nucleotides of SI, which were not part of the PBS.
The construct SIM5 contained a deletion from the second nucleotide in SI up to and including the second nucleotide in the palindrome of SII. SIM6 contained a deletion from the second G in the G tetrad in SI up to and including the second nucleotide in the palindrome of SII (position 398), and the G nucleotide at position 360 was replaced by a U. Both SIM5 and SIM6 were unable to dimerize (Fig. 2a, 11th and 12th lanes for SIM5, 13th and 14th lanes for SIM6). Since partial deletion of the palindrome reduced dimerization, some reduction might have been expected.
A third sequence, SIII (441 to 460), was also previously shown to be involved in HFV RNA dimerization in vitro (18). Two further mutants confirmed that SIII does not contribute to HFV dimerization in vitro. With the mutant SIIIM1, in which all nucleotides in SIII were randomly changed (Table 1), dimerization was only slightly reduced (Fig. 2b, seventh and eighth lanes). A similar reaction was obtained with mutant SIIIM2, in which nucleotides in SIII were changed such that the amino acid sequence remained the same as that of the wild type (not shown).
The effects of dimer mutants in cell culture.
A selection of the mutants, which differently affected dimer formation in vitro, was inserted into the wild-type virus backbone, and each was tested for its effect on virus replication in cell culture. Of SII, the constructs SIIM2, SIIM8, and M32 were chosen since they significantly reduced dimer formation in vitro (Fig. 1a), and the constructs SIIM6 and SIIM7 abolished dimer formation in vitro (Fig. 1b). In contrast, SIIM4 was chosen because it had no adverse effect on in vitro dimerization (Fig. 1a). Of SI and SIII, the constructs SIIIM2 (slightly reduced dimer formation in vitro) and SIM1 (intermediate band) were also tested in cell culture.
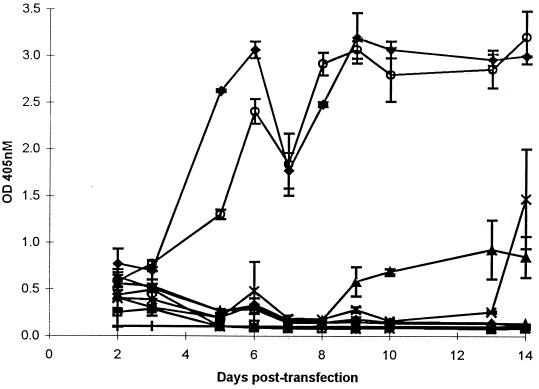
Wild-type (pHSRV-2) and mutant DNA were transfected into BHK-21 cells, and the infection was monitored by light microscopy for syncytium induction for 14 days. A cytopathic effect was first seen in the HSRV-2- and SIIM4-infected cultures on day 2 p.t., and by day 10 p.t. all cells in these cultures were clearly infected. Supernatant from each culture was assayed for RT activity (Fig. 3). The mutant SIIM4 and wild-type virus had similar RT activity profiles. Low RT activity was detected in all mutants on day 2 p.t. This subsequently declined in SIM1, SIIM7, SIIM6, SIIM8, M32, and SIIM2. For SIIM6, SIIM7, SIIM8, and M32 no RT activity above background levels was detected for the remainder of the assay (14 days). However, for SIM1 and SIIIM2 the RT activity increased on days 9 and 13 p.t., respectively, indicating that these two mutations were not lethal to the virus (Fig. 3). The slight drop observed for SIM1 on day 14 p.t. is probably due to cell death in the aging culture.
FIG. 3.
Comparison of RT levels produced by wild-type (HSRV-2) and dimer mutants in BHK-21 cells. BHK-21 cells seeded at 2 × 105 on the previous day were transfected with 10 μg of wild-type HSRV-2 (⧫), SIM1 (▴), SIIM2 (▵), SIIM4 (○), SIIM7 (∗), SIIM8 (◊), M32 (□), or SIIIM2 (x) DNA, and supernatant fluid was assayed for RT activity up to day 14 p.t. Supernatant from uninfected cells was also assayed (|). The means ± standard deviations of quadruplicate determinations for each construct are shown. OD 405 nM, optical density at 405 nM.
Extracellular virus titer was assessed on FAB cells. No virus was detected in all samples on day 3 p.t. or for the mutants SIIM6, SIIM7, and M32 on days 7 and 14 p.t. A virus titer of 10 was shown for SIM1 on day 14 p.t., and titers of 102 and 103 were observed for the wild-type virus and SIIM4 on days 7 and 14 p.t., respectively. These results correlated with the RT activities. The virus titer was not determined for SIIIM2 or SIIM8.
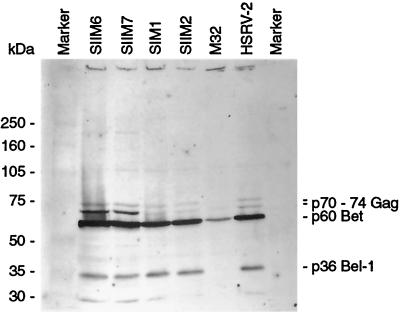
All constructs expressed viral proteins, as assessed by Western blotting (Fig. 4) (protein expression was not assessed for the mutants SIIM8 and SIIIM2). However, the relative levels of each protein compared with that of the wild-type virus were not quantified. No protein bands were observed with SIIM2, SIIM6, SIIM7, and M32 on days 6 and 14 p.t., correlating with the drop in RT activity, while some expression was seen for SIM1 on days 6 and 14 p.t. (not shown). Both wild-type virus and the mutant SIIM4 were able to produce proteins throughout the assay (not shown).
FIG. 4.
Comparison of viral protein expression levels between wild-type (HSRV-2) and mutants. BHK-21 cells (2 × 105) were transfected with 10 μg of wild-type (HSRV-2) or mutant DNA. The levels of viral protein expression were assessed on day 2 p.t. by Western blotting using HFV antibody-positive human serum. The first and last lanes are full-range molecular mass protein markers.
Mutants which are affected in dimerization can still package.
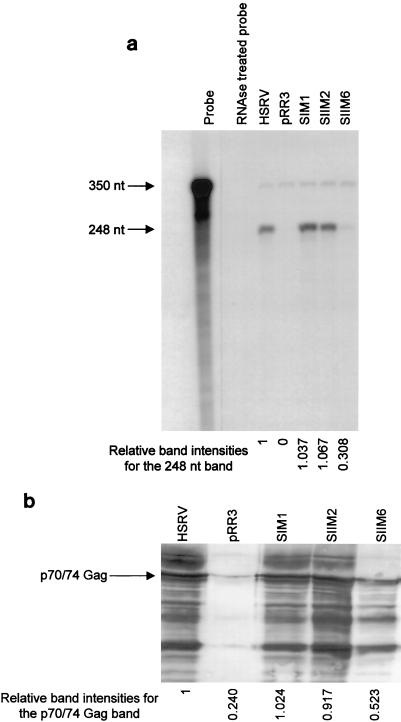
To investigate whether those dimerization mutants which failed to replicate in cell culture were unable to package RNA, RPAs were carried out. BHK/Bel-1 cells (7) were used, since the presence of the viral transactivator Tas (Bel-1) allows for a higher yield of viral antigens after transfection. Wild-type HFV (pHSRV-2) served as a replication-competent virus, whereas mutants SIM1, SIIM2, and SIIM6 were chosen as examples of replication-incompetent mutants. On BHK/Bel-1 cells, the replication-incompetent mutants again showed an initial cytopathic effect which eventually disappeared. In negative control experiments, BHK/Bel-1 cells were transfected with plasmid pRR3, which encodes Gag and Pol but lacks the env ORF (unpublished), ensuring that no virions are released into the supernatant (2, 50). Two days p.t. the supernatant was harvested and concentrated using Centricon columns and the virus was pelleted through a 20% sucrose cushion. The pellet was resuspended in 50 μl of H2O, 10 μl of which was used in a Western blot and the remaining 40 μl of which was used for RPA. The doublet of the 70- and 74-kDa HFV Gag protein was readily observed on Western blots for all mutants tested, whereas no specific bands were detected for the negative control (Fig. 5a). Relative band intensities are also shown on the gel. To investigate whether the inability to replicate in cell culture was due to a packaging defect, RPAs were carried out. The antisense probe derived from the gag ORF detected only the unspliced genomic transcript. However, the replication-defective mutants were able to package the viral genome (Fig. 5b). The band intensities (Fig. 5b) indicate the extent of packaging relative to that of the wild type. Similar results were obtained when mutants SIM1, SIIM6, and SIIIM2 were tested. As demonstrated, SIIM6 is replication incompetent in cell culture, whereas SIM1 and SIIM2 replicate moderately (Fig. 4). These results are in agreement with Heinkelein et al. (29), who introduced deletions in the leader region that affected the dimerization sites but not packaging. Thus, a packaging defect does not underlie the inability of the dimerization mutants to replicate.
FIG. 5.
Analysis of virions released from wild-type virus (HSRV-2) and dimerization mutants. BHK/Bel-1 cells were transfected with 10 μg of the respective DNA, and the virus was pelleted 48 h later as described in Materials and Methods. (a) RPA of nucleic acid isolated from virions. The full-length probe and the protected fragment of 350 and 248 nucleotides (nt), respectively, are shown. The relative band intensities of each band compared with that of the positive control HSRV-2, determined using LabImage version 2.51, are shown at the bottom of the gel. (b) Western blot with foamy virus-infected antiserum. The 70- and 74-kDa doublet of the Gag protein is indicated. The relative band intensities of the 70- and 74-kDa Gag doublet compared with that of the positive control HSRV-2, determined using LabImage version 2.51, are shown at the bottom of the gel.
DISCUSSION
Previously we showed that three sites, SI (348 to 368), SII (391 to 410), and SIII (441 to 460), were required for efficient dimerization of HFV RNA in vitro (18). In transduction experiments, the region containing the DLS was found to be important for successful delivery of HFV-based vectors (19, 28, 58). In the present study the role of these sites was investigated further by the introduction of mutations and by testing for their ability to dimerize in vitro.
The palindromic sequence (positions 396 to 405) within SII was shown to be important, since dimer formation was abolished when it was changed to a nonpalindromic sequence (SIIM6). Mutations which resulted in a decreased palindrome (SIIM1 by two nucleotides and SIIM2 by six nucleotides) or an increased palindrome (SIIM3 by six nucleotides) reduced dimerization, indicating that the size of the palindrome is important. This was in agreement with results with the mutant M32, tested previously, which increased the palindrome by 12 nucleotides (18). When the nucleotides in the palindrome were changed to a different palindrome of equal length (SIIM7), again no dimerization was observed, indicating that the specific nucleotide composition of the palindrome is important for dimerization in vitro. However, the nonpalindromic sequences within SII are dispensable for dimer formation (SIIM4 and SIIM5) but not when they resemble part of the palindrome (SIIM8). Palindromic sequences have been shown to play a central role in the dimerization of retroviral RNA, including HIV-1 (34, 54) and MLV (26), in which they are part of the DLS. Dimerization is thought to be initiated via a loop-loop interaction through Watson-Crick base pairing of a palindrome to form an immature kissing-loop structure in HIV-1, avian leukosis-sarcoma virus, and MLV (22, 23, 34, 36, 54). In the case of HIV-1 and MLV this immature dimer is packaged by the Gag polyprotein. Following particle release and protein maturation, the nucleocapsid protein is thought to promote maturation of the dimer into a more stable structure (20, 24, 25). HIV-1 isolates are able to tolerate mutations in the palindromic loop that retain a palindromic sequence (47, 54). Mutations that disrupt the palindrome generally abolish dimerization in vitro, although some base changes can be tolerated, resulting in reduced dimer formation (12, 47, 54). Nucleotide changes can also be tolerated in the stem structure, provided that the stem is maintained (47, 54). The absence of a stem-loop structure for HFV and the lack of tolerance for nucleotide changes in SII suggest that the mechanism for HFV dimerization is different from that of HIV.
Other mechanisms proposed to be involved in dimerization include guanine tetrads (56) and the purine-rich sequence PuGGAPuA through the formation of quartets involving both guanine and adenine bases (41). Subsequent studies argued against this model, since efficient dimerization still occurs in HIV-1, HIV-2, and bovine leukemia virus mutants lacking these sequences in vitro (4, 30, 54) and for HIV-1 in vivo (6). In contrast, guanine-rich sequences, in addition to a palindromic sequence, were found to be important for efficient Moloney murine sarcoma virus replication (39). The present study demonstrates that the guanine quartet, within the PBS (positions 364 to 367), in SI is not involved in HFV dimer formation in vitro, since two mutants (SIM3 and SIM4) in which these nucleotides were changed dimerized at wild-type levels.
The effect of mutations in SII on virus replication in cell culture revealed that mutations resulting in dimer reduction in vitro inhibited virus spread in cell culture, even though the viral proteins (Gag, Tas, and Bet) were still produced.
Inhibition of virus growth was seen even when only one nucleotide in the palindrome was changed (SIIM1 and SIIM2) and when the palindrome was exchanged for a different palindrome of equal length (SIIM7). This suggests an essential role of the wild-type palindromic sequence, perhaps in initializing dimer formation. These results, taken together with the fact that no stem-loop structure was found by using a computer model, suggest that the mechanism for HFV dimerization is different from that of HIV-1 for several reasons. First, for HIV-1, mutations that partially disrupt the stem-loop palindrome can be tolerated by the virus in cell culture, although in many cases growth is severely impaired. Moreover, provided that the palindromic sequence is maintained, replication is delayed but not abrogated (5). In addition, if SII formed a stem structure, then nucleotide changes in the sequences surrounding the palindrome would be expected to reduce dimerization and virus replication. This was clearly not the case for SIIM4, where the mutant replicated at wild-type levels.
The mutations in SIM1 and SIIIM2 were not lethal to the virus, but replication was slower than it was for the wild-type virus. Sequence analysis showed that the mutant SIM1 acquired base changes that restored the wild-type sequence (with the addition of two extra nucleotides), accounting for the increase in virus replication on day 9 p.t. The four mutated nucleotides in SIM1 were not part of the PBS, and from these data their role in HFV replication is unclear. If, as suggested, SII is required to initiate dimer formation, then SI and SIII may be required to stabilize this complex. This would then explain why, in contrast to SII mutants which are lethal to the virus, those in SI and SIII result in slower virus replication, allowing the nucleotides to revert over time to form a more stable complex.
The results shown here demonstrate that efficient dimerization is a critical step for HFV to generate infectious virus. Although HFV virions can contain DNA, the initial nucleic acid packaged is RNA (2). This packaged RNA is then reverse transcribed intracellularly into DNA as a late event in the virus life cycle (42, 61). In contrast to HIV-1 and Moloney murine leukemia virus, replication-defective HFV dimerization mutants can package nucleic acid, even though the respective mutants are unable to replicate in cell culture, suggesting that packaging of genomic RNA is independent of dimerization. This is supported by the finding that RNA packaging still occurs after deletion of a large part of the HFV leader region (29). It remains a possibility that RNA interactions elsewhere in the genome may have formed dimeric structures which partly compensated for the loss of the main dimerization sequence.
The finding that dimerization may not be a prerequisite for packaging, however, is not unique to foamy viruses. Rapid-harvest Rous sarcoma virus (RSV) and visna virus particles were found to contain predominately monomeric RNA in early studies, although this may reflect methods available at the time (9–11). Another possible explanation for this is that newly packaged dimeric immature RNA is more unstable in these viruses than that packaged by HIV-1 and MLV (55). However, analysis of RSV protease mutants which ensure a pure population of immature virions found mainly monomeric RNA in mutant virions (46). This, taken together with the fact that dimeric RSV RNA has never been isolated from infected cells, suggests that monomeric RNA is encapsidated in RSV and dimerization occurs later (37, 45, 46).
The present study confirmed, by mutational analysis, that SI, SII, and SIII are involved in the dimerization of HFV RNA, both in vitro and for the generation of infectious virus in cell culture. However, although specific sequences were shown to be important for virus replication, further experiments will be needed to determine the exact nature of the observed defect. Since dimerization is thought to be involved in several steps in the HIV-1 and Moloney murine leukemia virus life cycles, this may also be true of foamy virus dimerization.
ACKNOWLEDGMENTS
This study was funded by the Wellcome Trust, the Jefferiss Trust, and the EU.
REFERENCES
- 1.Awang G, Sen D. Mode of dimerization of HIV-1 genomic RNA. Biochemistry. 1993;32:11453–11457. doi: 10.1021/bi00093a024. [DOI] [PubMed] [Google Scholar]
- 2.Baldwin D N, Linial M I. The roles of Pol and Env in the assembly pathway of human foamy virus. J Virol. 1998;72:3658–3665. doi: 10.1128/jvi.72.5.3658-3665.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bender W, Chien Y H, Chattopadhyay S, Vogt P K, Gardner M B, Davidson N. High-molecular-weight RNAs of AKR, NZB, and wild mouse viruses and avian reticuloendotheliosis virus all have similar dimer structures. J Virol. 1978;25:888–896. doi: 10.1128/jvi.25.3.888-896.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berkhout B, Essink B B, Schoneveld I. In vitro dimerization of HIV-2 leader RNA in the absence of PuGGAPuA motifs. FASEB J. 1993;7:181–187. doi: 10.1096/fasebj.7.1.8422965. [DOI] [PubMed] [Google Scholar]
- 5.Berkhout B, van Wamel J L. Role of the DIS hairpin in replication of human immunodeficiency virus type 1. J Virol. 1996;70:6723–6732. doi: 10.1128/jvi.70.10.6723-6732.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berkhout B, Das A T, van Wamel J L. The native structure of the human immunodeficiency virus type 1 RNA genome is required for the first strand transfer of reverse transcription. Virology. 1998;249:211–218. doi: 10.1006/viro.1998.9321. [DOI] [PubMed] [Google Scholar]
- 7.Bieniasz P D, Erlwein O, Aguzzi A, Rethwilm A, McClure M O. Gene transfer using replication-defective human foamy virus vectors. Virology. 1997;235:65–72. doi: 10.1006/viro.1997.8658. [DOI] [PubMed] [Google Scholar]
- 8.Bieth E, Gabus C, Darlix J L. A study of the dimer formation of Rous sarcoma virus RNA and of its effect on viral protein synthesis in vitro. Nucleic Acids Res. 1990;18:119–127. doi: 10.1093/nar/18.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brachic M, Vigne R. Properties of visna virus particles harvested at short time intervals: RNA content, infectivity, and ultrastructure. J Virol. 1975;15:1222–1230. doi: 10.1128/jvi.15.5.1222-1230.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Canaani E, Helm K V D, Duesberg P. Evidence for the 30–40s RNA as precursor of the 60–70s RNA of Rous sarcoma virus. Proc Natl Acad Sci USA. 1973;70:401–405. doi: 10.1073/pnas.70.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheung K S, Smith R E, Stone M P, Joklik W K. Comparison of immature (rapid harvest) and mature Rous sarcoma virus particles. Virology. 1972;50:851–864. doi: 10.1016/0042-6822(72)90439-4. [DOI] [PubMed] [Google Scholar]
- 12.Clever J L, Wong M I, Parslow T G. Requirements for kissing-loop-mediated dimerization of human immunodeficiency virus RNA. J Virol. 1996;70:5902–5908. doi: 10.1128/jvi.70.9.5902-5908.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clever J L, Parslow T G. Mutant human immunodeficiency virus type 1 genomes with defects in RNA dimerization or encapsidation. J Virol. 1997;71:3407–3414. doi: 10.1128/jvi.71.5.3407-3414.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Darlix J, Gabus C, Nugeyre M T, Clavel F, Barre Sinoussi F. cis elements and trans-acting factors involved in the RNA dimerization of the human immunodeficiency virus HIV-1. J Mol Biol. 1990;216:689–699. doi: 10.1016/0022-2836(90)90392-Y. [DOI] [PubMed] [Google Scholar]
- 15.Elkstrand D H L, Awad R J K, Kallander C F R, Gronowitz J S. A sensitive assay for the quantification of reverse transcriptase activity based on the use of carrier-bound template non-radioactive-product detection, with special reference to human-immunodeficiency-virus isolation. Biotechnol Appl Biochem. 1996;23:95–105. [PubMed] [Google Scholar]
- 16.Enssle J, Jordan I, Mauer B, Rethwilm A. Foamy virus reverse transcriptase is expressed independently from the Gag protein. Proc Natl Acad Sci USA. 1996;93:4137–4141. doi: 10.1073/pnas.93.9.4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Enssle J, Moebes A, Heinkelein M, Panhuysen M, Mauer B, Schweizer M, Neumann Haefelin D, Rethwilm A. An active foamy virus integrase is required for virus replication. J Gen Virol. 1999;80:1445–1452. doi: 10.1099/0022-1317-80-6-1445. [DOI] [PubMed] [Google Scholar]
- 18.Erlwein O, Cain D, Fischer N, Rethwilm A, McClure M O. Identification of the sites that act together to direct dimerisation of human foamy virus RNA in vitro. Virology. 1997;229:251–258. doi: 10.1006/viro.1997.8438. [DOI] [PubMed] [Google Scholar]
- 19.Erlwein O, Bieniasz P D, McClure M O. Sequences in pol are required for transfer of human foamy virus-based vectors. J Virol. 1998;72:5510–5516. doi: 10.1128/jvi.72.7.5510-5516.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feng Y X, Copeland T D, Henderson L E, Gorelick R J, Bosche W J, Levin J G, Rein A. HIV-1 nucleocapsid protein induces ‘maturation’ of dimeric retroviral RNA in vitro. Proc Natl Acad Sci USA. 1996;93:7577–7581. doi: 10.1073/pnas.93.15.7577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fischer N, Heinkelein M, Lindemann D, Enssle J, Baum C, Werder E, Zentgraf H, Muller J G, Rethwilm A. Foamy virus particle formation. J Virol. 1998;72:1610–1615. doi: 10.1128/jvi.72.2.1610-1615.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fisher J, Goff S P. Mutational analysis of stem-loops in the packaging signal of the Moloney murine leukemia virus. Virology. 1998;244:133–145. doi: 10.1006/viro.1998.9090. [DOI] [PubMed] [Google Scholar]
- 23.Fosse P, Motte N, Roumier A, Gabus C, Muriaux D, Darlix J L, Paoletti J. A short autocomplementary sequence plays an essential role in avian sarcoma-leukosis virus RNA dimerization. Biochemistry. 1996;35:16601–16609. doi: 10.1021/bi9613786. [DOI] [PubMed] [Google Scholar]
- 24.Fu W, Rein A. Maturation of dimeric viral RNA of Moloney murine leukemia virus. J Virol. 1993;67:5443–5449. doi: 10.1128/jvi.67.9.5443-5449.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fu W, Gorelick R J, Rein A. Characterization of human immunodeficiency virus type 1 dimeric RNA from wild-type and protease-defective virions. J Virol. 1994;68:5013–5018. doi: 10.1128/jvi.68.8.5013-5018.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Girard P M, Bonnet Mathoniere B, Muriaux D, Paoletti J. A short autocomplementary sequence in the 5′ leader region is responsible for dimerization of MoMuLV genomic RNA. Biochemistry. 1995;34:9785–9794. doi: 10.1021/bi00030a016. [DOI] [PubMed] [Google Scholar]
- 27.Haddrick M, Lear A L, Cann A J, Heaphy S. Evidence that a kissing loop structure facilitates genomic RNA dimerisation in HIV-1. J Mol Biol. 1996;259:58–68. doi: 10.1006/jmbi.1996.0301. [DOI] [PubMed] [Google Scholar]
- 28.Heinkelein M, Schmidt M, Fischer N, Moebes A, Lindemann D, Enssle J, Rethwilm A. Characterization of a cis-acting sequence in the Pol region required to transfer human foamy virus vectors. J Virol. 1998;72:6307–6314. doi: 10.1128/jvi.72.8.6307-6314.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heinkelein M, Thurow J, Dressler M, Imrich H, Neumann-Haefelin D, McClure M O, Rethwilm A. Complex effects of deletions in the 5′ untranslated region of primate foamy virus on viral gene expression and RNA packaging. J Virol. 2000;74:3141–3148. doi: 10.1128/jvi.74.7.3141-3148.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katoh I, Yasunaga T, Yoshinaka Y. Bovine leukemia virus RNA sequences involved in dimerization and specific gag protein binding: close relation to the packaging sites of avian, murine, and human retroviruses. J Virol. 1993;67:1830–1839. doi: 10.1128/jvi.67.4.1830-1839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kozac M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986;44:283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- 32.Kung H J, Hu S, Bender W, Bailey J M, Davidson N, Nicolson M O, McAllister R M. RD-114, baboon, and woolly monkey viral RNAs compared in size and structure. Cell. 1976;7:609–620. doi: 10.1016/0092-8674(76)90211-7. [DOI] [PubMed] [Google Scholar]
- 33.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 34.Laughrea M, Jette L. A 19-nucleotide sequence upstream of the 5′ major splice donor is part of the dimerization domain of human immunodeficiency virus 1 genomic RNA. Biochemistry. 1994;15:13464–13474. doi: 10.1021/bi00249a035. [DOI] [PubMed] [Google Scholar]
- 35.Laughrea M, Jette L. HIV-1 genome dimerization: kissing-loop hairpin dictates whether nucleotides downstream of the 5′ splice junction contribute to loose and tight dimerization of human immunodeficiency virus RNA. Biochemistry. 1997;36:9501–9508. doi: 10.1021/bi970862l. [DOI] [PubMed] [Google Scholar]
- 36.Laughrea M, Jette L, Mak J, Kleiman L, Liang C, Wainberg M A. Mutations in the kissing-loop hairpin of human immunodeficiency virus type 1 reduce viral infectivity as well as genomic RNA packaging and dimerization. J Virol. 1997;71:3397–3406. doi: 10.1128/jvi.71.5.3397-3406.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lear A L, Haddrick M, Heaphy S. A study of the dimerization of Rous sarcoma virus RNA in vitro and in vivo. Virology. 1995;212:47–57. doi: 10.1006/viro.1995.1452. [DOI] [PubMed] [Google Scholar]
- 38.Linial M L. Foamy viruses are unconventional retroviruses. J Virol. 1999;73:1747–1755. doi: 10.1128/jvi.73.3.1747-1755.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ly H, Nierlich D P, Olsen J C, Kaplan A H. Moloney murine sarcoma virus genomic RNAs dimerize via a two-step process: a concentration-dependent kissing-loop interaction is driven by initial contact between consecutive guanines. J Virol. 1999;73:7255–7261. doi: 10.1128/jvi.73.9.7255-7261.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Malmesten A, Elkstrand D H L, Akerbolm L, Gronowitz J S, Kallander C F R, Bendinelli M, Matteucci D. A colorimetric reverse transcriptase (RT) assay optimised for Moloney murine leukemia virus, and its use for characterization of RTs of unknown identity. J Virol Methods. 1998;75:9–20. doi: 10.1016/s0166-0934(98)00091-3. [DOI] [PubMed] [Google Scholar]
- 41.Marquet R, Baudin F, Gabus C, Darlix J L, Mougel M, Ehresmann C, Ehresmann B. Dimerization of human immunodeficiency virus (type 1) RNA: stimulation by cations and possible mechanism. Nucleic Acids Res. 1991;19:2349–2357. doi: 10.1093/nar/19.9.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moebes A, Enssle J, Bieniasz P D, Heinkelein M, Lindemann D, Bock M, McClure M O, Rethwilm A. Human foamy virus reverse transcription that occurs late in the viral replication cycle. J Virol. 1997;71:7305–7311. doi: 10.1128/jvi.71.10.7305-7311.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mougel M, Zhang Y, Barklis E. cis-active structural motifs involved in specific encapsidation of Moloney murine leukemia virus RNA. J Virol. 1996;70:5043–5050. doi: 10.1128/jvi.70.8.5043-5050.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murti K G, Bondurant M, Tereba A. Secondary structural features in the 70S RNAs of Moloney murine leukemia and Rous sarcoma viruses as observed by electron microscopy. J Virol. 1981;37:411–419. doi: 10.1128/jvi.37.1.411-419.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oertle S, Spahr P F. Role of the Gag polyprotein precursor in packaging and maturation of Rous sarcoma virus genomic RNA. J Virol. 1990;64:5757–5763. doi: 10.1128/jvi.64.12.5757-5763.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ortiz-Conde B A, Hughes S H. Studies of the genomic RNA of leukosis viruses: implications for RNA dimerization. J Virol. 1999;73:7165–7174. doi: 10.1128/jvi.73.9.7165-7174.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paillart D C, Marquet R, Skripkin E, Ehresmann B, Ehresmann C. Mutational analysis of the bipartite dimer linkage structure of human immunodeficiency virus type 1 genomic RNA. J Biol Chem. 1994;269:27486–27493. [PubMed] [Google Scholar]
- 48.Paillart J C, Berthoux L, Ottmann M, Darlix J L, Marquet R, Ehresmann B, Ehresmann C. A dual role of the putative RNA dimerization initiation site of human immunodeficiency virus type 1 in genomic RNA packaging and proviral DNA synthesis. J Virol. 1996;70:8348–8354. doi: 10.1128/jvi.70.12.8348-8354.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paillart J C, Marquet R, Skripkin E, Ehresmann C, Ehresmann B. Dimerization of retroviral genomic RNAs: structural and functional implications. Biochimie. 1996;78:639–653. doi: 10.1016/s0300-9084(96)80010-1. [DOI] [PubMed] [Google Scholar]
- 50.Pietschmann T, Heinkelein M, Heldmann M, Zentgraf H, Rethwilm A, Lindemann D. Foamy virus capsids require the cognate envelope protein for particle release. J Virol. 1999;73:2613–2621. doi: 10.1128/jvi.73.4.2613-2621.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Prats A C, Roy C, Wang P A, Erard M, Housset V, Gabus C, Paoletti C, Darlix J L. cis elements and trans-acting factors involved in dimer formation of murine leukemia virus RNA. J Virol. 1990;64:774–783. doi: 10.1128/jvi.64.2.774-783.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rethwilm A, Baunach G, Netzer K O, Maurer B, Borisch B, ter Meulen V. Infectious DNA of the human spumaretrovirus. Nucleic Acids Res. 1990;18:733–738. doi: 10.1093/nar/18.4.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rethwilm A. Regulation of foamy virus gene expression. Curr Top Microbiol Immunol. 1995;193:1–23. doi: 10.1007/978-3-642-78929-8_1. [DOI] [PubMed] [Google Scholar]
- 54.Skripkin E, Paillart J C, Marquet R, Ehresmann B, Ehresmann C. Identification of the primary site of the human immunodeficiency virus type 1 RNA dimerization in vitro. Proc Natl Acad Sci USA. 1994;91:4945–4949. doi: 10.1073/pnas.91.11.4945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stolfus C M, Snyder P N. Structure of the B77 sarcoma virus RNA: stabilization of RNA after packaging. J Virol. 1975;16:1161–1170. doi: 10.1128/jvi.16.5.1161-1170.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sundquist W I, Heaphy S. Evidence for interstrand quadruplex formation in the dimerization of human immunodeficiency virus 1 genomic RNA. Proc Natl Acad Sci USA. 1993;15:3393–3397. doi: 10.1073/pnas.90.8.3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vagner S, Waysbort A, Marenda M, Gensac M C, Amalric F, Prats A C. Alternative translation of the Moloney murine leukemia virus mRNA controlled by internal ribosomal entry involving the p57/PTB splicing factor. J Biol Chem. 1995;270:20376–20383. doi: 10.1074/jbc.270.35.20376. [DOI] [PubMed] [Google Scholar]
- 58.Wu M, Chari S, Yanchis T, Mergia A. cis-acting sequences required for simian foamy virus type 1 vectors. J Virol. 1998;72:3451–3454. doi: 10.1128/jvi.72.4.3451-3454.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yu S F, Linial M L. Analysis of the role of the bel and bet open reading frames of human foamy virus by using a new quantitative assay. J Virol. 1993;67:6618–6624. doi: 10.1128/jvi.67.11.6618-6624.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yu S F, Baldwin D N, Gwynn S R, Yendapalli S, Linial M L. Human foamy virus replication: a pathway distinct from that of retroviruses and hepadnaviruses. Science. 1996;271:1579–1582. doi: 10.1126/science.271.5255.1579. [DOI] [PubMed] [Google Scholar]
- 61.Yu S F, Sullivan M D, Linial M L. Evidence that the human foamy virus genome is DNA. J Virol. 1999;73:1565–1572. doi: 10.1128/jvi.73.2.1565-1572.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]