Abstract
In the native skeletal muscle, capillaries reside in close proximity to muscle stem cells (satellite cells, SCs) and regulate SC numbers and quiescence through partially understood mechanisms difficult to study in vivo. This challenge could be addressed by the development of a 3-dimensional (3D) in vitro model of vascularized skeletal muscle harboring both a pool of quiescent SCs and a robust network of capillaries. Still, studying interactions between SCs and endothelial cells (ECs) within a tissue-engineered muscle environment has been hampered by the incompatibility of commercially available EC media with skeletal muscle differentiation. In this study, we first optimized co-culture media and cellular ratios to generate highly functional vascularized human skeletal muscle tissues (“myovascular bundles”) with contractile properties (~10 mN/mm2) equaling those of avascular, muscle-only tissues (“myobundles”). Within one week of muscle differentiation, ECs in these tissues formed a dense network of capillaries that co-aligned with muscle fibers and underwent initial lumenization. Incorporating vasculature within myobundles increased the total SC number by 82%, with SC density and quiescent signature being increased proximal (≤20μm) to EC networks. In vivo, at two weeks post-implantation into dorsal window chambers in nude mice, vascularized myobundles exhibited improved calcium handling compared to avascular implants. In summary, we engineered highly functional myovascular tissues that enable studies of the roles of EC-SC crosstalk in human muscle development, physiology, and disease.
Keywords: skeletal muscle, tissue engineering, vascularization, satellite cell, window chamber
Graphical Abstract

1. Introduction:
Skeletal muscle comprises an average of 30-50% of the human body by mass and generates contractile forces responsible for locomotion and respiration. The high regenerative capacity of skeletal muscle is enabled by the existence of muscle stem cells, termed satellite cells (SCs), which in healthy muscle express the transcription factor Pax7, are negative for MyoD, and reside in a quiescent, non-cycling (Ki67−) state in specialized niches abutting myofibers [1-3]. Upon injury, SCs activate (expressing MyoD), proliferate, and either (1) return to quiescence or (2) commit to differentiation to repair damaged or form new myofibers [4, 5]. SC fate in skeletal muscle is regulated by cell-matrix interactions and both juxtacrine and paracrine signals from myofibers and resident non-muscle cells [1, 4-6]. In particular, SCs and endothelial cells (ECs) are found in close proximity [7-9] where reciprocal juxtacrine [10] and paracrine [7, 9, 10] interactions have been shown to maintain SC quiescence [7, 9, 10] and capillary patterning [10] in homeostasis, or promote synergistic angio-myogenesis during regeneration [11-13]. Additionally, SC density is directly correlated with vascular density in vivo [9, 14, 15] and dysfunction of one cell type impairs function of the other via paracrine signals [16, 17]. However, direct mechanistic studies of SC-EC interactions in vivo are hindered by the inability to study muscle biology in the absence of vasculature and by local and systemic confounding variables present in the in vivo environment.
The modular nature of in vitro tissue-engineered platforms permits tightly controlled cellular crosstalk studies of ECs with multiple cell types in a physiologically relevant, three-dimensional (3D) culture environment. In our original studies, we have described engineering of highly functional 3D neonatal [18] and adult-derived rat skeletal muscle tissues harboring a quiescent SC pool with regenerative capacity that could be augmented by the inclusion of macrophages [19]. More recently, we have developed engineered human skeletal muscle tissues (“myobundles”) using primary or iPSC-derived muscle progenitor cells which allowed us to study human muscle response to exercise [20, 21], disease [20, 22], injury [23], and drug treatment [22, 24]. Recently, we have shown that human primary SCs, which are typically activated during in vitro culture, undergo deactivation in 3D myobundles to attain a quiescent (Pax7+/MyoD−/Ki67−) but heterogeneous phenotype akin to that of the native SCs in vivo [25]. Yet, interactions between SCs and microvasculature have not been studied within homeostatic tissue-engineered skeletal muscles, though in 2D culture, dysfunctional ECs have been shown to impair SC proliferation and differentiation [17].
To date, in vitro vascularization of skeletal muscle tissues has been mainly utilized to accelerate their perfusion, viability, and engraftment efficiency after implantation in vivo [26-29]. However, functionality of such vascularized muscle models has been significantly hindered by inability to define proper coculture media. Simple mixing of EC- and muscle-specific growth media does not resolve this issue, yielding suboptimal EC viability and vascular tube formation and decreased muscle differentiation and function [30, 31]. Recently, human plasma-like media (HPLM), comprised of human physiologically relevant macro- and micronutrient levels, has been developed to more accurately model human circulating factors [32]. Compared to traditional media, HPLM better replicates clinical drug responses [32-35] and T cell function in vitro [34], but its potential to support coculture of incompatible cell types has not been established. Alternatively, EC-muscle cocultures have been engineered to mimic the compartmentalization of fluid within vasculature [30, 36]. In these models, a hollow EC channel surrounded by engineered muscle tissue allows for use of cell-specific media inside and outside the channel, which improves muscle functionality compared to use of mixed media [30, 36]. Still, compared to native muscle tissue, ECs in these models only line the muscle surface, limiting their utility for studies of complex EC-SC interactions found in the native muscle. As such, the roles of ECs in SC niche formation and function are yet to be studied in a homeostatic 3D muscle tissue environment in vitro.
To study the effect of vasculature on the human SC niche, we first optimized media for coculture of primary myogenic cells and ECs and generated vascularized myobundles with contractile function comparable to that of avascular myobundles. In these “myovascular tissues”, we then evaluated the effect of vasculature on abundance of Pax7+ cells, their quiescence signature, and proximity to formed vessels. Finally, avascular and myovascular tissues were implanted into mouse dorsal skin-fold window chambers to assess the longevity of implanted vascular networks and the effects of in vitro pre-vascularization on myobundle perfusion throughout the duration of implantation and Ca2+ handling 2 weeks post-implantation.
2. Materials and Methods
2.1. Human Myogenic Cell Isolation and Culture
Human skeletal muscle tissue was acquired from surgical waste of deidentified donors with no known pathology per Duke University Institutional Review Board approved protocols (Pro00052540, Pro00054162) and myogenic cells were isolated as previously described [24, 25]. Briefly, freshly harvested human muscle tissue was washed in PBS with 1X Anti-Anti and finely minced with a scalpel. Tissue fragments were partially enzymatically digested in 0.05% Trypsin for 30 minutes on a rocker at 37°C. Following digestion, trypsin was neutralized using Fetal Bovine Serum (FBS) (Hyclone), the remaining fragments were spun down and resuspended in DMEM Low Glucose (Sigma) supplemented with 1X Anti-Anti (Gibco), then pre-plated in 1 T-175 for 1 Hour at 37°C. The tissue fragment containing media was then collected, spun down, and the tissue fragments were resuspended in skeletal muscle 2D growth media (DMEM Low Glucose, 10% FBS, 0.4ng/mL Dexamethasone (Sigma), 10ng/mL epidermal growth factor (EGF, Prospecio), and 0.064mg/mL Penicillin, supplemented with 2X Anti-Anti) and plated on Matrigel (Corning) coated plates to allow for cell outgrowth for approximately 1 week (with media changes every 2 days). During and following outgrowth, SCs from muscle tissue fragments proliferate and spontaneously differentiate into myoblasts, which can be repeatedly passaged and cultured or frozen for downstream use. These myogenic cells were cultured on Matrigel coated flasks in skeletal muscle growth media, passaged with 0.025% Trypsin (Thermo Fisher) at ~85% confluency, frozen at earlier passages if needed, and used at passage 5 for all shown studies. Cells from three human donors were used, and each experiment was repeated using cells from at least two independent donors.
2.2. Human Endothelial Progenitor Cell Isolation and Culture
Human endothelial progenitor cells (EPCs) were generously provided by the Truskey Lab at Duke University. As previously described, EPCs were isolated from human cord blood (sourced from the Carolina Cord Blood Bank, Duke IRB protocol Pro00058324) [37]. The whole blood was diluted 1:1 with Hanks Balanced Salt Solution and Histopaque-1077 (Sigma). The suspension was spun at 740G for 30 minutes to create a density gradient and mononuclear cells were collected and plated in endothelial growth media (EBM2 (Lonza) supplemented with EGM-2 bullet kit and 10% FBS) on Collagen Type I (rat tail, BD Biosciences) coated flasks. Media was changed daily, and endothelial colonies were visible within 7-10 days of plating. EPC identity was confirmed via flow cytometry for CD31 and cells were passaged or frozen for later use. EPCs were cultured in endothelial growth media (EGM-2, Lonza) and were passaged with 0.05% Trypsin through passage 10.
2.3. Lentivirus Production and Cell Transduction
Lentiviral plasmids were designed, generated, and lentivirus was produced as previously described [38-40]. Briefly, to generate a CMV-mCherry lentivirus, a fluorescent mCherry reporter gene was inserted into a pRRL-CMV plasmid (a gift from Inder Verma, Salk Instute). To generate an MHCK7-GCaMP6 lentivirus, the muscle-specific promotor MHCK7 [41] and the fluorescent calcium reporter GCaMP6 [42] were inserted into the pRRL backbone plasmid. HEK293T cells were cultured in high glucose DMEM supplemented with 10% fetal bovine serum and 1% Penicillin/Streptomycin. At 70-80% confluency, HEKs were co-transfected with pRRL-CMV-mCherry or pRRL-MHCK7-GCaMP6, VSVG, and Pax2 plasmids using Jetprime. HEK media was changed 16 hours post-transfection and media containing virus was collected 2-3 days following the initial media change. Virus was purified by precipitation with addition of 33% LentiX- Concentrator at 4°C on an orbital shaker and pelleted by centrifugation at 1500xg for 45 minutes. Precipitated virus was then resuspended in PBS, aliquoted, and stored at −80°C. To label EPCs with mCherry flourescence, cells were transduced with the CMV-mCherry lentivirus (4.8 vp/cell) at the time of plating and media was changed 24 hours later. Transduced EPCs were cultured for 3-4 days to allow for visual confirmation of lentiviral expression, before being used for myovascular bundle fabrication. For GCaMP6 transduction of myoblasts, pRRL-MHCK7-GCaMP6 lentivirus was added to the cell suspension (0.17 vp/cell) at the time of myobundle fabrication.
2.4. Human Primary Myobundle Fabrication
Human myobundles were fabricated in polydimethylsiloxane PDMS molds with two 5-mm long, 1-mm wide semi-circular channels, as previously described [21, 22, 24, 25]. Briefly, molds were coated with 2% (w/v) pluronic F-127 (Invitrogen) for 30 minutes at room temperature to prevent myobundle adhesion. Cerex® (nylon) frames were placed in the molds to serve as anchor points for the myobundles and support uniaxial tension. A cell solution (375K myogenic cells – with or without 18.75K EPCs – in 9.3μL media and 1μL of 50 U/mL thrombin (Sigma) in 1% BSA in PBS per myobundle) and a gelling solution (1.75μL media, 5μL Matrigel, and 5μL of 22mg/mL fibrinogen (Sigma) in PBS per myobundle) were prepared separately on ice in vials to make 4 myobundles at a time. Cell solution was added to the gelling solution, pipetted into the channels of the PDMS molds, and left to polymerize for 30 minutes at 37°C prior to the addition of media. Myobundles were then cultured dynamically on a rocker in in skeletal muscle 3D growth media (2D growth media supplemented with 1.5mg/mL 6-aminocaproic acid (ACA, Sigma) and 10ng/mL human VEGF-165 (Peprotech)) for 4 days, after which media was switched to custom-designed human plasma like media (HPLM) (Sup Table 1) supplemented with 10 ng/mL human VEGF-165 for an additional 7-10 days of differentiation. At culture day 2, frames with myobundles were removed from PDMS molds and cultured in free-floating conditions [43]. Media was changed every other day through both the growth and differentiation phases.
2.5. Force Testing
Contractile function of myobundles was tested between days 7 and 9 of differentiation using a custom force measurement set-up, as previously described [21, 24, 25, 44]. Briefly, individual myobundles were placed in the testing chamber in DMEM Low Glucose media and maintained at 37°C, pinned to a fixed PDMS block on one end and a PDMS float attached to a force transducer (mounted on a computer-controlled linear actuator (Thor Labs)) on the other. The myobundle frame was cut to allow myobundle stretching (via the linear actuator) and tissue was stimulated with 90V/cm 5ms electrical pulses using parallel platinum electrodes submerged in the chamber. Twitch contraction was measured at 0, 5, 10, and 15% stretch. At the stretched length yielding the maximum twitch contractile force, myobundles were stimulated at frequencies of 1, 5, 10, 20, and 40Hz to record force-frequency response. Recorded force traces were analyzed using a custom MATLAB script to determine the maximum active force, time to peak twitch, and half-relaxation time [21, 24, 25, 44].
2.6. Ca2+ Transient Imaging
At the time of myobundle formation, myoblasts were transduced with an MHCK7-GCaMP6 lentivirus, as previously described [19, 24, 38]. Electrically stimulated Ca2+ transients were quantified after 1 week of differentiation or after additional 2 weeks post-implantation into mouse dorsal skinfold window chambers [19]. Myobundles were transferred into a custom glass-bottom chamber filled with 37°C Tyrode’s solution and stimulated using parallel carbon electrodes. GCaMP6 fluorescence movies were acquired through a FITC filter set at 50 fps rate under 4X magnification using an Andor iXon camera affixed to a Nikon microscope. Upon background subtraction, relative change in fluorescence ΔF/F0 within a region of interest encompassing the majority of the myobundle was calculated as (F-F0)/F0 with F0 being the resting fluorescence level. Ca2+ transient amplitude was calculated as the peak ΔF/F0 induced by electrical stimulation. All analysis was performed using the Andor Solis software and a custom Matlab script [19].
2.7. Immunohistology and Image Analysis
Myobundles were fixed with 2% paraformaldehyde in PBS overnight at 4°C. Following fixation, samples were washed with PBS and either immunostained as whole tissues or embedded in Optimum Cooling Temperature (OCT) Compound (Sakura) for cryosectioning and cross-sectional immunostaining. For cross-sectional images, myobundles were embedded in OCT perpendicular to the cutting surface, snap frozen in liquid nitrogen, cut in 10 μm sections, and mounted on slides. For antigen retrieval (M-cadherin staining), slides were submerged in citrate buffer containing slide holders and placed in a 2100 Antigen Retriever (Aptum Biologics) for the duration of the retrieval cycle. Samples were then blocked for 30 minutes at room temperature with a solution of 5% donkey serum, 0.1% Triton X-100 and 0.03% Sodium Azide in PBS. Primary antibodies were applied overnight at 4°C followed by secondary antibodies applied for 2 hours at room temperature. For whole-tissue stains, samples were blocked overnight at 4°C with a solution of 5% donkey serum (Abcam), 0.3% Triton X-100 (Thermo Fisher), and .03% Sodium Azide in PBS. Both primary and secondary antibodies (Sup Table 2) were applied overnight. Samples were then washed and mounted for imaging using ProLong Glass Antifade Mountant (Invitrogen) and were imaged using an Andor Dragonfly spinning disk confocal microscope.
Immunoflourescent images were analyzed using Image J or custom CellProfiler Pipelines. Generally, primary objects were identified using Huang global thresholding (Image J) or robust background adaptive thresholding (CellProfiler). For whole myobundle quantifications (CD31+ Area, %Pax7+ Nuclei, Pax7+ cell distance to vessels, %Ki67+ SC), images were captured through the depth of the myobundle in 1mm Z-slices. A minimum of 3 representative Z-slices were then quantified and averaged for each tissue. For 2D myotube diameter measurements, 20 manual measurements of diameter were averaged per monolayer. Each diameter measurement was made perpendicular to the longitudinal axis of the myotube and intersected a minimum of 1 myogenin+ nucleus. Vascular morphology was quantified using the ImageJ tool “Angiogenesis Analyzer” [45], which identified vascular structures from thresholded whole-mount CD31-stained images and calculated total vessel length. The average vessel length was calculated by dividing the total vessel length by the number of independent vascular structures, counted manually. The vessel alignment was quantified using the ImageJ “Directionality” tool to analyze alignment of vessel “trees” generated by Angiogenesis Analyzer. Three representative Z slices were quantified and averaged for each tissue. For concentric vessel density analysis myobundle cross-sectional area was outlined manually and automatically segmented into three segments with equal thickness (center, middle, and outer) using a custom Cell Profiler pipeline. Vessel density in each segment was calculated by dividing CD31+ area within the segment by the segment area.
2.8. Dorsal Skinfold Window Chamber Implantation
Animal experiments were approved by the Duke University Institutional Animal Care and Use committee and followed all clinical and ethical regulations as detailed by the NIH Guide for Care and Use of Laboratory Animals. MHCK7-GCaMP6 transduced avascular (control) and myovascular bundles were implanted into athymic nude mice dorsal skinfold window chambers as previously described [18, 19, 38]. Briefly, mice (~10 weeks of age; 25-35g; male) were anaesthetized via an intraperitoneal injection of ketamine/xylazine (100 mg kg−1/ 10 mg kg−1) and were attached to a temporary “C-frame” which was used to demarcate where the forward-facing skin needed to be resected. All layers of the forward-facing skin (cutis, subcutis, retractor and panniculus carnosis muscles, and associated fascia) were dissected away and the titanium dorsal skinfold window chamber was assembled around the dissected skin. The frame with myobundles was then laid over the panniculus carnosis muscle of the rear-facing skin and a sterile cover glass was placed over the implanted tissue while the chamber was superfused with sterile PBS. The mice were then removed from the “C-frame”, the cover glass was secured with a ring clamp, and the chamber was secured with sutures along the top line. Pre-operatively, mice were injected subcutaneously with buprenorphine ER and were left to recover on a heat pad under observation. The mice were checked daily for signs of discomfort or infection with antibiotics administered orally as needed.
2.9. Intravital Imaging of Implanted Myobundles
Intravital imaging of implanted avascular and vascularized myobundles was performed on days 4, 7, 11, and 14 post-implantation in dorsal window chambers using our previously published methods [18, 19, 46]. Briefly, mice were anesthetized by isoflurane and positioned onto a custom frame mounted above the microscope objective. The body of the mouse was covered with a 37°C heating pad. All images were captured by a 2.5X objective (Zeiss), a tunable filter (Cambridge Research & Instrumentation, Inc.), and a DVC Camera (DVC-1412M, ThorLabs). Brightfield images were captured at 510 nm and mCherry images were captured at 610 nm. To quantify blood vessel density (BVD), images were processed using local contrast enhancement in Image J (CLAHE, FIJI), myobundle borders were manually identified, and large blood vessels located behind the implanted myobundles were manually excluded. BVD was calculated by dividing total vessel area by the myobundle area.
2.10. Statistical Methods
Data was analyzed using Graphpad Prism Version 10.2.0 and shown as mean ± SEM. For immunohistochemistry quantification and contractile force measurements, individual data points represent separate myobundles or 2D cultures. Outlier tests (ROUT, Q=2%) were run on all data points. Paired or unpaired T-tests were used for comparisons between 2 groups with normal distributions depending on experimental design. Mann-Whitney tests were used for comparison between 2 groups with non-normal distributions. 1- or 2-way ANOVA’s were used for multiple comparisons (based on experimental design) followed by Sidak post-hoc testing as applicable. Ex vivo GCaMP quantifications were analyzed using a 2-way ANOVA main effects model with repeated measures.
3. Results
3.1. Optimized culture conditions enable the formation of vascular networks with lumen-like structures within engineered myobundles
Generation of high-fidelity in vitro vascularized engineered muscle tissues has long proved challenging due to differing EC and muscle media requirements [30, 31]. To enable the culture of vascularized human myobundles, we first optimized media conditions for both human primary endothelial progenitor cells (EPCs) and human primary myogenic cells in 2D monoculture. The effects of 3D skeletal muscle growth media (3DG) on EPC viability and proliferation were assessed by culturing EPCs in 2D for 4 days (Sup Fig 1a) to mimic the 4-day growth phase of myobundle culture (Fig 1a). While commercially available EGM-2 EC media resulted in continuous EPC proliferation, 3DG media resulted in progressive cell death (Sup Fig 1b, c). This cell death was prevented by supplementing 3DG with 10 ng/mL of VEGF-165, the most abundant and biologically active VEGF isoform [47, 48], which maintained EPC numbers through at least 4 days of culture (Sup Fig 1b,c).
Figure 1: Development of aligned and lumenized endothelial cell (EC) vessels within vascularized myobundles.
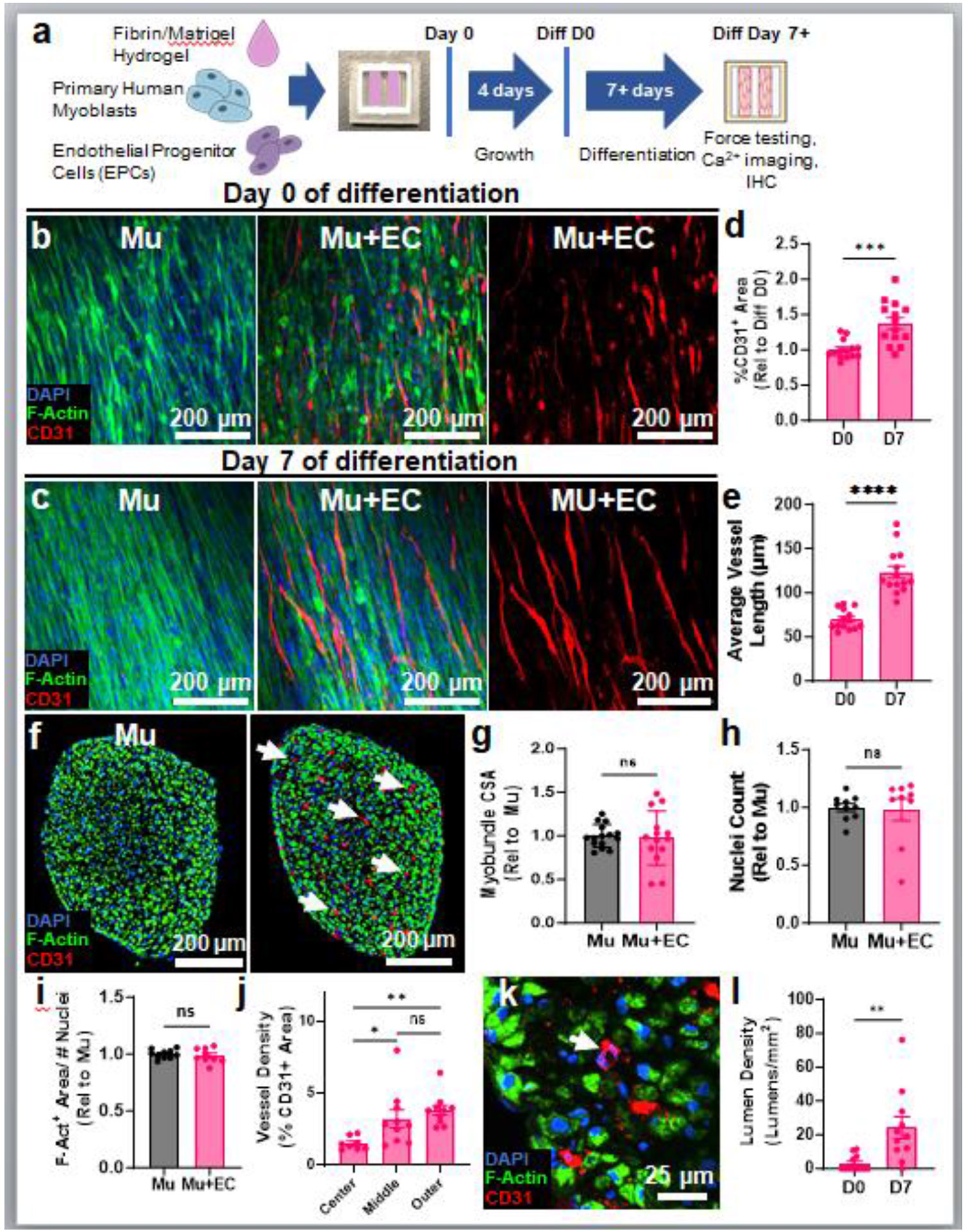
a, Experimental schematic of myovascular bundle formation, culture, and analysis (IHC, immunohistochemistry). b-e, Representative whole-mount images of avascular, muscle-only (Mu) and vascularized (Mu+EC) myobundles just prior to switch to HPLM differentiation media (D0, b) and on day 7 of differentiation (D7, c; note co-alignment with F-actin+ myofibers) and corresponding quantifications of CD31+ (EC) area (n=14 myobundles, ***p<0.001, d) and vessel length (n=14 myobundles, ****p<0.0001, e). f-i, Representative images of Mu and Mu+EC myobundle cross-sections (f) and corresponding quantifications of myobundle cross-sectional area (n=14-15 myobundles, ns: p>0.05, g), nuclei count per cross-section (n=9-10 myobundles, ns: p>0.05, h), F-Actin area per nucleus (n=9-10, ns: p>0.05, i), and regional CD31+ (EC) density in myovascular bundles (n=11 myobundles ns: p>0.05, *p<0.05, **p<0.01, j) on D7 of differentiation. k-l, Representative image of myovascular bundle cross-section showing formation of CD31+ lumens (white arrow, k) and corresponding quantification of lumen density at D0 and D7 (n=11-12, **p<0.01, I).
Typically, vascularized engineered muscle tissues have been generated by mixing skeletal muscle and EC media in varying ratios resulting in compromised engineered muscle functionality [36]. To overcome this limitation, we developed a serum-free human plasma-like media (HPLM) that better mimics nutrients and metabolites found in healthy plasma (Sup Table 1). Specifically, by utilizing the human blood metabolome database [49], we identified 34 metabolites expressed at greater than 1μM in healthy adult blood and included those with physiological levels of amino acids and established levels of inorganic salts, trace elements, and vitamins previously utilized in serum-containing HPLM [32-34]. We then identified and added 25 additional hormones, antioxidants, lipids, and growth factors that support differentiation of human myoblasts including IGF-1 [50] and circulating factors such as bFGF [51], EGF [52, 53], thyroid hormone [54-58], and heparin [59, 60], which modify growth factor activity and support both myogenesis [50, 57-59] and vasculogenesis [51-55, 60]. Addition of VEGF to HPLM supported the survival of EPCs for at least 6 days of 2D monoculture (Sup Fig 1b), with cell numbers that were comparable to cell numbers during culture in 3DG+VEGF media (Sup Fig 1d).
We next determined the effects of the tested culture media on primary human myogenic cell differentiation for 5 days in 2D culture by immunostaining for sarcomeric α-actinin (SAA) and the terminal muscle differentiation transcription factor myogenin. We found that EGM-2 alone limited fusion and differentiation capacity of human myogenic cells, as evident from reductions in percentage of myogenin+ nuclei incorporated into SAA+ myotubes and average myotube diameter (Sup Fig 2a-c). Furthermore, use of EGM2 media yielded the formation of non-contractile myobundles (data not shown). In contrast, use of HPLM enabled robust myoblast fusion as shown by the formation of greater than 60 μm thick SAA+ myotubes (Sup Fig 2c). Similar to previous studies with use of traditional muscle differentiation media [61], mixing of EGM-2 with HPLM resulted in a dose-dependent loss of myoblast fusion capacity (Sup Fig 2b) and decrease of myotube diameter (Sup Fig 2c).
For subsequent 3D culture, we characterized the composition of cells used for myobundle fabrication by staining for muscle specific transcription factors Pax7 and MyoD, the EC marker CD31, and the pericyte marker PDGFRβ. This cell population consisted of predominantly myogenic progenitors (90.4±1.65% MyoD+ or Pax7+, Sup Fig 3a,b), a small fraction of PDGFRβ+ cells (1.6±0.24%, Sup Fig 3c-e), no CD31+ ECs (Sup Fig 3f-h), and the reminder of vimentin+ cells (Sup Fig 3i,j). To generate 3D vascularized myobundles (“myovascular bundles”), EPCs were lentivirally transduced with mCherry to facilitate identification during culture and supplemented into the myogenic cell suspension used for myobundle fabrication (Fig 1a). We first optimized the EPC: ratio and found that the 1:20 ratio (5%) enabled formation of vascular networks (Sup Fig 4a,b) without detrimental effects on muscle contractile function (Sup Fig 4c). We then compared EC network formation and muscle force generation between HPLM and our traditional (N2) muscle differentiation media [25] both supplemented with VEGF. In 1-week differentiated myobundles, HPLM supported greater EC network density (Sup Fig 5a,b) and contractile force amplitude (Sup Fig 5c) compared to traditional muscle differentiation media. Based on these findings, we proceeded to characterize myovascular bundles comprised of 5% EPCs and cultured in 3DG growth media followed by HPLM differentiation media, both supplemented with VEGF-165. On day 0 of differentiation (D0), densely packed F-actin+ differentiating myotubes and developing mCherry+ EPC networks were present within the myovascular bundles but lacked the organization present in native muscle (Fig 1b). However, by day 7 of differentiation (D7), F-actin and SAA immunostaining revealed uniaxially aligned, cross-striated multinucleated myotubes present in in both avascular and vascularized myobundles (Fig 1c, Sup Fig 6a,b), indicating that the presence of vasculature did not impact muscle architecture or sarcomere organization. Additionally, mCherry+ EPC networks aligned within myobundles starting from D0 (Sup Fig 6c), thus recapitulating the structure of native muscle-resident vasculature [28]. We further found that CD31+ area in vascularized myobundles increased from D0 to D7 (Fig 1d), confirming the findings from our 2D studies that optimized media conditions prevent loss of EC viability. Additionally, with time of culture, organization of formed vascular networks was improved, as evident from the increased average vessel length at D7 of differentiation (Fig 1e).
To further assess morphological differences between avascular and vascularized myobundles, we performed cross-sectional stainings of D7 tissues (Fig 1f) and found no differences in gross tissue morphology, including tissue cross-sectional area (CSA) (Fig 1g), nuclear density (Fig 1h), F-Actin+ CSA (Sup Fig 6d), or F-Actin+ CSA per nucleus (indicating myotube size, Fig 1i). In myovascular bundles, we found CD31+ vessels to be the most abundant at the outer tissue region (Fig 1f,j), indicating potential roles of oxygen and mass transfer gradients in the vessel formation. Further analysis of CD31+ cells in cross-sections of D0 and D7 tissues revealed an EC network density of 185.4±60.34 CD31+ cords/mm2 at D7 (Sup Fig 6e), comparable to capillary density of native skeletal muscle [62, 63]. Capillary development in vivo is characterized by the formation of vascular cord-like structures which subsequently mature to form lumens and ultimately support blood flow [64]. Notably, from D0 to D7 of differentiation, we observed the formation of lumen-like structures at a density of 24.23±21.61 lumens/mm2 (Fig 1k, l), which despite being significantly lower than in native muscle [62, 63], suggested progressive maturation of vascular networks with time of culture.
3.2. Myovascular bundles maintain contractile function comparable to avascular myobundles
To assess the effects of vascularization on muscle function, we next compared isometric force and Ca2+ transient generation in D7 avascular and myovascular tissues. We found no difference in the maximum active force, maximum specific force, or passive force generation in avascular vs. vascularized myobundles (Fig 2a-d). Analysis of twitch kinetics revealed a small but significant decrease in time to peak contractile force in vascularized myobundles (Mu: 75.45 ± 1.872 ms, Mu+EC: 68.00 ± 1.214 ms) (Fig 2e), while no changes were observed in half-relaxation time (HRT, Fig 2f). We next measured electrically induced Ca2+ transients in D7 myobundles and myovascular bundles transduced with a muscle-specific MHCK7-driven GCaMP6 lentivirus, as previously described [18, 19, 38]. In agreement with similar muscle mass and contractile strength of myobundles and myovascular bundles, we found no differences in maximal Ca2+ transient amplitude or kinetics (not shown) during twitch and tetanic contraction (Fig 2h-j). Collectively, these findings indicate successful vascularization of human myobundles with no detrimental effects on high contractile strength achieved in muscle-only tissues.
Figure 2: Functional characterization of myovascular bundles.

a-f, Representative traces of twitch (1Hz stimulation) and tetanic (40Hz stimulation) contractions in avascular (Mu) and myovascular (Mu+EC) tissues at differentiation day D7 (a) and corresponding quantifications of maximum active (tetanic) force (n=28-31 myobundles, ns: not significant, b), specific tetanic force (n=10-12, ns: not significant, c), passive force generation at 5%, 10%, and 15% stretch (n=7-12 myobundles, ns: not significant, d), time to peak twitch (n=22-23, **p<0.01, e), and half-relaxation time (HRT, n=22-26, ns: not significant, f). g, Representative snapshots of baseline and peak GCaMP6 fluorescence during twitch and tetanic contraction in Mu and Mu+EC myobundles. h-j, Representative traces of twitch and tetanic Ca2+ transients (shown as relative GCaMP6 fluorescence, ΔF/F0, h) and corresponding quantifications of twitch (n=16, ns: not significant, i) and tetanic (n=16, ns: not significant, j) Ca2+ transient amplitudes shown relative to Mu group.
3.3. Vascularization of myobundles results in increased number, juxtavascular positioning, and decreased proliferation of Pax7+ SCs
In native skeletal muscle, ECs and SCs correlate in numbers [9] and capillaries reside near SCs to form juxtavascular niches where they regulate SC quiescence and activation [10]. In our engineered tissues, the presence of Pax7+ cells expressing the niche marker M-cadherin (Sup Fig 7a,b) suggested the formation of SC niches [25]. Quantitative image analysis further revealed that compared to avascular bundles, myovascular bundles exhibited an 82% increase in Pax7+ SC number by D7, while no significant differences were observed at D0 (Fig 3a,b). We next assessed Pax7+ cell cycle activity (a hallmark of SC quiescence) by immunostaining for the proliferation marker Ki67 and found no difference in SC Ki67 expression between control and vascularized myobundles on either D0 or D7 (Fig 3c). As SCs are typically found within 20μm of ECs in healthy native muscle [9], we next examined if this preferential SC-EC proximity was replicated in myovascular bundles by quantifying SC density within and beyond 20μm of the nearest CD31+ vessel (Fig 3d). Consistent with average SC density results (Fig 3b), SC density in vascularized myobundles was increased both within and beyond 20μm of the nearest CD31+ vessel (Fig 3e). Additionally, it was significantly increased within vs. beyond 20μm of the nearest CD31+ vessel (Fig 3e). We then corroborated these findings by analyzing images of “mock” Mu+EC myobundles generated by overlaying representative CD31+ vessel masks derived from myovascular bundle images onto images of avascular myobundles stained for Pax7 (Sup Fig 7c). In this case, we found no preferential SC localization near overlaid vessels either through Pax7 density analysis (Sup Fig 7d) or when comparing distance histograms of mock vs. true myovascular myobundles (Sup Fig 7e,f). Furthermore, analysis of Pax7+ nuclei in myovascular bundles revealed significantly decreased cell cycle activity (assessed by the fraction of Ki67+/Pax7+ nuclei) within 20μm of the nearest vessel (Fig 3f), consistent with the enhanced quiescence of juxtavascular SCs observed in vivo [10]. Together, these findings show that the presence of EC networks within myovascular bundles increases SC numbers and decreases SC proliferation near formed vessels.
Figure 3: Effects of vascularization on abundance and location of Pax7+ cells in myobundles.

a-c, Representative whole-mount images of myobundles on D0 and D7 of differentiation and corresponding quantifications of percentage of Pax7+ nuclei (relative to Mu group, n=11-26 myobundles, ****: p<0.0001, ns: not significant, b) and Pax7+ cells that are Ki67+ (n=11-26, ns: not significant, c). d, Representative whole-mount images of Mu and Mu+EC myobundles. Yellow arrows in myovascular bundles denote Pax7+ nuclei in juxtavascular positions. e, Quantification of Pax7+ density in Mu myobundles and within 20μm and beyond 20μm from the nearest CD31+ vessels in vascularized myobundles, all shown relative to Mu group (n=16, *p<0.05, **p<0.01, ****p<0.0001), h, Quantification of percentage Pax7+ cells that are Ki67+ within 20μm and beyond 20μm from the nearest CD31+ vessels in vascularized myobundles (n=13, *p<0.05).
3.4. Factors secreted by vascularized myobundles do not increase SC abundance in avascular myobundles
EC regulation of SCs is known to be multifactorial involving both juxtacrine and paracrine signaling [7, 9, 10]. To assess if paracrine signaling was sufficient to increase SC numbers as observed in vascularized tissues, we treated avascular myobundles daily with media conditioned for 24hrs by either avascular myobundles (control) or myovascular bundles (Fig 4a). Whole-tissue immunostaining revealed no effect of myovascular bundle conditioned media on SC abundance (Fig 4b,c) or cell cycling (Fig 4d) in avascular myobundles. Furthermore, unlike previous reports suggesting that paracrine signaling from ECs can improve engineered muscle contractility [65], we found no effects of conditioned media from either control or vascularized myobundles on contractile force amplitude (Fig 4e) or kinetics (Sup Fig 8).
Figure 4: Effects of vascular paracrine signals on SC abundance in myobundles.

a, Schematic of experimental design for media conditioning experiments. b-e, Representative whole-mount images of Mu myobundles, Mu+EC myobundles, Mu myobundles conditioned with Mu myobundle media, and Mu myobundles conditioned with Mu+EC myobundle media (b) and corresponding quantifications of the percent of Pax7+ nuclei shown relative to Mu group (n=13-16 myobundles, **p<0.01, c), percentage of Pax7+ cells that are Ki67+ (n=13-16, ns: not significant, d) and maximum tetanic force (40Hz stimulation) shown relative to Mu group (n=14-16, ns: not significant, e).
3.5. In vitro vascularization improves myobundle calcium handling following implantation into mouse dorsal skinfold window chambers
Engineered muscle tissues have long held promise as a therapeutic approach to regenerate skeletal muscle following large injuries or genetic diseases [66]. We have previously implanted myobundles transduced with MHCK7-GCaMP lentivirus into athymic nude mouse dorsal skinfold window chambers, to allow live, minimally invasive measurements of implant vascularization and Ca2+ handling [18, 19, 46]. Building on this work, we utilized the window chamber implantation model to assess the longevity of engineered myovascular bundles in vivo and study how the presence of in vitro engineered vasculature affects myobundle vascularization/perfusion and Ca2+ handling post-implantation (Fig 5a,b). Specifically, by intravital in situ imaging, we found progressive increase in perfused blood vessel density (BVD) within both avascular and pre-vacularized myobundles over 14 days post-implantation (Fig 5c,d). Interestingly, in vitro vascularization of myobundles showed no significant effects on perfused vessel ingrowth at any point post-implantation compared to vessel ingrowth in avascular implants (Fig 5c,d). By specifically tracking the implanted vasculature via mCherry fluorescence, we further found that implanted mCherry+ vessels remained stable after implantation (Fig 5e,f). After two weeks, implanted myobundles were explanted and electrically stimulated to assess potential effects on Ca2+ handling properties. Despite no differences in time course of in vivo vascularization, Ca2+ transient amplitude was significantly increased in myovascular vs. avascular myobundle explants (Fig 5g-i) and associated with a faster Ca2+ transient rise (Fig 5j). Together, these studies showed that compared to muscle-only implants, in vitro pre-vascularization of engineered muscle tissues can result in improved muscle Ca2+ handling upon implantation in vivo.
Figure 5: Improved functionality of myovascular bundles implanted in mouse dorsal window chambers.

a, Schematic of dorsal skinfold window chamber experimental design, timeline, and end point. b, Photograph of myobundles implanted into a dorsal window chamber in a nude mouse. c-d, Representative intravital images of blood vessel ingrowth into initially avascular and pre-vascularized myobundles on day 4 and 14 of post-implantation (c) and corresponding quantification of blood vessel density (BVD, n=18-28 myobundles, significant increasing trend, p<0.0001, d). e-f, Representative intravital images of mCherry fluorescence at post-implantation D4, 7, 11, and 14 (e), and corresponding quantification of % mCherry+ area within myobundle (n=24-26 myobundles, ns: not significant, f). g, Representative ex vivo recorded snapshots of baseline and peak GCaMP6 fluorescence during twitch (1Hz stimulation) and tetanic (40 Hz stimulation) contraction in Mu and Mu+EC myobundle explants. h-j, Representative traces of twitch and tetanic Ca2+ transients (shown as relative GCaMP6 fluorescence, ΔF/F0, h) and corresponding quantifications of twitch and tetanic Ca2+ transient amplitudes shown relative to Mu group (n=17-18 myobundles, **p<0.01, i) and time to peak twitch (n=17-18 myobundles, *p<0.05, j).
4. Discussion
In this study, we have developed an in vitro model of vascularized skeletal muscle with similar contractile function and increased SC density compared to avascular, muscle-only tissues. In vitro engineering of functional vascularized muscle tissues has been hindered by incompatibility between muscle and EC culture media, compromising both tissue myogenesis and vasculogenesis [31, 36, 65]. In agreement, use of EC growth media (EGM-2) in our study, even when diluted, inhibited muscle differentiation and fusion (Sup Fig 2). We thus optimized a custom-made serum-free HPLM differentiation media which when supplemented with VEGF and other pro-myoangiogenic factors (e.g. bFGF and EGF) supported both muscle (Sup Fig 2, Fig 2) and EPC viability and capillary network formation (Sup Fig 1, Fig 1). Previous studies with HPLM media reported more clinically predictive in vitro drug responses and altered cellular metabolism, including rewiring of urea metabolism in cancer cells [32-35]. HPLM has also been shown to drastically alter T lymphocyte transcriptome, cytokine secretion, and improve their activation response [34]. To our knowledge, this is the first report where HPLM was used for co-culture of cell types with distinct media requirements. Underlying mechanisms of how HPLM supports successful engineering of myovascular bundles remain to be explored. Importantly, the use of customized HPLM for the first time allowed generation of highly functional vascularized muscle tissues with similar levels of muscle differentiation and contractile strength compared to muscle-only controls (Fig 2).
In particular, the specific forces of myovascular bundles (~10mN/mm2) are orders of magnitude higher than previously reported (10-40 μN/mm2) for engineered vascularized skeletal muscle [30, 36, 67]. Previous compartmentalized muscle-endothelium models have demonstrated that human umbilical vein ECs (HUVECs) can increase contractile force of C2C12 [65] and primary human [30, 36] engineered muscle, presumably via secretion of trophic paracrine factors. In contrast, we found that media conditioned by myovascular bundles had no effect on force generation of avascular myobundles (Fig 4). The reason for this difference may be distinct secretomes of different EC sources used, different culture media, high myobundle functionality limiting potential for additional force increase, or the much lower endothelial:myogenic cell ratio (1:20) used in this compared to other conditioned media studies [65]. Nevertheless, the myovascular bundles developed here permit simple 3D co-culture of human myogenic and endothelial cells characterized by robust muscle function, native vascular density, and no need for use of complex compartmentalized fluid systems.
Previously, our group and others have shown that engineered 3D muscle tissues can form and maintain a niche-like environment in which Pax7+ SC-like cells attain a non-cycling state and approximate in vivo SC transcriptome and injury response [18, 25, 68-70]. In vivo, the function of SC niches is influenced by adjacent capillaries whereby SCs secrete VEGFA to attract ECs, while ECs maintain SC quiescence though expression of a Notch ligand DLL4 [10]. Here we show, for the first time in engineered skeletal muscles, that the presence of EC capillary structures increases SC numbers (Fig 3b). Furthermore, we find that SCs are preferentially located within 20 μm of formed vessels (Fig 3e), where SCs adopt a less proliferative state (Fig 3f), as observed in vivo [10]. These findings support reciprocal paracrine [7, 9, 10] and juxtacrine [10] interactions suggested to direct SC fate and EC patterning. Our conditioned media studies (Fig 4) and similar rates of SC proliferation at D0 and D7 (Fig 3) further suggest that increased SC numbers are likely not due to secreted ECs paracrine factors promoting SC proliferation [9, 17]. However, as SC numbers are increased within 20μm of capillaries, the potential of local secreted paracrine factors increasing SC numbers cannot be ruled out. Alternatively, the main factor leading to increased SC numbers in myovascular bundles may be EC juxtacrine signaling which may act in part through Notch signaling [10] to increase cell survival [71], inhibit fusion to myofibers [72], and support SC quiescence [73, 74] and self-renewal [75].
In addition to robust contractile function, lumen density in vascularized myobundles (~24 lumens/mm2) was higher than previously reported for in vitro engineered muscles (~7.5 lumens/mm2) [31], but still significantly lower than in native muscle [62, 63]. Interestingly, while the human vascular networks in myobundles were robustly maintained for at least two additional weeks after implantation in vivo (Fig 5f), this was not the case beyond two weeks of 3D co-culture in vitro. Timed addition of growth factors, such as angiopoietin [76] and platelet derived growth factor-BB [77], may further improve the density, lumenization, and longevity of myobundle vessels in vitro. Furthermore, incorporation of additional cell types, such as pericytes, to support vessel stability [78], macrophages, to complement myovascular interactions and improve muscle regenerative capacity [11, 19], fibro-adipogenic progenitors (FAPs), as a source of VEGFA [10, 79], or functional motoneurons [80, 81], to model the intricate neuromyovascular milieu of skeletal muscle in vivo, could all improve the physiological relevance of myovascular bundles which currently lack distinct fiber types [82] and only approximate the native extracellular matrix [83, 84] and SC niche [4, 85]. Beyond the studies of homeostatic roles of ECs in healthy skeletal muscle, myovascular bundles could also serve to investigate muscle-EC crosstalk in the context of muscle development [86, 87], exercise [15, 88], regeneration [19, 25], and various myovascular pathologies, including Duchenne’s muscular dystrophy [89, 90], myositis [91, 92], and peripheral vascular disease [93, 94]. Moreover, as incorporation of ECs in 3D engineered tissues [95, 96] and use of HPLM to culture cancer cells [32, 33, 35] alter in vitro drug responses, use of myovascular bundles may allow for more clinically predictive drug screening in the future.
Previous studies have shown that engineered C2C12 [28, 97] and human [26] muscle tissues pre-vascularized in vitro exhibit enhanced vascularization, perfusion, and survival in vivo. In our 2-week implantation study, in vitro pre-vascularization of human myobundles did not accelerate blood vessel ingrowth compared to that of avascular implants (Fig 5d), potentially due to use of a different scaffold, cell types, or an implantation model compared to previous studies. For example, we utilized blood-derived EPCs as opposed to HUVECs, commonly used as a model of mature ECs in vascularized engineered tissues [28, 98-100]. While EPCs show significant expansion potential [101], network forming capacity [102], and pro-angiogenic factor secretion [103] in vitro, there have been conflicting reports about their ability to maintain network stability post-implantation [104, 105]. In our studies, mCherry-labelled EPC networks within implanted myobundles remained stable for two weeks in vivo, but their ability to stimulate ingrowth of host capillaries and form anastomoses relative to other available EC sources remains to be studied within the same in vivo setting. Moreover, while EPCs are commonly utilized in various in vitro applications [106, 107], the EC function is known to be organ-specific [108, 109], thus warranting the future studies with muscle-resident ECs [110], ideally using methods that also enable perfusion of engineered vessels [111] in myobundles.
Notably, we for the first time demonstrate that in vitro pre-vascularization of engineered muscle tissues improves their Ca2+ transient amplitude and kinetics after implantation (Fig 5g-j). This improved Ca2+ handling without improved vascularization in vivo, may suggest an increase in regenerative capacity due to increased SC numbers or the presence of ECs. We have previously shown that upon implantation in window chambers, myobundles undergo a transient ischemic insult, likely due to no mass transfer through the window chamber glass coverslip and the switch from dynamic environment in vitro to static environment in vivo. In such conditions, increased SC density and myobundle regenerative capacity may eventually improve implanted muscle Ca2+ handling in vivo [18, 19]. Simultaneously, in transient hypoxic conditions post-implantation, EPCs could secrete factors that promote myofiber survival, SC proliferation, and/or myogenesis, similar to their responses to native muscle injury [9, 11, 17, 112]. Together, these findings warrant future mechanistic studies of how in vitro vascularization yields improved Ca2+ handling of implanted myobundles. Additionally, in vivo Ca2+ transient studies remain to be complemented with measurements of implant contractile force, resistance to fatigue, and neuromuscular integration to fully elucidate the effects of in vitro vascularization on muscle functionality post-implantation.
In summary, we have developed a biomimetic in vitro model of human vascularized skeletal muscle tissue that maintains robust functional metrics of avascular myobundles, exhibits increased SC density, mimics the native proximity between ECs and SCs, and results in improved Ca2+ handling compared to muscle-only tissues after implantation in vivo. In the future, this 3D cell culture platform could be used to model and study roles of myovascular crosstalk in muscle growth and disease/injury response, and, ultimately, enable the development of engineered skeletal muscle tissues with enhanced therapeutic potential.
Supplementary Material
Statement of Significance.
In native skeletal muscle, intricate relationships between vascular cells and muscle stem cells (“satellite cells”) play critical roles in muscle growth and regeneration. Current methods for in vitro engineering of contractile skeletal muscle do not recreate capillary networks present in vivo. Our study for the first time generates in vitro robustly vascularized, highly functional engineered human skeletal muscle tissues. Within these tissues, satellite cells are more abundant and, similar as in vivo, they are more dense and less proliferative proximal to endothelial cells. Upon implantation in mice, vascularized engineered muscles show improved calcium handling compared to muscle-only implants. We expect that this versatile in vitro system will enable studies of muscle-vasculature crosstalk in human development and disease.
Acknowledgements
We thank Drs. George Truskey and Ellery Jones at Duke University for generously providing the human endothelial progenitor cells used in this work and Abbigail Helfer for contributing to graphic design and illustrations. We also thank Dr. Sharon Gerecht and Emily Warren for providing the hiPSC-derived pericytes as a positive control for validation of the PDGFRβ antibody.
Funding
This work was supported by the National Institutes of Health grants [UG3TR002142 (NB), U01EB028901 (NB), R01AR070543 (NB), R01AR079223 (NB), R01AR082979 (NB), R01AR083155 (NB), 1F31AR080574 (TB), and T32GM00855 (TB)].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Competing Interests
All authors declare no conflicts of interest.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data Availability
The raw/processed data required to reproduce these findings are available upon request.
References
- [1].Yin H, Price F, Rudnicki MA, Satellite cells and the muscle stem cell niche, Physiol Rev 93(1) (2013) 23–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Loreti M, Sacco A, The jam session between muscle stem cells and the extracellular matrix in the tissue microenvironment, NPJ Regen Med 7(1) (2022) 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Relaix F, Bencze M, Borok MJ, Der Vartanian A, Gattazzo F, Mademtzoglou D, Perez-Diaz S, Prola A, Reyes-Fernandez PC, Rotini A, Taglietti t., Perspectives on skeletal muscle stem cells, Nat Commun 12(1) (2021) 692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bentzinger CF, Wang YX, Dumont NA, Rudnicki MA, Cellular dynamics in the muscle satellite cell niche, EMBO Rep 14(12) (2013) 1062–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wosczyna MN, Rando TA, A Muscle Stem Cell Support Group: Coordinated Cellular Responses in Muscle Regeneration, Dev Cell 46(2) (2018) 135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Evano B, Tajbakhsh S, Skeletal muscle stem cells in comfort and stress, NPJ Regen Med 3 (2018) 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Latroche C, Gitiaux C, Chretien F, Desguerre I, Mounier R, Chazaud B, Skeletal Muscle Microvasculature: A Highly Dynamic Lifeline, Physiology (Bethesda) 30(6) (2015) 417–27. [DOI] [PubMed] [Google Scholar]
- [8].Schmalbruch H, Hellhammer U, The number of nuclei in adult rat muscles with special reference to satellite cells, Anat Rec 189(2) (1977) 169–75. [DOI] [PubMed] [Google Scholar]
- [9].Christov C, Chretien F, Abou-Khalil R, Bassez G, Vallet G, Authier FJ, Bassaglia Y, Shinin V, Tajbakhsh S, Chazaud B, Gherardi RK, Muscle satellite cells and endothelial cells: close neighbors and privileged partners, Mol Biol Cell 18(4) (2007) 1397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Verma M, Asakura Y, Murakonda BSR, Pengo T, Latroche C, Chazaud B, McLoon LK, Asakura A, Muscle Satellite Cell Cross-Talk with a Vascular Niche Maintains Quiescence via VEGF and Notch Signaling, Cell Stem Cell 23(4) (2018) 530–543 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Latroche C, Weiss-Gayet M, Muller L, Gitiaux C, Leblanc P, Liot S, Ben-Larbi S, Abou-Khalil R, Verger N, Bardot P, Magnan M, Chretien F, Mounier R, Germain S, Chazaud B, Coupling between Myogenesis and Angiogenesis during Skeletal Muscle Regeneration Is Stimulated by Restorative Macrophages, Stem Cell Reports 9(6) (2017) 2018–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Rhoads RP, Johnson RM, Rathbone CR, Liu X, Temm-Grove C, Sheehan SM, Hoying JB, Allen RE, Satellite cell-mediated angiogenesis in vitro coincides with a functional hypoxia-inducible factor pathway, Am J Physiol Cell Physiol 296(6) (2009) C1321–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Germani A, Di Carlo A, Mangoni A, Straino S, Giacinti C, Turrini P, Biglioli P, Capogrossi MC, Vascular endothelial growth factor modulates skeletal myoblast function, Am J Pathol 163(4) (2003) 1417–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Emslie-Smith AM, Engel AG, Microvascular changes in early and advanced dermatomyositis: a quantitative study, Ann Neurol 27(4) (1990) 343–56. [DOI] [PubMed] [Google Scholar]
- [15].Jensen L, Bangsbo J, Hellsten Y, Effect of high intensity training on capillarization and presence of angiogenic factors in human skeletal muscle, The Journal of physiology 557(Pt 2) (2004) 571–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hettinger ZR, Kargl CK, Shannahan JH, Kuang S, Gavin TP, Extracellular vesicles released from stress-induced prematurely senescent myoblasts impair endothelial function and proliferation, Exp Physiol 106(10) (2021) 2083–2095. [DOI] [PubMed] [Google Scholar]
- [17].Kargl CK, Nie Y, Evans S, Stout J, Shannahan JH, Kuang S, Gavin TP, Factors secreted from high glucose treated endothelial cells impair expansion and differentiation of human skeletal muscle satellite cells, The Journal of physiology 597(20) (2019) 5109–5124. [DOI] [PubMed] [Google Scholar]
- [18].Juhas M, Engelmayr GC Jr., Fontanella AN, Palmer GM, Bursae N, Biomimetic engineered muscle with capacity for vascular integration and functional maturation in vivo, Proc Natl Acad Sci U S A 111(15) (2014) 5508–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Juhas M, Abutaleb N, Wang JT, Ye J, Shaikh Z, Sriworarat C, Qian Y, Bursae N, Incorporation of macrophages into engineered skeletal muscle enables enhanced muscle regeneration, Nat Biomed Eng 2(12) (2018) 942–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Chen Z, Li B, Zhan RZ, Rao L, Bursae N, Exercise mimetics and JAK inhibition attenuate IFN-gamma-induced wasting in engineered human skeletal muscle, Sci Adv 7(4) (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Khodabukus A, Madden L, Prabhu NK, Koves TR, Jackman CP, Muoio DM, Bursae N, Electrical stimulation increases hypertrophy and metabolic flux in tissue-engineered human skeletal muscle, Biomaterials 198 (2019) 259–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wang J, Zhou CJ, Khodabukus A, Tran S, Han SO, Carlson AL, Madden L, Kishnani PS, Koeberl DD, Bursae N, Three-dimensional tissue-engineered human skeletal muscle model of Pompe disease, Commun Biol 4(1) (2021) 524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Khodabukus A, Kaza A, Wang J, Prabhu N, Goldstein R, Vaidya VS, Bursae N, Tissue-Engineered Human Myobundle System as a Platform for Evaluation of Skeletal Muscle Injury Biomarkers, Toxicol Sci 176(1) (2020) 124–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Madden L, Juhas M, Kraus WE, Truskey GA, Bursae N, Bioengineered human myobundles mimic clinical responses of skeletal muscle to drugs, Elife 4 (2015) e04885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wang J, Broer T, Chavez T, Zhou CJ, Tran S, Xiang Y, Khodabukus A, Diao Y, Bursae N, Myoblast deactivation within engineered human skeletal muscle creates a transcriptionally heterogeneous population of quiescent satellite-like cells, Biomaterials 284 (2022) 121508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Gholobova D, Terrie L, Mackova K, Desender L, Carpentier G, Gerard M, Hympanova L, Deprest J, Thorrez L, Functional evaluation of prevascularization in one-stage versus two-stage tissue engineering approach of human bio-artificial muscle, Biofabrication 12(3) (2020) 035021. [DOI] [PubMed] [Google Scholar]
- [27].Yan G, Yan R, Chen C, Chen C, Zhao Y, Qin W, Veldman MB, Li S, Lin S, Engineering vascularized skeletal muscle tissue with transcriptional factor ETV2-induced autologous endothelial cells, Protein Cell 10(3) (2019) 217–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Koffler J, Kaufman-Francis K, Shandalov Y, Egozi D, Pavlov DA, Landesberg A, Levenberg S, Improved vascular organization enhances functional integration of engineered skeletal muscle grafts, Proc Natl Acad Sci U S A 108(36) (2011) 14789–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Gholobova D, Gerard M, Terrie L, Desender L, Shansky J, Vandenburgh H, Thorrez L, Coculture Method to Obtain Endothelial Networks Within Human Tissue-Engineered Skeletal Muscle, Methods Mol Biol 1889 (2019) 169–183. [DOI] [PubMed] [Google Scholar]
- [30].Kim H, Osaki T, Kamm RD, Asada H, Multiscale engineered human skeletal muscles with perfusable vasculature and microvascular network recapitulating the fluid compartments, Biofabrication (2022) [DOI] [PubMed] [Google Scholar]
- [31].Levenberg S, Rouwkema J, Macdonald M, Garfein ES, Kohane DS, Darland DC, Marini R, van Blitterswijk A, Mulligan RC, D'Amore PA, Langer R, Engineering vascularized skeletal muscle tissue, Nat Biotechnol 23(7) (2005) 879–84. [DOI] [PubMed] [Google Scholar]
- [32].Cantor JR, Abu-Remaileh M, Kanarek N, Freinkman E, Gao X, Louissaint A Jr., Lewis CA, Sabatini M, Physiologic Medium Rewires Cellular Metabolism and Reveals Uric Acid as an Endogenous Inhibitor of UMP Synthase, Cell 169(2) (2017) 258–272 e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Vande Voorde J, Ackermann T, Pfetzer N, Sumpton D, Mackay G, Kalna G, Nixon C, Blyth K, Gottlieb E, Tardito S, Improving the metabolic fidelity of cancer models with a physiological cell culture medium, Sci Adv 5(1) (2019) eaau7314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Leney-Greene MA, Boddapati AK, Su HC, Cantor JR, Lenardo MJ, Human Plasma-like Medium Improves T Lymphocyte Activation, iScience 23(1) (2020) 100759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Senkowski W, Gall-Mas L, Falco MM, Li Y, Lavikka K, Kriegbaum MC, Oikkonen J, Bulanova D, Pietras EJ, Vossgrone K, Chen YJ, Erkan EP, Dai J, Lundgren A, Gronning Hog MK, Larsen IM, Lamminen T, Kaipio K, Huvila J, Virtanen A, Engelholm L, Christiansen P, Santoni-Rugiu E, Huhtinen K, Carpen O, Hynninen J, Hautaniemi S, Vaharautio A, Wennerberg K, A platform for efficient establishment and drug-response profiling of high-grade serous ovarian cancer organoids, Dev Cell 58(12) (2023) 1106–1121 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kim H, Osaki T, Kamm RD, Asada HH, Tri-culture of spatially organizing human skeletal muscle cells, endothelial cells, and fibroblasts enhances contractile force and vascular perfusion of skeletal muscle tissues, FASEB J 36(8) (2022) e22453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kang SD, Carlon TA, Jantzen AE, Lin FH, Ley MM, Allen JD, Stabler TV, Haley NR, Truskey GA, Achneck HE, Isolation of functional human endothelial cells from small volumes of umbilical cord blood, Ann Biomed Eng 41(10) (2013) 2181–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Rao L, Qian Y, Khodabukus A, Ribar T, Bursae N, Engineering human pluripotent stem cells into a functional skeletal muscle tissue, Nat Commun 9(1) (2018) 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Strash N, DeLuca S, Janer Carattini GL, Heo SC, Gorsuch R, Bursae N, Human Erbb2-induced Erk activity robustly stimulates cycling and functional remodeling of rat and human cardiomyocytes, Elife 10 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Strash N, DeLuca S, Janer Carattini GL, Chen Y, Wu T, Heifer A, Scherba J, Wang I, Jain M, Naseri R, Bursae N, Time-dependent effects of BRAF-V600E on cell cycling, metabolism, and function in engineered myocardium, Sci Adv 10(4) (2024) eadh2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Salva MZ, Himeda CL, Tai PW, Nishiuchi E, Gregorevic P, Allen JM, Finn EE, Nguyen QG, Blankinship MJ, Meuse L, Chamberlain JS, Hauschka SD, Design of tissue-specific regulatory cassettes for high-level rAAV-mediated expression in skeletal and cardiac muscle, Mol Ther 15(2) (2007) 320–9. [DOI] [PubMed] [Google Scholar]
- [42].Chen TW, Wardill TJ, Sun Y, Pulver SR, Renninger SL, Baohan A, Schreiter ER, Kerr RA, Orger MB, Jayaraman V, Looger LL, Svoboda K, Kim DS, Ultrasensitive fluorescent proteins for imaging neuronal activity, Nature 499(7458) (2013) 295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Jackman CP, Carlson AL, Bursae N, Dynamic culture yields engineered myocardium with near-adult functional output, Biomaterials 111 (2016) 66–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Bian W, Bursae N, Soluble miniagrin enhances contractile function of engineered skeletal muscle, FASEB J 26(2) (2012) 955–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Carpentier G, Berndt S, Ferratge S, Rasband W, Cuendet M, Uzan G, Albanese P, Angiogenesis Analyzer for ImageJ - A comparative morphometric analysis of "Endothelial Tube Formation Assay" and "Fibrin Bead Assay", Sci Rep 10(1) (2020) 11568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Shadrin IY, Allen BW, Qian Y, Jackman CP, Carlson AL, Juhas ME, Bursae N, Cardiopatch platform enables maturation and scale-up of human pluripotent stem cell-derived engineered heart tissues, Nat Commun 8(1) (2017) 1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Holmes DI, Zachary I, The vascular endothelial growth factor (VEGF) family: angiogenic factors in health and disease, Genome Biol 6(2) (2005) 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Peach CJ, Mignone VW, Arruda MA, Alcobia DC, Hill SJ, Kilpatrick LE, Woolard J, Molecular Pharmacology of VEGF-A Isoforms: Binding and Signalling at VEGFR2, Int J Mol Sci 19(4) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Wishart DS, Guo A, Oler E, Wang F, Anjum A, Peters H, Dizon R, Sayeeda Z, Tian S, Lee BL, Berjanskii M, Mah R, Yamamoto M, Jovel J, Torres-Calzada C, Hiebert-Giesbrecht M, Lui VW, Varshavi D, Varshavi D, Allen D, Arndt D, Khetarpal N, Sivakumaran A, Harford K, Sanford S, Yee K, Cao X, Budinski Z, Liigand J, Zhang L, Zheng J, Mandal R, Karu N, Dambrova M, Schioth HB, Greiner R, Gautam V, HMDB 5.0: the Human Metabolome Database for 2022, Nucleic Acids Res 50(D1) (2022) D622–D631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Jacquemin V, Furling D, Bigot A, Butler-Browne GS, Mouly V, IGF-1 induces human myotube hypertrophy by increasing cell recruitment, Exp Cell Res 299(1) (2004) 148–58. [DOI] [PubMed] [Google Scholar]
- [51].Richardson TP, Peters MC, Ennett AB, Mooney DJ, Polymeric system for dual growth factor delivery, Nat Biotechnol 19(11) (2001) 1029–34. [DOI] [PubMed] [Google Scholar]
- [52].Hirata A, Ogawa S, Kometani T, Kuwano T, Naito S, Kuwano M, Ono M, ZD1839 (Iressa) induces antiangiogenic effects through inhibition of epidermal growth factor receptor tyrosine kinase, Cancer Res 62(9) (2002) 2554–60. [PubMed] [Google Scholar]
- [53].Okamura K, Morimoto A, Hamanaka R, Ono M, Kohno K, Uchida Y, Kuwano M, A model system for tumor angiogenesis: involvement of transforming growth factor-alpha in tube formation of human microvascular endothelial cells induced by esophageal cancer cells, Biochem Biophys Res Commun 186(3) (1992) 1471–9. [DOI] [PubMed] [Google Scholar]
- [54].Mousa SA, Davis FB, Mohamed S, Davis PJ, Feng X, Pro-angiogenesis action of thyroid hormone and analogs in a three-dimensional in vitro microvascular endothelial sprouting model, Int Angiol 25(4) (2006) 407–13. [PubMed] [Google Scholar]
- [55].Davis PJ, Davis FB, Mousa SA, Thyroid hormone-induced angiogenesis, Curr Cardiol Rev 5(1) (2009) 12–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Panaite PA, Barakat-Walter I, Thyroid hormone enhances transected axonal regeneration and muscle reinnervation following rat sciatic nerve injury, J Neurosci Res 88(8) (2010) 1751–63. [DOI] [PubMed] [Google Scholar]
- [57].Lee JW, Kim NH, Milanesi A, Thyroid Hormone Signaling in Muscle Development, Repair and Metabolism, J Endocrinol Diabetes Obes 2(3) (2014) 1046. [PMC free article] [PubMed] [Google Scholar]
- [58].Butler-Browne GS, Barbet JP, Thornell LE, Myosin heavy and light chain expression during human skeletal muscle development and precocious muscle maturation induced by thyroid hormone, Anatomy and embryology 181(6) (1990) 513–22. [DOI] [PubMed] [Google Scholar]
- [59].Sangaj N, Kyriakakis P, Yang D, Chang CW, Arya G, Varghese S, Heparin mimicking polymer promotes myogenic differentiation of muscle progenitor cells, Biomacromolecules 11(12) (2010) 3294–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Ashikari-Hada S, Habuchi H, Kariya Y, Kimata K, Heparin regulates vascular endothelial growth factor165-dependent mitogenic activity, tube formation, and its receptor phosphorylation of human endothelial cells. Comparison of the effects of heparin and modified heparins, J Biol Chem 280(36) (2005) 31508–15. [DOI] [PubMed] [Google Scholar]
- [61].Kim H, Osaki T, Kamm RD, Asada HH, Multiscale engineered human skeletal muscles with perfusable vasculature and microvascular network recapitulating the fluid compartments, Biofabrication 15(1) (2022). [DOI] [PubMed] [Google Scholar]
- [62].McGuire BJ, Secomb TW, Estimation of capillary density in human skeletal muscle based on maximal oxygen consumption rates, Am J Physiol Heart Circ Physiol 285(6) (2003) H2382–91. [DOI] [PubMed] [Google Scholar]
- [63].Zoladz JA, Semik D, Zawadowska B, Majerczak J, Karasinski J, Kolodziejski L, Duda K, Kilarski WM, Capillary density and capillary-to-fibre ratio in vastus lateralis muscle of untrained and trained men, Folia Histochem Cytobiol 43(1) (2005) 11–7. [PubMed] [Google Scholar]
- [64].Lammert E, Axnick J, Vascular lumen formation, Cold Spring Harb Perspect Med 2(4) (2012) a006619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Osaki T, Sivathanu V, Kamm RD, Crosstalk between developing vasculature and optogenetically engineered skeletal muscle improves muscle contraction and angiogenesis, Biomaterials 156 (2018) 65–76. [DOI] [PubMed] [Google Scholar]
- [66].Wang J, Khodabukus A, Rao L, Vandusen K, Abutaleb N, Bursae N, Engineered skeletal muscles for disease modeling and drug discovery, Biomaterials 221 (2019) 119416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Choi YJ, Jun YJ, Kim DY, Yi HG, Chae SH, Kang J, Lee J, Gao G, Kong JS, Jang J, Chung WK, Rhie JW, Cho DW, A 3D cell printed muscle construct with tissue-derived bioink for the treatment of volumetric muscle loss, Biomaterials 206 (2019) 160–169. [DOI] [PubMed] [Google Scholar]
- [68].Pinton L, Khedr M, Lionello VM, Sarcar S, Maffioletti SM, Dastidar S, Negroni E, Choi S, Khokhar N, Bigot A, Counsell JR, Bernardo AS, Zammit PS, Tedesco FS, 3D human induced pluripotent stem cell-derived bioengineered skeletal muscles for tissue, disease and therapy modeling, Nat Protoc 18(4) (2023) 1337–1376. [DOI] [PubMed] [Google Scholar]
- [69].Tiburcy M, Markov A, Kraemer LK, Christalla P, Rave-Fraenk M, Fischer HJ, Reichardt HM, Zimmermann WH, Regeneration competent satellite cell niches in rat engineered skeletal muscle, FASEB Bioadv 1(12) (2019) 731–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Fleming JW, Capel AJ, Rimington RP, Wheeler P, Leonard AN, Bishop NC, Davies OG, P Lewis M, Bioengineered human skeletal muscle capable of functional regeneration, BMC Biol 18(1) (2020) 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Liu L, Charville GW, Cheung TH, Yoo B, Santos PJ, Schroeder M, Rando TA, Impaired Notch Signaling Leads to a Decrease in p53 Activity and Mitotic Catastrophe in Aged Muscle Stem Cells, Cell Stem Cell 23(4) (2018) 544–556 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Esteves de Lima J, Blavet C, Bonnin MA, Hirsinger E, Havis E, Relaix F, Duprez D, TMEM8C-mediated fusion is regionalized and regulated by NOTCH signalling during foetal myogenesis, Development 149(2) (2022). [DOI] [PubMed] [Google Scholar]
- [73].Buas MF, Kabak S, Kadesch T, The Notch effector Hey1 associates with myogenic target genes to repress myogenesis, J Biol Chem 285(2) (2010) 1249–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Bjornson CR, Cheung TH, Liu L, Tripathi PV, Steeper KM, Rando TA, Notch signaling is necessary to maintain quiescence in adult muscle stem cells, Stem Cells 30(2) (2012) 232–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Wen Y, Bi P, Liu W, Asakura A, Keller C, Kuang S, Constitutive Notch activation upregulates Pax7 and promotes the self-renewal of skeletal muscle satellite cells, Mol Cell Biol 32(12) (2012) 2300–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Saharinen P, Eklund L, Alitalo K, Therapeutic targeting of the angiopoietin-TIE pathway, Nat Rev Drug Discov 16(9) (2017) 635–661. [DOI] [PubMed] [Google Scholar]
- [77].Gianni-Barrera R, Burger M, Wolff T, Heberer M, Schaefer DJ, Gurke L, Mujagic E, Banfi A, Long-term safety and stability of angiogenesis induced by balanced single-vector co-expression of PDGF-BB and VEGF164 in skeletal muscle, Sci Rep 6 (2016) 21546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Peters EB, Liu B, Christoforou N, West JL, Truskey GA, Umbilical Cord Blood-Derived Mononuclear Cells Exhibit Pericyte-Like Phenotype and Support Network Formation of Endothelial Progenitor Cells In Vitro, Ann Biomed Eng 43(10) (2015) 2552–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Groppa E, Martini P, Derakhshan N, Theret M, Ritso M, Tung LW, Wang YX, Soliman H, Hamer MS, Stankiewicz L, Eisner C, Erwan LN, Chang C, Yi L, Yuan JH, Kong S, Weng C, Adams J, Chang L, Peng A, Blau HM, Romualdi C, Rossi FMV, Spatial compartmentalization of signaling imparts source-specific functions on secreted factors, Cell Rep 42(2) (2023) 112051. [DOI] [PubMed] [Google Scholar]
- [80].Afshar Bakooshli M, Lippmann ES, Mulcahy B, Iyer N, Nguyen CT, Tung K, Stewart BA, van den Dorpel H, Fuehrmann T, Shoichet M, Bigot A, Pegoraro E, Ahn H, Ginsberg H, Zhen M, Ashton RS, Gilbert PM, A 3D culture model of innervated human skeletal muscle enables studies of the adult neuromuscular junction, Elife 8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Rimington RP, Fleming JW, Capel AJ, Wheeler PC, Lewis MP, Bioengineered model of the human motor unit with physiologically functional neuromuscular junctions, Sci Rep 11(1) (2021) 11695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Kadi F, Charifi N, Henriksson J, The number of satellite cells in slow and fast fibres from human vastus lateralis muscle, Histochem Cell Biol 126(1) (2006) 83–7. [DOI] [PubMed] [Google Scholar]
- [83].Schuler SC, Liu Y, Dumontier S, Grandbois M, Le Moal E, Cornelison D, Bentzinger CF, Extracellular matrix: Brick and mortar in the skeletal muscle stem cell niche, Front Cell Dev Biol 10 (2022) 1056523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Hocking DC, Titus PA, Sumagin R, Sarelius IH, Extracellular matrix fibronectin mechanically couples skeletal muscle contraction with local vasodilation, Circ Res 102(3) (2008) 372–9. [DOI] [PubMed] [Google Scholar]
- [85].Thomas K, Engler AJ, Meyer GA, Extracellular matrix regulation in the muscle satellite cell niche, Connect Tissue Res 56(1) (2015) 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Murray B, Wilson DJ, Muscle patterning, differentiation and vascularisation in the chick wing bud, J Anat 190 (Pt 2)(Pt 2) (1997) 261–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Ontell M, Kozeka K, The organogenesis of murine striated muscle: a cytoarchitectural study, Am J Anat 171(2) (1984) 133–48. [DOI] [PubMed] [Google Scholar]
- [88].Hoier B, Hellsten Y, Exercise-induced capillary growth in human skeletal muscle and the dynamics of VEGF, Microcirculation 21(4) (2014) 301–14. [DOI] [PubMed] [Google Scholar]
- [89].Podkalicka P, Mucha O, Dulak J, Loboda A, Targeting angiogenesis in Duchenne muscular dystrophy, Cell Mol Life Sci 76(8) (2019) 1507–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Shen Y, Kim IM, Hamrick M, Tang Y, Uncovering the Gene Regulatory Network of Endothelial Cells in Mouse Duchenne Muscular Dystrophy: Insights from Single-Nuclei RNA Sequencing Analysis, Biology (Basel) 12(3) (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Honda M, Shimizu F, Sato R, Mizukami Y, Watanabe K, Takeshita Y, Maeda T, Koga M, Kanda T, Jo-1 Antibodies From Myositis Induce Complement-Dependent Cytotoxicity and TREM-1 Upregulation in Muscle Endothelial Cells, Neurol Neuroimmunol Neuroinflamm 10(4) (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Lemmer D, Schmidt J, Kummer K, Lemmer B, Wrede A, Seitz C, Balcarek P, Schwarze K, Muller GA, Patschan D, Patschan S, Impairment of muscular endothelial cell regeneration in dermatomyositis, Front Neurol 13 (2022) 952699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].McDermott MM, Ferrucci L, Gonzalez-Freire M, Kosmac K, Leeuwenburgh C, Peterson CA, Saini S, Sufit R, Skeletal Muscle Pathology in Peripheral Artery Disease: A Brief Review, Arterioscler Thromb Vasc Biol 40(11) (2020) 2577–2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Pipinos II, Sharov VG, Shepard AD, Anagnostopoulos PV, Katsamouris A, Todor A, Filis KA, Sabbah HN, Abnormal mitochondrial respiration in skeletal muscle in patients with peripheral arterial disease, J Vasc Surg 38(4) (2003) 827–32. [DOI] [PubMed] [Google Scholar]
- [95].Takeda M, Miyagawa S, Ito E, Harada A, Mochizuki-Oda N, Matsusaki M, Akashi M, Sawa Y, Development of a drug screening system using three-dimensional cardiac tissues containing multiple cell types, Scientific reports 11(1) (2021) 5654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Ardalani H, Sengupta S, Harms V, Vickerman V, Thomson JA, Murphy WL, 3-D culture and endothelial cells improve maturity of human pluripotent stem cell-derived hepatocytes, Acta Biomater 95 (2019) 371–381. [DOI] [PubMed] [Google Scholar]
- [97].Nakayama KH, Quarta M, Paine P, Alcazar C, Karakikes I, Garcia V, Abilez OJ, Calvo NS, Simmons CS, Rando TA, Huang NF, Treatment of volumetric muscle loss in mice using nanofibrillar scaffolds enhances vascular organization and integration, Commun Biol 2 (2019) 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Gilbert-Honick J, Grayson W, Vascularized and Innervated Skeletal Muscle Tissue Engineering, Adv Healthc Mater 9(1) (2020) e1900626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Correia C, Grayson WL, Park M, Hutton D, Zhou B, Guo XE, Niklason L, Sousa RA, Reis RL, Vunjak-Novakovic G, In vitro model of vascularized bone: synergizing vascular development and osteogenesis, PLoS One 6(12) (2011) e28352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Maiullari F, Costantini M, Milan M, Pace V, Chirivi M, Maiullari S, Rainer A, Bad D, Marei HE, Seliktar D, Gargioli C, Bearzi C, Rizzi R, A multi-cellular 3D bioprinting approach for vascularized heart tissue engineering based on HUVECs and iPSC-derived cardiomyocytes, Sci Rep 8(1) (2018) 13532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Hur J, Yoon CH, Kim HS, Choi JH, Kang HJ, Hwang KK, Oh BH, Lee MM, Park YB, Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis, Arterioscler Thromb Vasc Biol 24(2) (2004) 288–93. [DOI] [PubMed] [Google Scholar]
- [102].Joo HJ, Song S, Seo HR, Shin JH, Choi SC, Park JH, Yu CW, Hong SJ, Lim DS, Human endothelial colony forming cells from adult peripheral blood have enhanced sprouting angiogenic potential through up-regulating VEGFR2 signaling, Int J Cardiol 197 (2015) 33–43. [DOI] [PubMed] [Google Scholar]
- [103].Urbich C, Aicher A, Heeschen C, Dernbach E, Hofmann WK, Zeiher AM, Dimmeler S, Soluble factors released by endothelial progenitor cells promote migration of endothelial cells and cardiac resident progenitor cells, J Mol Cell Cardiol 39(5) (2005) 733–42. [DOI] [PubMed] [Google Scholar]
- [104].Au P, Daheron LM, Duda DG, Cohen KS, Tyrrell JA, Lanning RM, Fukumura D, Scadden DT, Jain RK, Differential in vivo potential of endothelial progenitor cells from human umbilical cord blood and adult peripheral blood to form functional long-lasting vessels, Blood 111(3) (2008) 1302–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Finkenzeller G, Graner S, Kirkpatrick CJ, Fuchs S, Stark GB, Impaired in vivo vasculogenic potential of endothelial progenitor cells in comparison to human umbilical vein endothelial cells in a spheroid-based implantation model, Cell Prolif 42(4) (2009) 498–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Peters EB, Endothelial Progenitor Cells for the Vascularization of Engineered Tissues, Tissue Eng Part B Rev 24(1) (2018) 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Finkenzeller G, Torio-Padron N, Momeni A, Mehlhorn AT, Stark GB, In vitro angiogenesis properties of endothelial progenitor cells: a promising tool for vascularization of ex vivo engineered tissues, Tissue Eng 13(7) (2007) 1413–20. [DOI] [PubMed] [Google Scholar]
- [108].Wust R, Terrie L, Muntefering T, Ruck T, Thorrez L, Efficient co-isolation of microvascular endothelial cells and satellite cell-derived myoblasts from human skeletal muscle, Front Bioeng Biotechnol 10 (2022) 964705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Rafii S, Butler JM, Ding BS, Angiocrine functions of organ-specific endothelial cells, Nature 529(7586) (2016) 316–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Urbanczyk M, Zbinden A, Schenke-Layland K, Organ-specific endothelial cell heterogenicity and its impact on regenerative medicine and biomedical engineering applications, Adv Drug Deliv Rev 186 (2022) 114323. [DOI] [PubMed] [Google Scholar]
- [111].Skylar-Scott MA, Uzel SGM, Nam LL, Ahrens JH, Truby RL, Damaraju S, Lewis JA, Biomanufacturing of organ-specific tissues with high cellular density and embedded vascular channels, Sci Adv 5(9) (2019) eaaw2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Nederveen JP, Joanisse S, Snijders T, Thomas ACQ, Kumbhare D, Parise G, The influence of capillarization on satellite cell pool expansion and activation following exercise-induced muscle damage in healthy young men, The Journal of physiology 596(6) (2018) 1063–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw/processed data required to reproduce these findings are available upon request.


