Abstract
Among the 7.8 million women with breast cancer worldwide, at least 33% to 44% of them are affected by lymphatic pain. Lymphatic pain refers to co-occurring pain (e.g., pain, aching or soreness) and swelling. Pharmacological approaches, such as the uses of NSAIDS, opioids, antiepileptics, ketamine and lidocaine, have very limited effects on lymphatic pain. Limited research in this field has made it difficult for patients and clinicians to differentiate lymphatic pain from other types of pain. Precision assessment to distinguish different types of pain is essential for finding efficacious cure for pain. Innovative behavioral interventions to promote lymph flow and reduce inflammation are promising to reduce lymphatic pain. The goal of this review is to provide a comprehensive understanding of lymphatic pain through research evidence-based knowledge and insights into precision assessment and therapeutic behavioral intervention for lymphatic pain.
Keywords: lymphatic, pain, assessment, behavioral, breast cancer, fluid accumulation
Introduction
Lymphatic pain refers to co-occurring pain, or sensations of aching, soreness, or tenderness, and swelling.1–2 For breast cancer survivors, lymphatic pain occurs usually in the ipsilateral body or upper limb.1–2 While breast cancer mortality has been significantly decreased over years,3–4 lymphatic pain remains one of the most common and long-term and debilitating effects of cancer treatment.5–6 Currently, at least 33% to 44% of the more than 7.8 million women treated for breast cancer worldwide are affected by lymphatic pain.1,3,5
The concept of lymphatic pain has emerged in recent research.1–2,5–6 Historically, the concept of cancer-related pain has been used to study chronic pain associated with cancer or cancer treatment.7–9 Cancer-related pain refers to persistent pain that continues more than three months after active cancer treatment.7–10 Researchers have operationalized cancer-related pain in terms of occurrence and severity of general bodily pain in any body location.7–10 This line of research has increased our understanding of cancer-related chronic pain, yet, it has not been able to distinguish different types of pain after cancer treatment, such as lymphatic pain due to fluid accumulation and inflammation, general bodily pain, postmastectomy pain, chemotherapy-induced peripheral neuropathy, or arthralgias related to hormonal treatments.7–10 Consequently, opportunities are limited to explore the underlying physiological and psychosocial mechanisms of different types of pain and develop efficacious pain treatments.
Lymphatic pain is caused by abnormal lymph fluid accumulation.1–2 Mainstream pharmacological approaches (e.g., NSAIDS, opioids, antiepileptics, ketamine and lidocaine) have very limited effects on lymphatic pain.7,11–12 Recent research demonstrates that some behavioral interventions that promote lymph flow and reduce inflammation are efficacious for lymphatic pain.2 However, limited research has made it difficult for patients and clinicians to differentiate lymphatic pain from other types of pain. The goal of this review is to provide a comprehensive understanding of lymphatic pain through research evidence-based knowledge and insights into precision assessment and therapeutic behavioral interventions for lymphatic pain.
Impact of Lymphatic Pain
Lymphatic pain negatively impacts breast cancer survivors’ physical function, emotional health, and overall health. Lymphatic pain significantly interferes individuals’ activities of daily living (ADLs).1 ADLs are essential daily activities for individuals to live an independent life and are important measures for daily living function.13–15 Impairment in ADLs occurs when individuals are not able to perform the essential daily living activities, resulting in poor quality of life (QOL) and lack of independent living. A recently study of 568 breast cancer survivors detailed that patients with lymphatic pain reported impairments in 45% of ADLs and had a significantly increased risk of having difficulty in performing all the 13 ADLs (i.e., cooking, using a knife, writing/typing, cleaning, vacuuming, laundry, carrying objects, yard work, dressing self, bathing self, driving, making bed, and taking care of children) when compared to patients with only pain but no swelling.1 Patients with lymphatic pain were 4.74 times more likely to have impaired ADLs (OR = 4.74, 95% CI = [2.65–8.50], P < 0.001) compared to patients with only pain but no swelling.16 Noticeably, when patients with lymphatic pain reported co-occurring fatigue, their risk of having impaired ADLs increased 24.43 times (OR = 24.43, 95% CI = [5.44–109.67], P < 0.001).16 This undergirds the importance of assessing the incremental impact of co-occurring lymphatic pain and other symptoms.
Lymphatic pain also increases emotional distress among breast cancer survivors.1,16 Emotional distress are negative emotions evoked by an individual’s experience of physical symptoms, such as pain.17–20 These negative emotions include frustration, sadness, guilt/self-blame, being worried, irritation, fear, anger, loneliness, helplessness, hopelessness, anxiety, and depression.17–20 A recent study of 354 breast cancer survivors found that the odds of having emotional distress were 12.82 times higher in patients with lymphatic pain (OR = 12.82, 95%CI = [6.72–24.46], P < 0.001). The risk of having emotional distress for patients with lymphatic pain increased tremendously if fatigue also co-occurred (OR = 26.52, 95%CI = [9.64–72.90], P < 0.001).16 While emotional distress is influenced by several factors related to cancer treatment, such as having a mastectomy and a lumpectomy, recent research demonstrates that lymphatic pain is a very important and significant predictor for emotional distress in breast cancer survivors.1,16 Further research is needed to detail how lymphatic pain impacts breast cancer survivors’ physical functioning, emotional well-being, and overall health.
Etiology of Lymphatic Pain
Lymphatic pain and lymphedema share similar etiologies of abnormal fluid accumulation. 1,6,21–25 Breast cancer treatment interrupts normal functions of lymphatic system, which causes an accumulation of lymph fluid and creates pain, or aching, or soreness along with swelling.1,6,21–22 Lymphatic pain can occur in patients either with or without a diagnosis of lymphedema.1–2,23–24 Lymphedema following breast cancer treatment is a chronic and incurable condition.11,22 The hallmark of lymphedema is swelling which is often defined and quantitatively operationalized as an increase in limb size or girth or lymph fluid level.11,23–24 Lymphatic pain often indicates an early stage of lymphedema for breast cancer survivors without a diagnosis of lymphedema.1–6,23–27 For breast cancer survivors with lymphedema, lymphatic pain is part of “living with a perpetual discomfort”28: p.853 and the exacerbation of lymphatic pain indicates the worsening of lymphedema.28 Future research is needed to further explicate the etiology and underlying mechanism of lymphatic pain.
Risk Factors for Lymphatic Pain
The major risk factors for lymphatic pain are associated with cancer treatment, such as surgical removal of lymph nodes which interrupt the normal function of lymphatic system and radiation exposure which is associated with trauma to the lymphatic system.1 Other non-cancer treatment related risk factors include lymphedema diagnosis, financial hardship, obesity, and younger age.1 The odds of having lymphatic pain were 9.68 times higher in breast cancer survivors with a lymphedema diagnosis (OR = 9.68, P < 0.001, 95% CI = [5.78–16.63]).1 Limited research has explored the genetic or genomic influence on lymphatic pain. Research on heterogeneity of lymphedema phenotype21 found that the odds of having pain were 4.70 times higher in patients with the genotype VEGF-C rs3775203 heterozygous A/C compared to those with homozygous C/C genotype. Similarly, the odds of having pain were 6.29 times higher in patients with genotype IL13 rs1800952 homozygous T/T and 2.04 times higher in patients with heterozygous T/C had 2.04 compared to the homozygous C/C genotype. Notably, patients whose genotype contained both VEGF-C rs3775203 and IL13 rs1800952 variants had 12.86 times higher odds of experiencing pain. More research is needed to elucidate the genetic and genomic impact on lymphatic pain.
It is noteworthy that a recent study of 568 breast cancer survivors was the first to identify financial hardship (i.e., not having enough income to make ends meet) as one of the major risk factors for lymphatic pain.1 Patients with financial hardship were 4.64 times more likely to have lymphatic pain (OR = 4.64, P = 0.001, 95% CI = [1.99–11.32]). This provides evidence that social determinants of health (e.g., financial status) have negative impact on lymphatic pain, suggesting that assessment of patient’s financial status is important in identifying a patient’s risk of lymphatic pain. In the United States, racially minoritized women have delays in breast cancer treatment as well as inadequate treatment.31–32 Later stage diagnosis and delayed treatment lead to higher risk of pain and lymphedema due to the need to have more aggressive surgical treatment, more lymph nodes removed, and radiation.22,31–32
Obesity increases the risk of lymphatic pain.1,11,5 Patients with a body mass index (BMI) ≥30 kg/m2 were 3.49 times more likely to have lymphatic pain (OR 3.49, 95%CI 1.87–6.50; P < 0.001).5 Obesity and lymphatic pain are inflammatory conditions, and more research studies are needed to explore the role of inflammatory pathways that contribute to lymphatic pain. In the United States, obesity is more likely to occur among women living in rural and economically disadvantaged communities.33–34 Thus, assessing place-based disadvantages for the risk of lymphatic pain is essential.
An early study30 found that younger age was a risk factor for lymphedema. Similarly, younger age is also a significant risk for lymphatic pain (OR = 0.97, P = 0.011, 95% CI = [0.96–0.99]).1 Compared to older women, women of younger age may have jobs outside the home, share more parenting responsibilities, care for elderly parents, and do more household chores; all of which may contribute to stress and chronic inflammation that could lead to increased risk of lymphatic pain and lymphedema. More research is needed to explore stress and actual physical labor on the lymphatic system and fluid accumulation.
Precision Assessment of Lymphatic Pain
As a cluster of co-occurring symptoms of pain, or sensations of aching, soreness, or tenderness and swelling, lymphatic pain is a subjectively perceived indicator that reflects abnormal biological or physiological changes that may or may not be observed objectively.35 The subjective nature of lymphatic pain entails its assessment using patient-reported outcome measures (PROMs). PROMs are patient’s direct reports about his/her health condition without clinician or other people’s interpretation of the patient’s response.36–37 As a component of lymphatic pain, swelling may be measured by an objective measure of limb volume or circumference differences or fluid level. However, these objective measurements of swelling are less associated with QOL than patient-reported symptoms (e.g., pain, aching, soreness, and swelling).11 Therefore, PROMs are optimal measures for lymphatic pain, a subjective phenomenon.36–37
Lymphatic pain following breast cancer has been operationalized as the patient-report of co-occurring pain, or aching, or soreness, or tenderness and swelling.1 The Breast Cancer and Lymphedema Symptom Experience Index (BCLE-SEI) Part I has been used to assess lymphatic pain (i.e., pain, aching, soreness, tenderness, and swelling) and additional symptoms related to lymph fluid accumulation or lymphedema.1–2,5 BCLE-SEI is valid and reliable patient-report instrument with a Cronbach’s alpha of 0.92 for symptom occurrence.6,19–20,26–27 A response frame can be adjusted (e.g., “now,” “past seven days”, or “past three months”) to indicate whether lymphatic pain occurs currently or has been persistent. Each item is rated on a 5-point Likert scale (i.e., 0 = no presence of a given symptom to 4 = greatest severity of a given symptom). Higher scores indicate more severe lymphatic pain. A recent large study of 568 breast cancer survivors used BCLE-SEI and identified four pain phenotypes: 33.1% were classified as lymphatic pain phenotype (i.e., presence of pain, aching, or soreness, and swelling), 35.9% as pain without swelling phenotype (i.e., only pain, aching, or soreness without arm/hand swelling), 5.9% as only swelling phenotype (i.e., only arm/hand swelling without pain, aching, or soreness), and 25% as phenotype of no symptom (i.e., absence of pain, aching, soreness, and arm/hand swelling). This study found that 41% of patients in the lymphatic pain group had >5% interlimb volume differences using an objective measure of limb volume by infra-red perometer (Perometry 350S). This supports the clinical observation that lymphatic pain often precedes changes in limb volume or lymph fluid. Thus, self-reported lymphatic pain can be considered an important marker for early lymphedema. Given that the major causes of lymphatic pain are inflammation and fluid accumulation; future research should explore genotypes, biomarkers, and fluid levels as potential objective markers of lymphatic pain.
Therapeutic Behavioral Intervention
Pharmacologic interventions have very limited effects on cancer-related pain and lymphatic pain, including the use of NSAIDS, opioids, antiepileptics, ketamine and lidocaine and long-term use of the medications in breast cancer patients can be problematic.11 Behavioral strategies are also used for cancer-related pain.7–10 Lymphedema is usually treated through manual lymph drainage, physical therapy, compression garments, upper extremity exercise, focusing on swelling reduction.11 Like lymphedema, lymphatic pain is associated with increased lymph fluid accumulation evidenced by increased lymph fluid level and inter-limb volume differences,1−2; and associated inflammatory responses.21,25 Effective treatment of lymphatic pain can decrease the risk of developing lymphedema and lessen lymphedema severity.26–27 However, there are persistent and worldwide challenges for patients (e.g., availability of interventions, cost and time for clinical visits) to receive timely and effective interventions for managing lymphatic pain.
The-Optimal-Lymph-Flow (TOLF) is a web- and mobile- based program and one of the available behavioral interventions for lymphatic pain. TOLF intervention is based on physiological (fluid accumulation and inflammation) and cognitive principles (low self-efficacy for pain management) to promote lymph flow.2,26–27,29,38–40 TOLF program consists of therapeutic lymphatic exercises, healthy diet (i.e., nutrition-balanced, portion-appropriate diet, adequate hydration), and proper sleep. Table 1 details the TOLF intervention strategies. TOLF therapeutic lymphatic exercises to promote lymph flow include muscle-tightening deep breathing, muscle-tightening pumping, and limb mobility exercises. In addition, TOLF provides patients with knowledge about lymphatic system, lymphedema, and daily self-assessment.
Table 1.
| Keep a Healthy Weight | ||
|---|---|---|
| Strategies | Rationale | Actions |
|
Home-Based Muscle-Tightening Exercises ➢ Muscle-Tightening Deep Breathing ➢ Muscle-Tightening Pumping |
✓ Muscle-tightening-deep-breathing stimulates lymphatic ducts and help the lymphatic system to absorb lipids and decrease inflammation. ✓ Muscle-tightening pumping exercises create muscle pumping. This helps lymph fluid flow and decreases inflammation and helps to absorb lipids. ✓ Muscle-tightening-deep-breathing and pumping exercises help to relax the body and decrease stress and pain. |
✓ Twice a day in the morning and at night before brushing teeth or as much as the patient wants throughout the day. ✓ Sedentary lifestyle: At least every 4 hours |
|
Diet ➢ Eat nutrition-balanced Diet ➢ Maintain Portion -appropriate Diet |
✓ Eat a nutrition-balanced diet: more vegetables and fruits as well as quality proteins. ✓ Maintain portion-appropriate diet: Cease eating when feeling 75% full for each meal. |
✓ Each meal |
| Stay Hydrated | ✓ People may actually be thirsty, not hungry. | ✓ Drink 6 to 8 glasses of water daily in the morning, before and during meals, and throughout the day. ✓ Avoid drinks with calories (e.g. juices). ✓ Drink green tea to boost metabolism. |
|
Large Muscle Exercises ➢ Walking, running, dancing, swimming, Yoga |
✓ Large muscle exercises (e.g. walking, running, swimming, dancing, Yoga) help to burn more calories. ✓ Large muscle exercises also promote lymph flow by creating muscle pumps. |
✓ 30-minutes 3 times a week or daily. |
| Get Enough Sleep | ✓ Lack of sleep increases the production of the stress hormone cortisol, creates hunger, and leads to overeating. ✓ Getting just one more hour of sleep per night reduces belly fat accumulation. |
✓ 7–8 hours of sleep per night. |
Guided by the self-efficacy theory (Figure 1) and based on physiological-cognitive- behavioral principles, TOLF provides self-management strategies to activate the lymphatic system and promote lymph flow to decrease lymphatic pain and reduce the risk and severity of lymphedema.2,26–27,38–40 TOLF intervention focuses on training patient to implement self-management skills in their daily lives outside clinical settings without professionally administered therapy (e.g., by therapists or nurses). In other words, to achieve the therapeutic effects, patients execute home-based self-management skills. Self-efficacy for TOLF intervention refers to a breast cancer survivor’s belief in her ability to execute TOLF self-management skills.2,26–27,38–42 Therefore, building patients’ skills to manage lymphatic pain would increase their self-efficacy for TOLF interventions.
Figure 1.
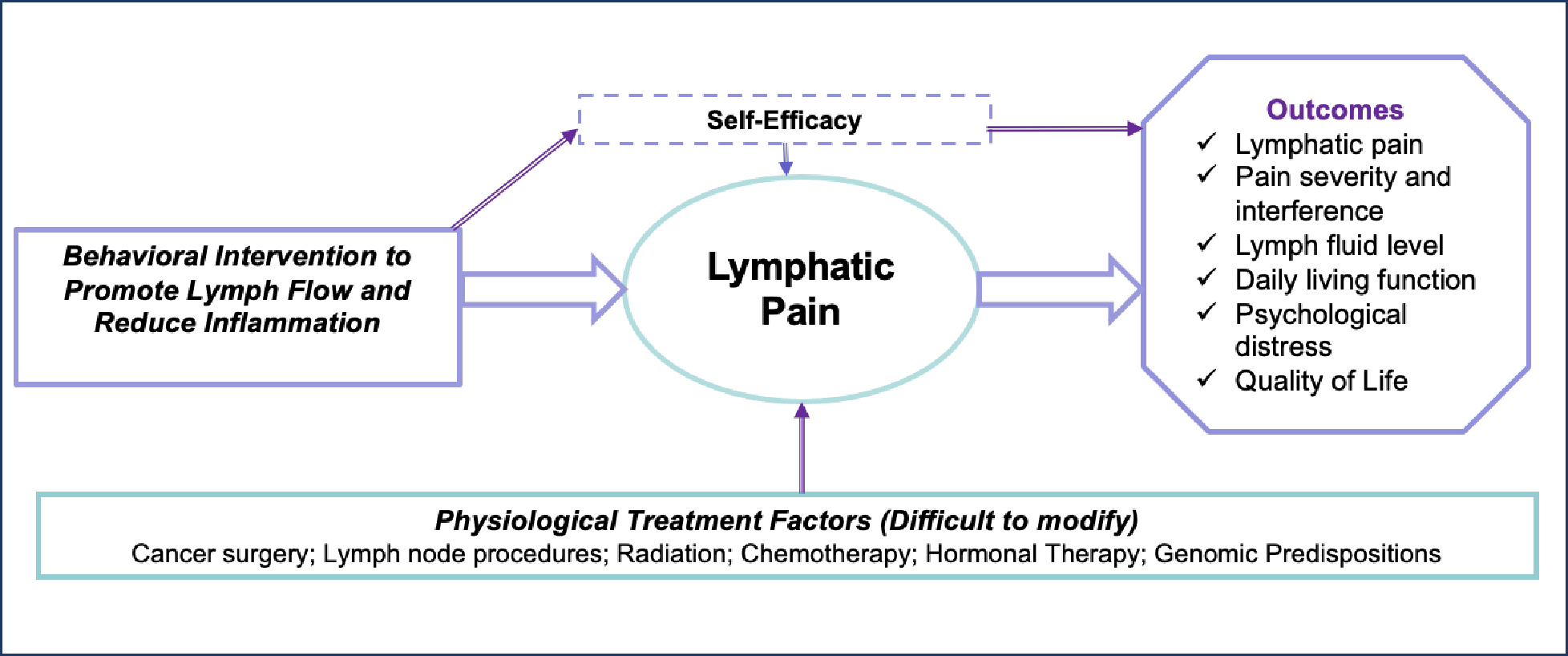
Theoretical Model for Lymphatic Pain Management
In a clinical trial, TOLF intervention was effective in preventing lymph fluid accumulation and maintaining pre-surgical limb volume among over 90% of 140 patients after breast cancer surgery.38 A recently single-arm trial focusing on the immediate effects of TOLF lymphatic exercises2 demonstrated a significant reduction in lymph fluid levels in bioimpedance mean L-Dex scores ( (Mean Changes) = −2.68, 95% CI = [−4.67, −0.69], P = 0.010). TOLF intervention demonstrated greater effects in patients with abnormal lymph fluid levels (i.e., L-Dex 7.1) in mean L-Dex scores ( = −5.19, 95% CI = [−1.75, −8.63], P = 0.008).2
A larger, 12-week, parallel Randomized Clinical Trial (RCT)29 randomized 120 patients either into Arm Precaution (AP) control to improve limb mobility or TOLF intervention to promote lymph flow. At the end of the trial, significantly fewer patients in the TOLF intervention group reported chronic pain (i.e., lymphatic pain) (49% vs. 71%; OR=0.39, 95% CI = [0.17, 0.90], P = 0.021). TOLF intervention was effective in achieving complete pain reduction in 50% of patients (50% vs 22%; OR=3.56, 95% CI = [1.39, 9.76], P = 0.005) compared to the AP control group of 22%. TOLF intervention was effective in lowering significantly median severity scores (Med) of pain (= 0, Interquartile Range [IQR] = 0–1 vs = 1, IQR = 0–2; P = 0.024) and general bodily pain (= 1, IQR = 0–1.5 vs =1, IQR = 1–3; P = 0.040).14 In addition, significantly fewer patients in TOLF group reported arm/hand swelling (P = 0.038). TOLF intervention achieved a 13% reduction in the proportion of patients who took pain medications compared to the AP control group which had a 5% increase. Results of a single-arm trial2 supported the immediate effects of TOLF lymphatic exercises13 on reduction in lymphatic pain ( = -1.00, 95% CI = [−1.5, −0.1], P = 0.004), and arm/hand swelling ( = −1.00, 95% CI = [−1.5, −0.5], P = 0.004).
Recommendations for Research and Clinical Practice
To deploy the emerging knowledge regarding lymphatic pain and different types of pain, the following recommendations are posited to translate and integrate this knowledge into research and clinical practice.
Recommendation for research.
Given that the mechanism of lymphatic pain is associated with lymph fluid accumulation and inflammation, it is possible that lymphatic pain may have different underlying mechanisms compared to other pain phenotypes (e.g., chemotherapy-induced peripheral neuropathy, or arthralgias related to hormonal treatments, pain without swelling). Emerging research evidence presents new insights to differentiate lymphatic pain from other types of pain, which is essential in finding a cure. Future research should explore the unique underlying mechanisms of lymphatic pain through biomarker and genomic approaches as well as associated demographic and socioeconomic determinants (e.g., age, financial status, ethnicity).
Current research suggests that behavioral interventions (e.g., TOLF intervention) designed to promote lymph flow produce significant benefits in reducing lymphatic pain, general bodily pain, and specific lymphedema symptoms (e.g., arm/hand swelling, heaviness, limited movement in shoulder and arm). 2,26–27,29,38–40 Future research should investigate the effects of different behavioral interventions on different types of pain, including lymphatic pain. Research should also focus on developing targeted and effective therapeutic interventions for pain by investigating which therapeutic approaches, such as pharmacological, low-dose laser, or behavioral approaches, are most efficacious for different types of pain with different etiologies.
Recommendations for clinical practice.
To differentiate lymphatic pain from other types of pain, clinicians may use the reliable and valid patient outcome measures (e.g., BCLE-SEI) to evaluate and track lymphatic pain in clinical practice to ensure timely interventions.43–44 Research-based behavioral interventions to promote lymph flow and inflammation (e.g., TOLF intervention) are able to induce immediate and long-term therapeutic effects to relieve lymphatic pain, swelling, and lymphedema symptoms. 2,26–27,29,38–40 Such interventions can be prescribed to patients to reduce not only lymphatic pain, but also general bodily pain and other symptoms associated with fluid accumulation.
Conclusion
At least one-third of breast cancer survivors have suffered lymphatic pain that is defined as co-occurring pain or sensations of aching, soreness, or tenderness and swelling.1,16 Importantly, lymphatic pain results in greater impairments in ADLs, emotional distress, and overall health.1,5,16 Precision assessment that enables clinicians to distinguish different types of pain is imperative to find a cure for pain. Behavioral interventions to promote lymph flow are safe, efficacious, and affordable. Such interventions are beneficial for millions of women treated for breast cancer worldwide to reduce lymphatic pain, general bodily pain, and other symptoms related to fluid accumulations and chronic inflammation.
Highlights.
More than one third of women treated for breast cancer are affected by lymphatic pain.
Lymphatic pain refers to co-occurring pain, or sensations of aching or soreness or tenderness, and swelling. Pharmacological approaches, such as NSAIDS, opioids, antiepileptics, ketamine and lidocaine, have very limited effects on lymphatic pain.
Innovative behavioral interventions to promote lymph flow and reduce inflammation are promising to reduce lymphatic pain.
Funding:
This review was part of study funded by Oncology Nursing Foundation (2022 ONF RE33) with Mei R Fu as the principal investigator and the National Institute of Health /National Science Foundation /National Cancer Institute (1R01CA214085–01) with Mei R Fu and Yao Wang as the multiple principal investigators. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the funders. The funders had no role in the preparation of the manuscript and decision to publish.
Footnotes
Conflicts of Interest
The authors declare no conflict of interest.
Human and Animal Rights and Informed Consent
Not Applicable
Compliance with Ethical Standards: Not Applicable
Contributor Information
Jeanna Mary Qiu, Harvard Medical School, 25 Shattuck Street, Boston, MA 02115.
Mei Rosemary Fu, The Dorothy and Dale Thompson Missouri Endowed Professor in Nursing, Associate Dean for Research, School of Nursing and Health Studies, University of Missouri-Kansas City, 2464 Charlotte Street, 2nd Floor, Room 2326, Kansas City, Missouri 64108.
Catherine S. Finlayson, Lienhard School of Nursing, College of Health Professions, Pace University, Wright Cottage, 861 Bedford Road, Pleasantville, NY 10570.
Charles P. Tilley, Rutgers University, School of Nursing-Camden.
Rubén Martín Payo, Faculty of Medicine & Health Sciences, University of Oviedo, Cristo Campus, 33006, Oviedo, Principality of Asturias, Spain.; Principality of Asturias Health Research Institute (ISPA), University Hospital Avenue, 33011, Oviedo, Principality of Asturias, Spain.
Stephanie Korth, University Health Kansas City, Building #1, 2101 Charlotte Street, Suite #110, Kansas City, Missouri 64108.
Howard L. Kremer, University Health Kansas City.
Cynthia L. Russell Lippincott, School of Nursing and Health Studies, University of Missouri-Kansas City, 2464 Charlotte, Kansas City, MO 64108.
References
- 1.Fitzgerald Jones K, Fu MR, McTernan ML, et al. Lymphatic Pain in Breast Cancer Survivors. Lymphat Res Biol. 2022;20(5):525–532. doi: 10.1089/lrb.2021.0017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fu MR, McTernan ML, Qiu JM, et al. The Effects of Kinect-Enhanced Lymphatic Exercise Intervention on Lymphatic Pain, Swelling, and Lymph Fluid Level. Integr Cancer Ther. 2021;20:15347354211026757. doi: 10.1177/15347354211026757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization, 2023. Breast cancer. Available on https://www.who.int/news-room/fact-sheets/detail/breast-cancer. (Accessed February 3, 2024).
- 4.American Cancer Society (ACS). Breast Cancer Facts & Figures. Atlanta, 2022–2024: American Cancer Society, Inc. Accessed February 3, 2024. URL: https://www.cancer.org/research/cancer-facts-statistics/breast-cancer-facts-figures.html [Google Scholar]
- 5.Fu MR, Axelrod D, Guth A, McTernan ML, Qiu JM, Zhou Z, Ko E, Magny-Normilus C, Scagliola J, Wang Y. The Effects of Obesity on Lymphatic Pain and Swelling in Breast Cancer Patients. Biomedicines. 2021. Jul 14;9(7):818. doi: 10.3390/biomedicines9070818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fu MR, Axelrod D, Cleland CM, Qiu Z, Guth AA, Kleinman R, Scagliola J, Haber J. Symptom report in detecting breast cancer-related lymphedema. Breast Cancer (Dove Med Press). 2015. Oct 15;7:345–52. doi: 10.2147/BCTT.S87854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paice JA. Pain in Cancer Survivors: How to Manage. Curr Treat Options Oncol. 2019;20(6):48. Published 2019 May 6. doi: 10.1007/s11864-019-0647-0 [DOI] [PubMed] [Google Scholar]
- 8.Treede RD, Rief W, Barke A, et al. Chronic pain as a symptom or a disease: the IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11). Pain. 2019;160(1):19–27. doi: 10.1097/j.pain.0000000000001384 [DOI] [PubMed] [Google Scholar]
- 9.Jiang C, Wang H, Wang Q, Luo Y, Sidlow R, Han X. Prevalence of Chronic Pain and High-Impact Chronic Pain in Cancer Survivors in the United States. JAMA Oncol. 2019;5(8):1224–1226. doi: 10.1001/jamaoncol.2019.1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones KF, Fu MR, Wood Magee L, et al. “It Is So Easy For Them to Dismiss”: A Phenomenological Study of Cancer Survivors with Chronic Cancer-Related Pain [published online ahead of print, 2023 Mar 21]. J Palliat Med. 2023;10.1089/jpm.2022.0538. doi: 10.1089/jpm.2022.0538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Armer JA, Ostby P, Ginex P, et al. ONS Guidelines for Cancer treatment-related lymphedema. Onco Nurs Forum. 2020; 47(5), 518–538. DOI 10.1188/20.ONF.518-538. [DOI] [PubMed] [Google Scholar]
- 12.Dowell D, Ragan KR, Jones CM, et al. Prescribing opioids for pain—The New CDC Clinical Practice Guideline. N Engl J Med 2022;387(22):2011–2013; doi: 10.1056/NEJMp2211040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sweeney C, Schmitz KH, Lazovich D, Virnig BA, Wallace RB, Folsom AR. Functional limitations in elderly female cancer survivors. J Natl Cancer Inst 2006;98:521–529. [DOI] [PubMed] [Google Scholar]
- 14.O’Toole JA, Ferguson CM, Swaroop MN, et al. The impact of breast cancer-related lymphedema on the ability to perform upper extremity activities of daily living. Breast Cancer Res Treat 2015;150:381–388. [DOI] [PubMed] [Google Scholar]
- 15.Park JH, Merriman J, Brody A, et al. Limb volume changes and activities of daily living: A prospective study. Lymphat Res Biol 2020. doi: 10.1089/Irb.2020.0077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fu MR, McTernan ML, Qiu JM, et al. Co-occurring Fatigue and Lymphatic Pain Incrementally Aggravate Their Negative Effects on Activities of Daily Living, Emotional Distress, and Overall Health of Breast Cancer Patients. Integr Cancer Ther. 2022;21:15347354221089605. doi: 10.1177/15347354221089605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fu MR, Kang Y. Psychosocial impact of living with cancer-related lymphedema. Semin Oncol Nurs. 2013;29(1):50–60. doi: 10.1016/j.soncn.2012.11.007 [DOI] [PubMed] [Google Scholar]
- 18.Reis JC, Antoni MH, Travado L. Emotional distress, brain functioning, and biobehavioral processes in cancer patients: a neuroimaging review and future directions. CNS Spectr. 2020;25(1):79–100. doi: 10.1017/S1092852918001621 [DOI] [PubMed] [Google Scholar]
- 19.Shi S, Lu Q, Fu MR, et al. Psychometric properties of the Breast Cancer and Lymphedema Symptom Experience Index: The Chinese version. Eur J Oncol Nurs. 2016;20:10–16. doi: 10.1016/j.ejon.2015.05.002 [DOI] [PubMed] [Google Scholar]
- 20.Cachero-Rodríguez J, Menéndez-Aller Á, Fu MR, Llaneza-Folgueras A, Fernández-Alvarez MM, Martín-Payo R. Psychometric Properties of the Spanish Version of Breast Cancer and Lymphedema Symptom Experience Index. Psicothema. 2022;34(2):291–298. doi: 10.7334/psicothema2021.388 [DOI] [PubMed] [Google Scholar]
- 21.Fu MR, Conley YP, Axelrod D, et al. Precision assessment of heterogeneity of lymphedema phenotype, genotypes and risk prediction. Breast. 2016;29:231–240. doi: 10.1016/j.breast.2016.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kwan ML, Yao S, Lee VS, Roh JM, Zhu Q, Ergas IJ, Liu Q, Zhang Y, Kutner SE, Quesenberry CP Jr, Ambrosone CB, Kushi LH. Race/ethnicity, genetic ancestry, and breast cancer-related lymphedema in the Pathways Study. Breast Cancer Res Treat. 2016. Aug;159(1):119–29. doi: 10.1007/s10549-016-3913-x. Epub 2016 Jul 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fu MR, Wang Y, Li C, et al. Machine learning for detection of lymphedema among breast cancer survivors. Mhealth. 2018;4:17. Published 2018 May 29. doi: 10.21037/mhealth.2018.04.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wei X, Lu Q, Jin S, Li F, Zhao Q, Cui Y, Jin S, Cao Y, Fu MR (2021, online ahead of print). Developing and validating a prediction model for lymphedema detection in breast cancer survivors. European Journal of Oncology Nursing, 10.1016/j.ejon.2021.102023. [DOI] [PubMed] [Google Scholar]
- 25.Fu MR, Aouizerat BE, Yu G, et al. Model-Based Patterns of Lymphedema Symptomatology: Phenotypic and Biomarker Characterization. Curr Breast Cancer Rep. 2021;13(1):1–18. doi: 10.1007/s12609-020-00397-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu F, Li F, Fu MR, et al. Self-management Strategies for Risk Reduction of Subclinical and Mild Stage of Breast Cancer-Related Lymphedema: A Longitudinal, Quasi-experimental Study. Cancer Nurs. 2021;44(6):E493–E502. doi: 10.1097/NCC.0000000000000919 [DOI] [PubMed] [Google Scholar]
- 27.Du X, Li Y, Fu L, et al. Strategies in activating lymphatic system to promote lymph flow on lymphedema symptoms in breast cancer survivors: A randomized controlled trial. Front Oncol. 2022;12:1015387. Published 2022 Oct 24. doi: 10.3389/fonc.2022.1015387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fu MR, Rosedale M. Breast cancer survivors’ experiences of lymphedema-related symptoms. J Pain Symptom Manage. 2009;38(6):849–859. doi: 10.1016/j.jpainsymman.2009.04.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fu MR, Axelrod D, Guth AA, et al. A Web- and Mobile-Based Intervention for Women Treated for Breast Cancer to Manage Chronic Pain and Symptoms Related to Lymphedema: Results of a Randomized Clinical Trial. JMIR Cancer. 2022;8(1):e29485. Published 2022 Jan 17. doi: 10.2196/29485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Armer J, Fu MR. Age differences in post-breast cancer lymphedema signs and symptoms. Cancer Nurs. 2005;28(3):200–209. doi: 10.1097/00002820-200505000-00007 [DOI] [PubMed] [Google Scholar]
- 31.Buki LP, Rivera-Ramos ZA, Kanagui-Muñoz M, Heppner PP, Ojeda L, Lehardy EN, Weiterschan KA. “I never heard anything about it”: Knowledge and psychosocial needs of Latina breast cancer survivors with lymphedema. Women’s Health (Lond). 2021. Jan-Dec;17:17455065211002488. doi: 10.1177/17455065211002488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eversley R, Estrin D, Dibble S, Wardlaw L, Pedrosa M, Favila-Penney W. Post-treatment symptoms among ethnic minority breast cancer survivors. Oncol Nurs Forum. 2005. Mar 5;32(2):250–6. doi: 10.1188/05.ONF.250-256. [DOI] [PubMed] [Google Scholar]
- 33.Miller JW, Smith JL, Ryerson AB, Tucker TC, Allemani C. Disparities in breast cancer survival in the United States (2001–2009): Findings from the CONCORD-2 study. Cancer. 2017. Dec 15;123 Suppl 24(Suppl 24):5100–5118. doi: 10.1002/cncr.30988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wielen LM, Gilchrist EC, Nowels MA, Petterson SM, Rust G, Miller BF. Not Near Enough: Racial and Ethnic Disparities in Access to Nearby Behavioral Health Care and Primary Care. J Health Care Poor Underserved. 2015. Aug;26(3):1032–47. doi: 10.1353/hpu.2015.0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fu MR, LeMone P, McDaniel RW. An integrated approach to an analysis of symptom management in patients with cancer. Oncol Nurs Forum. 2004;31(1):65–70. doi: 10.1188/04.ONF.65-70 [DOI] [PubMed] [Google Scholar]
- 36.Washington AE, Lipstein SH The patient-centered outcomes research institute--promoting better information, decisions, and health. N. Engl. J. Med. 2011;365:e31. doi: 10.1056/NEJMp1109407. [DOI] [PubMed] [Google Scholar]
- 37.Helyer LK, Varnic M, Le LW, Leong W, McCready D Obesity is a risk factor for developing postoperative lymphedema in breast cancer patients. Breast J. 2010;16:48–54. doi: 10.1111/j.1524-4741.2009.00855.x. [DOI] [PubMed] [Google Scholar]
- 38.Fu MR, Axelrod D, Guth AA, et al. Proactive approach to lymphedema risk reduction: a prospective study. Ann Surg Oncol. 2014;21(11):3481–3489. doi: 10.1245/s10434-014-3761-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fu MR, Axelrod D, Guth AA, et al. mHealth self-care interventions: managing symptoms following breast cancer treatment. Mhealth. 2016;2:28. doi: 10.21037/mhealth.2016.07.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fu MR, Axelrod D, Guth AA, et al. Usability and feasibility of health IT interventions to enhance self-care for lymphedema symptom management in breast cancer survivors. Internet Interv. 2016;5:56–64. doi: 10.1016/j.invent.2016.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anderson KO, Dowds BN, Pelletz RE, Edwards WT, Peeters-Asdourian C. Development and initial validation of a scale to measure self-efficacy beliefs in patients with chronic pain. Pain. 1995. Oct;63(1):77–84. [DOI] [PubMed] [Google Scholar]
- 42.Porter LS, Keefe FJ, Garst J, McBride CM, Baucom D. Self-efficacy for managing pain, symptoms, and function in patients with lung cancer and their informal caregivers: associations with symptoms and distress. Pain. 2008. Jul 15;137(2):306–315. doi: 10.1016/j.pain.2007.09.010. Epub 2007 Oct 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nahum JL, Fu MR, Scagliola J, et al. Real-time electronic patient evaluation of lymphedema symptoms, referral, and satisfaction: a cross-sectional study. Mhealth. 2021;7:20. Published 2021 Apr 20. doi: 10.21037/mhealth-20-118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bustos VP, Friedman R, Pardo JA, Granoff M, Fu MR, Singhal D. Tracking Symptoms of Patients With Lymphedema Before and After Power-Assisted Liposuction Surgery. Ann Plast Surg. 2023;90(6):616–620. doi: 10.1097/SAP.0000000000003430 [DOI] [PubMed] [Google Scholar]


