Abstract
OBJECTIVE:
To evaluate the association between continuous glucose monitoring in pregnant people with type 2 diabetes and perinatal outcomes.
METHODS:
This was a retrospective cohort study of pregnant people with type 2 diabetes who received prenatal care and delivered singleton, nonanomalous neonates at a single academic tertiary care center from November 1, 2019, to February 28, 2023. The primary outcome was a composite of neonatal morbidity, including hypoglycemia, hyperbilirubinemia, shoulder dystocia, large for gestational age at birth, preterm birth, neonatal intensive care unit (NICU) admission, or perinatal death. Demographics and outcomes were compared by type of monitoring (continuous glucose monitoring vs intermittent self-monitoring of blood glucose), and multivariable logistic regression estimated the association between continuous glucose monitoring use and perinatal outcomes.
RESULTS:
Of 360 pregnant people who met the inclusion criteria, 82 (22.7%) used continuous glucose monitoring. The mean gestational age at continuous glucose monitoring initiation was 21.3±6.4 weeks. The use of continuous glucose monitoring was associated with lower odds of the primary composite neonatal morbidity (65.9% continuous glucose monitoring vs 77.0% self-monitoring of blood glucose, adjusted odds ratio [aOR] 0.48, 95% CI, 0.24–0.94). Continuous glucose monitoring use was also associated with lower odds of preterm birth (13.4% vs 25.2%, aOR 0.48, 95% CI, 0.25–0.93) and NICU admission (33.8% vs 47.6%, aOR 0.36, 95% CI, 0.16–0.81).
CONCLUSION:
In pregnant people with type 2 diabetes, continuous glucose monitoring use was associated with less neonatal morbidity, fewer preterm births, and fewer NICU admissions.
Pregestational diabetes complicates about 1–2% of pregnancies1,2 and is associated with significant maternal and neonatal morbidity.3,4 The increased risk of morbidity among pregnant people with diabetes is directly related to their glycemic control.5 Therefore, pregnant people with diabetes are instructed to check blood glucose levels at least four times daily with frequent prenatal visits for medication adjustments to achieve glucose targets.1 However, intermittent self-monitoring of blood glucose can be cumbersome and provides only snapshots of glycemic control throughout the day.
Advances in medical technology and the development of continuous glucose monitoring may overcome many of the limitations of traditional self-monitoring of blood glucose and provide detailed data about not only the glucose level but also the direction and rate of change. Continuous glucose monitoring allows real-time monitoring of blood glucose levels and can be used to inform adjustments to insulin therapy, diet, and physical activity to achieve optimal glycemic control, which is especially important in pregnancy.
In pregnant people with type 1 diabetes, the use of continuous glucose monitoring has been shown to decrease the rates of large-for-gestational-age (LGA) neonates, neonatal intensive care unit (NICU) admissions, and neonatal hypoglycemia, with improved maternal glycemic control compared with self-monitoring of blood glucose.6 However, the effect of continuous glucose monitoring in pregnant people with type 2 diabetes is unknown.7 To address this, our objective was to evaluate the association between continuous glucose monitoring use in pregnant people with type 2 diabetes and perinatal outcomes.
METHODS
This was a retrospective cohort study of pregnant people with type 2 diabetes who received prenatal care and delivered at a single academic tertiary care center in the United States from November 1, 2019, to February 28, 2023. People with pregnancy loss at less than 20 weeks of gestation, a multifetal gestation, or at least one major or two minor fetal anomalies were excluded.8 Baseline characteristics and outcome data were obtained through a combination of a validated extraction protocol and individual review of electronic medical records for missing data and neonatal outcomes, including hypoglycemia, hyperbilirubinemia, mechanical ventilation, intraventricular hemorrhage, necrotizing enterocolitis, sepsis, and perinatal death. Race and ethnicity were included given the known racial and ethnic disparities in type 2 diabetes prevalence and diabetes-related adverse outcomes.
All pregnant people with type 2 diabetes at our center receive prenatal care, including diabetes management, provided by a group practice of maternal–fetal medicine clinicians but may be seen by obstetrics and gynecology residents, maternal–fetal medicine fellows, advanced practice clinicians, or attending faculty at prenatal visits. Management, including nutrition counseling and diabetic education by certified diabetes care and education specialists, laboratory evaluation, frequency of visits to review glycemic control, pharmacotherapy initiation and titration, ultrasound surveillance, corticosteroids for threatened preterm birth before 34 weeks of gestation, and delivery, is standardized by an institutional protocol, which aligns with recommended guidelines from the American College of Obstetricians and Gynecologists, the Society for Maternal-Fetal Medicine, and the American Diabetes Association.1,9
Pregnant people were grouped according to glucose monitoring method: use of continuous glucose monitoring, including Dexcom G6 or Freestyle Libre 2 device, at any time during pregnancy or intermittent self-monitoring of blood glucose with a glucometer. The institutional protocol does not specifically recommend the use of continuous glucose monitoring, and any decision to prescribe continuous glucose monitoring was at the discretion of the obstetric clinician. Regardless of the method of glucose monitoring, glycemic control was reviewed every 1–2 weeks in person or by phone with medication adjustments to achieve recommended glucose goals.
For pregnant people performing self-monitoring of blood glucose, glucometers or paper glucose logs were reviewed, and goals of fasting glucose lower than 95 mg/dL and 2-hour postprandial glucose lower than 120 mg/dL were used to titrate initiation and adjustment of medications. For those using continuous glucose monitoring, continuous glucose monitoring data were uploaded to online continuous glucose monitoring portals, and ambulatory glucose profiles were reviewed with medication adjustments to achieve the recommended goals of time in range above 70%, time below range below 4%, and time above range below 25%. We used a target range of 65–140 mg/dL because we cannot customize the lower limit of the target range to the recommended 63 mg/dL because of current limitations of available continuous glucose monitoring systems.10 People using continuous glucose monitoring were not asked to keep separate logs of fasting and postprandial glucose values, but these may be estimated from the continuous glucose monitoring reports and used for management at the discretion of the obstetric clinician.
The primary outcome was a composite of neonatal morbidity, which included hypoglycemia, hyperbilirubinemia, shoulder dystocia, LGA at birth, preterm birth, NICU admission, or perinatal death. This composite was selected because it is similar to outcomes evaluated in recent randomized clinical trials of pregnant people with type 2 diabetes.3,4 Hypoglycemia was defined as neonatal glucose less than 40 mg/dL within the first 24 hours of life. Hyperbilirubinemia was defined according to receipt of phototherapy. We defined LGA as birth weight greater than the 90th percentile for gestational age according to sex based on a unified standard for fetal growth curves.11 Preterm birth was defined as delivery at less than 37 weeks of gestation, and perinatal death included stillbirth after 20 weeks of gestation or neonatal death before hospital discharge. Secondary neonatal outcomes included components of the primary composite outcome, preterm birth before 34 weeks of gestation, gestational age at delivery, birth weight, small for gestational age or birth weight less than the 10th percentile, and severe neonatal morbidity. Severe neonatal morbidity included mechanical ventilation, intraventricular hemorrhage, necrotizing enterocolitis, early-onset sepsis, and perinatal death. Secondary maternal outcomes included hemoglobin A1c (Hb A1c) before delivery, change in Hb A1c from baseline to before delivery, gestational weight gain, preeclampsia, cesarean delivery, postpartum length of stay, and postpartum readmission.
Baseline demographic, medical, and obstetric characteristics were first compared between pregnant people who used continuous glucose monitoring and those who did not with the χ2, Fisher exact, and Wilcoxon rank-sum tests as appropriate. Maternal and neonatal outcomes were compared in a similar manner. Multivariable logistic and linear regression models were used to estimate the association between the use of continuous glucose monitoring and the primary and secondary outcomes compared with self-monitoring of blood glucose. Covariates that differed by continuous glucose monitoring use with P<.05 in bivariable analysis were included in the initial multivariable models, and those found to be significant confounders with P<.10 were retained in the final models to avoid model overfitting given the relatively small sample size and relatedness of many covariates. In addition, we evaluated for effect modification by baseline Hb A1c.
Approval was obtained from the University of Alabama at Birmingham IRB (IRB 300010419) before initiation of the study. Analyses were performed with STATA 17.0, and all tests were two-tailed with statistical significance defined as P<.05. No imputation for missing data was performed, and no adjustments for multiple comparisons were made. Therefore, analyses of secondary outcomes were considered to be exploratory and hypothesis generating. An a priori analysis was performed to determine study feasibility after identification of individual group sizes before additional analyses were conducted. On the basis of group sizes of 82 people who used continuous glucose monitoring and 278 who did not use continuous glucose monitoring, an estimated primary outcome rate of 70%, 90% power, and 2-sided α of 0.05, we calculated that we would be able to detect a 30% relative reduction in the primary outcome, which seemed reasonable based on effect sizes observed in prior clinical trials.3,12
RESULTS
Of 12,779 pregnant people who delivered at or after 20 weeks of gestation, 410 had type 2 diabetes (Fig. 1). After the exclusion of those with multifetal pregnancies (n=12) and fetal anomalies (n=38), a total of 360 pregnant people were eligible for inclusion. Of these, 82 (22.7%) used continuous glucose monitoring and 278 (77.2%) used self-monitoring of blood glucose. Of those who used continuous glucose monitoring, 73 used Dexcom G6 continuous glucose monitoring devices and nine used Freestyle Libre 2 devices, with a mean gestational age at initiation of 21.3±6.4 weeks. No people were using continuous glucose monitoring before pregnancy.
Fig. 1.
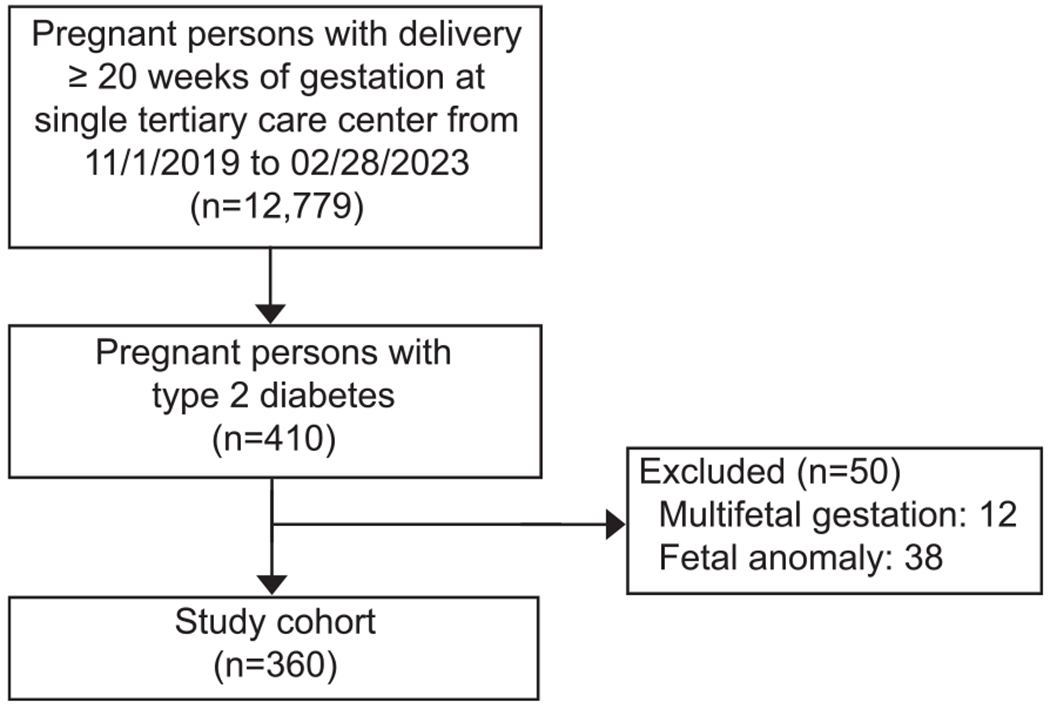
Study cohort flow diagram.
Padgett. Continuous Glucose Monitoring for Type 2 Diabetes and Outcomes. Obstet Gynecol 2024.
Pregnant people who used continuous glucose monitoring were more likely to self-identify as non-Hispanic Black and less likely to identify as non-Hispanic White or Hispanic compared with those who used self-monitoring of blood glucose (Table 1). Marital status and insurance type were similar between groups. Pregnant people who used continuous glucose monitoring had higher baseline Hb A1c, but lower body mass index (BMI, calculated as weight in kilograms divided by height in meters squared) and were less likely to have chronic hypertension compared with those who used self-monitoring of blood glucose (Table 1). Pregnant people who used continuous glucose monitoring also presented for prenatal care at an earlier gestational age.
Table 1.
Baseline Demographic, Medical, and Obstetric Characteristics by Continuous Glucose Monitoring Use
| Characteristic | CGM (n=82) | SMBG (n=278) | P |
|---|---|---|---|
| Demographics | |||
| Maternal age (y) | 32.2 (28.0–35.7) | 32.7 (27.8–37.1) | .65 |
| Race | <.01 | ||
| Hispanic | 2 (2.5) | 46 (16.7) | |
| Non-Hispanic Black | 60 (74.1) | 167 (60.5) | |
| Non-Hispanic White | 15 (18.5) | 54 (19.6) | |
| None of the above | 4 (4.9) | 9 (3.3) | |
| Married | 27 (33.8) | 87 (32.1) | .78 |
| Government-assisted or no insurance | 48 (58.5) | 170 (61.2) | .67 |
| Medical comorbidities | |||
| BMI at first prenatal visit (kg/m2)* | 35.0 (30.4–38.8) | 39.1 (33.8–46.5) | <.001 |
| Baseline Hb A1c (%)* | 7.8 (6.8–10.2) | 7.0 (6.0–9.0) | <.01 |
| Insulin use | 71 (86.6) | 235 (84.5) | .65 |
| Chronic hypertension | 29 (35.4) | 134 (48.2) | .04 |
| Aspirin use | 67 (82.7) | 186 (72.4) | .06 |
| Obstetric characteristics | |||
| Nulliparous | 25 (30.5) | 92 (33.1) | .66 |
| Gestational age at first prenatal visit (wk) | 9.4 (7.6–15.6) | 12.6 (9.0–19.3) | <.001 |
| Male fetus | 35 (42.7) | 152 (54.7) | .06 |
CGM, continuous glucose monitoring; SMBG, intermittent self-monitoring of blood glucose; BMI, body mass index; Hb A1c, glycated hemoglobin.
Data are median (interquartile range) or n (%) unless otherwise specified.
Baseline defined as before pregnancy or at first prenatal visit.
With regard to the primary composite outcome, the most common components were hypoglycemia, hyperbilirubinemia, preterm birth (primarily indicated preterm birth because of preeclampsia), and NICU admission. Composite neonatal morbidity occurred less commonly among neonates born to those who used continuous glucose monitoring during pregnancy compared with those who used self-monitoring of blood glucose (65.9% vs 77.0%, P=.04, Table 2). There were significantly lower incidences of hyperbilirubinemia, preterm birth, and NICU admission among people who used continuous glucose monitoring. Although severe neonatal morbidity and perinatal death occurred less frequently among those who used continuous glucose monitoring, these differences were not statistically significant (Table 2).
Table 2.
Maternal and Neonatal Outcomes by Continuous Glucose Monitoring Use
| CGM (n=82) | SMBG (n=278) | P | |
|---|---|---|---|
| Primary neonatal outcome and composite components | |||
| Composite neonatal morbidity* | 54 (65.9) | 214 (77.0) | .04 |
| Hypoglycemia | 22 (27.5) | 85 (31.6) | .49 |
| Hyperbilirubinemia | 20 (25.0) | 101 (37.6) | .04 |
| Shoulder dystocia | 2 (2.4) | 10 (3.6) | .61 |
| LGA at birth | 16 (19.5) | 54 (19.4) | .99 |
| Preterm birth before 37 wk | 28 (34.2) | 136 (48.9) | .02 |
| NICU admission | 27 (33.8) | 128 (47.6) | .03 |
| Stillbirth or neonatal death | 3 (3.7) | 12 (4.3) | .79 |
| Secondary neonatal outcomes | |||
| Preterm birth before 34 wk | 11 (13.4) | 70 (25.2) | .03 |
| Gestational age at delivery (wk) | 37.1 (35.9–38.0) | 37.0 (33.9–38.0) | .04 |
| SGA at birth | 12 (14.6) | 37 (13.3) | .76 |
| Birth weight (g) | 3,013 (2,540–3,450) | 2,845 (2,160–3,480) | .12 |
| Severe neonatal morbidity† | 6 (7.3) | 27 (9.7) | .51 |
| Mechanical ventilation | 4 (5.0) | 11 (4.1) | |
| Intraventricular hemorrhage | 1 (1.3) | 3 (1.1) | |
| Necrotizing enterocolitis | 0 | 2 (0.7) | |
| Early-onset sepsis | 0 | 0 | |
| Stillbirth or neonatal death | 3 (3.7) | 12 (4.3) | |
| Secondary maternal outcomes | |||
| Last Hb A1c before delivery (%) | 6.5 (6.0, 7.0) | 6.3 (5.8, 7.3) | .52 |
| Change in Hb A1c from baseline to delivery (%) | −1.7 (−3.3, −0.4) | −0.5 (−2.2, 0.0) | <.001 |
| Gestational weight gain (kg) | 8.9 (5.8, 14.2) | 6.7 (2.3, 11.2) | <.01 |
| Preeclampsia | 23 (28.1) | 109 (39.2) | .07 |
| Cesarean delivery | 54 (65.9) | 179 (64.4) | .81 |
| Postpartum length of stay (d) | 4 (3, 5) | 4 (3, 7) | .35 |
| Postpartum readmission | 5 (6.1) | 19 (6.8) | .81 |
CGM, continuous glucose monitoring; SMBG, intermittent self-monitoring of blood glucose; LGA, large for gestational age; NICU, neonatal intensive care unit; SGA, small for gestational age; Hb A1c, glycated hemoglobin.
Data are n (%) or median (IQR) unless otherwise specified.
Bold indicates statistical significance (P<.05).
Composite neonatal morbidity included hypoglycemia, hyperbilirubinemia, shoulder dystocia, LGA, preterm birth, NICU admission, and stillbirth or neonatal death.
Severe neonatal morbidity included mechanical ventilation, intraventricular hemorrhage, necrotizing enterocolitis, culture-proven early-onset sepsis, and stillbirth or neonatal death.
In multivariable analyses, continuous glucose monitoring use was associated with 52% lower adjusted odds of the primary composite neonatal morbidity (adjusted odds ratio 0.48, 95% CI, 0.24–0.94, Table 3). The observed association was consistent regardless of baseline Hb A1c with no evidence of effect modification (P=.39). Continuous glucose monitoring use was also significantly associated with lower adjusted odds of preterm birth before 37 weeks of gestation, preterm birth before 34 weeks of gestation, and NICU admission (Table 3). After adjustment for covariates, the association between continuous glucose monitoring use and other maternal and neonatal outcomes did not reach statistical significance (Table 3).
Table 3.
Association Between Continuous Glucose Monitoring Use and Maternal and Neonatal Outcomes
| OR or Mean Difference (95% CI) | aOR or Mean Difference (95% CI)* | |
|---|---|---|
| Primary neonatal outcome and composite components | ||
| Composite neonatal morbidity† | 0.58 (0.34–0.98) | 0.48 (0.24–0.94) |
| Hypoglycemia | 0.82 (0.47–1.43) | 0.85 (0.42–1.71) |
| Hyperbilirubinemia | 0.55 (0.32–0.97) | 0.78 (0.39–1.53) |
| Shoulder dystocia | 0.67 (0.14–3.12) | 2.20 (0.36–13.36) |
| LGA at birth | 1.00 (0.54–1.87) | 0.65 (0.28–1.54) |
| Preterm birth before 37 wk | 0.54 (0.32–0.90) | 0.48 (0.25–0.93) |
| NICU admission | 0.56 (0.33–0.95) | 0.36 (0.16–0.81) |
| Stillbirth or neonatal death | 0.84 (0.23–3.06) | 0.73 (0.15–3.61) |
| Secondary neonatal outcomes | ||
| Preterm birth before 34 wk | 0.46 (0.23–0.92) | 0.42 (0.18–0.99) |
| Gestational age at delivery (wk) | 0.82 (−0.08 to 1.72) | −0.10 (−1.31 to 1.11) |
| SGA at birth | 1.12 (0.55–2.26) | 0.86 (0.22–3.41) |
| Birth weight (g) | 197 (−45 to 439) | 42 (−332 to 415) |
| Severe neonatal morbidity‡ | 0.73 (0.29–1.84) | 0.97 (0.34–2.82) |
| Secondary maternal outcomes | ||
| Last Hb A1c before delivery (%) | −0.07 (−0.35 to 0.21) | −0.15 (−0.46 to 0.16) |
| Change in Hb A1c from baseline to delivery (%) | −0.95 (−1.56 to −0.35) | 0.15 (−0.49 to 0.78) |
| Gestational weight gain (kg) | 3.04 (1.10 to 4.98) | 2.26 (−0.14 to 4.67) |
| Preeclampsia | 0.60 (0.35–1.04) | 0.60 (0.28–1.31) |
| Cesarean delivery | 1.07 (0.64–1.79) | 0.66 (0.35–1.25) |
| Postpartum length of stay (d) | −0.31 (−1.87 to 1.25) | −0.80 (−2.57 to 0.97) |
| Postpartum readmission | 0.89 (0.32–2.45) | 1.01 (0.31–3.27) |
OR, odds ratio; aOR, adjusted odds ratio; LGA, large-for-gestational-age; NICU, neonatal intensive care unit; SGA, small for gestational age; Hb A1c, glycated hemoglobin.
Bold indicates statistical significance (P<.05).
Covariates that differed by continuous glucose monitoring use with P<.05 in bivariable analysis were included in the initial multivariable models, and those found to be significant confounders with P<.10 were retained in the final models. Final models were adjusted for baseline Hb A1c and chronic hypertension. Referent is no continuous glucose monitoring.
Composite neonatal morbidity included hypoglycemia, hyperbilirubinemia, shoulder dystocia, LGA, preterm birth, NICU admission, and stillbirth or neonatal death.
Severe neonatal morbidity included mechanical ventilation, intraventricular hemorrhage, necrotizing enterocolitis, culture-proven early-onset sepsis, and stillbirth or neonatal death.
DISCUSSION
In this cohort of pregnant people with type 2 diabetes, continuous glucose monitoring use during pregnancy was associated with 50% lower odds of a composite of neonatal morbidity and preterm birth and a nearly 70% reduction in NICU admission compared with intermittent self-monitoring of blood glucose. There was no association between continuous glucose monitoring use and our neonatal secondary outcomes, including gestational age at delivery, small for gestational age, and severe neonatal morbidity. Similarly, there was no association between continuous glucose monitoring use and any of our maternal outcomes.
Our findings extend the previous literature comparing continuous glucose monitoring with intermittent self-monitoring of blood glucose in pregnant people with type 2 diabetes, which was limited to only three studies and 138 people.13–15 In a recent systematic review and meta-analysis, pooled estimates for LGA neonates and preeclampsia were not different for people with type 2 diabetes who used continuous glucose monitoring compared with those who used intermittent self-monitoring of blood glucose.7 However, these findings were limited by use of older-generation continuous glucose monitoring devices, only intermittent continuous glucose monitoring use in pregnancy, and inclusion of a total of 109 people in the meta-analysis. Overall, the authors concluded that further research is needed given the small amount of data available for this population. Although our study is observational in nature, it provides a large cohort to begin to explore the association between the use of continuous glucose monitoring in pregnant people with type 2 diabetes and perinatal outcomes.
On the basis of data from the previous randomized clinical trial of continuous glucose monitoring for management of type 1 diabetes in pregnancy (CONCEPTT [Continuous Glucose Monitoring in Women With Type 1 Diabetes in Pregnancy Trial]), we were not surprised that we were unable to detect a significant difference in maternal glycemic control or LGA neonates with continuous glucose monitoring use compared with intermittent self-monitoring of blood glucose. Although the effects of continuous glucose monitoring use on neonatal outcomes such as neonatal hypoglycemia and NICU admission were quite profound in CONCEPTT with risk reductions of 46% and 37%, respectively, the reduction in LGA neonates was only 23%, and the difference in 34-week maternal Hb A1c was less than 0.2 percentage points (6.35% vs 6.53%). Thus, it is possible that we were simply not adequately powered to detect differences in these outcomes in our study.
Our study has several additional strengths. Our cohort is diverse with a large proportion of pregnant people who self-identify as non-Hispanic Black, more than half with government-assisted or no insurance, and varied prepregnancy Hb A1c levels. These characteristics thereby serve to increase the generalizability to people with type 2 diabetes from different social backgrounds and with varying degrees of prepregnancy glycemic control. In addition, our study reflects contemporary, clinical experience with continuous glucose monitoring in the United States, where many individuals with type 2 diabetes may not be able to obtain a continuous glucose monitoring device for glucose monitoring until the second trimester of pregnancy. Our cohort used newer-generation continuous glucose monitoring devices, and each device was prescribed to be used continuously until delivery. Finally, use of an institutional protocol for diabetes management without differential treatment of people using continuous glucose monitoring (ie, more frequent review of glycemic control or more telehealth visits) allowed us to evaluate the association between the method of glucose monitoring itself and our outcomes without confounding by other factors associated with differential management.
Our study also has limitations. The study was conducted at a single, tertiary care, academic referral center in the southeast United States and thus may not be generalizable to other prenatal care practices or geographic locations. As a retrospective cohort study, we cannot rule out the possibility of unmeasured confounding, in particular the clinical decision making that prompted a continuous glucose monitoring prescription. Although the comparison groups differed by multiple baseline characteristics, we would have expected some to have biased the results toward the null (higher baseline Hb A1c in the continuous glucose monitoring group) and others toward finding a difference (lower BMI, less chronic hypertension, and earlier gestational age at first prenatal visit in the continuous glucose monitoring group). We considered all of these factors as potential confounders in our multivariable analysis with final parsimonious models including adjustment for baseline Hb A1c and chronic hypertension. Only a randomized clinical trial will be able to ensure balance between groups, eliminate the possibility of confounding, and test for causality. Because of the nature of the study design, we were also not able to evaluate patient-centered outcomes such as satisfaction with method of glucose monitoring or anxiety related to diabetes care. Finally, although our cohort was larger than those from previously published studies, we still had limited power to detect differences in less common outcomes (ie, shoulder dystocia, severe neonatal morbidity, perinatal death) and could not evaluate for differences by type of continuous glucose monitoring.
With the rapid advancements of continuous glucose monitoring technology and growing patient desire to use these wearable devices, it is imperative that we understand the risks and benefits of continuous glucose monitoring for different populations, including pregnant people with type 2 diabetes. Although the findings of this study have demonstrated potential for improved pregnancy outcomes with continuous glucose monitoring use, important risks of continuous glucose monitoring use that should also be considered include increased patient anxiety, skin irritation, alarm fatigue, and potential inaccuracies.16 The findings from this study help expand the existing knowledge surrounding continuous glucose monitoring use in pregnancies complicated by type 2 diabetes, but large, multicenter, randomized controlled trials are needed to confirm our results. Furthermore, future studies will also help to elucidate evidence-based continuous glucose monitoring targets for pregnant people with type 2 diabetes, clarify how patients and clinicians should interpret and act on continuous glucose monitoring data, and describe the long-term benefits and risks. Overall, this retrospective cohort study demonstrates promise for continuous glucose monitoring to improve the health of pregnant people with type 2 diabetes and their neonates, but randomized controlled trials are needed to definitively test continuous glucose monitoring in this growing population at high risk.
Acknowledgments
Ashley N. Battarbee was supported by K23HD103875 during the study period.
Footnotes
Each author has confirmed compliance with the journal’s requirements for authorship.
Financial Disclosure:
The authors did not report any potential conflicts of interest.
REFERENCES
- 1.Pregestational diabetes mellitus. ACOG Practice Bulletin No. 201. American College of Obstetricians and Gynecologists. Obstet Gynecol 2018;132:e228–48. doi: 10.1097/AOG.0000000000002960 [DOI] [PubMed] [Google Scholar]
- 2.Deputy NP, Kim SY, Conrey EJ, Bullard KM. Prevalence and changes in preexisting diabetes and gestational diabetes among women who had a live birth–United States, 2012-2016. MMWR Morb Mortal Wkly Rep 2018;67:1201–7. doi: 10.15585/mmwr.mm6743a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boggess KA, Valint A, Refuerzo JS, Zork N, Battarbee AN, Eichelberger K, et al. Metformin plus insulin for preexisting diabetes or gestational diabetes in early pregnancy the MOMPOD randomized clinical trial. JAMA 2023;330:2182–90. doi: 10.1001/jama.2023.22949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feig DS, Donovan LE, Zinman B, Sanchez JJ, Asztalos E, Ryan EA, et al. Metformin in women with type 2 diabetes in pregnancy (MiTy): a multicentre, international, randomised, placebo-controlled trial. Lancet Diabetes Endocrinol 2020;8:834–44. doi: 10.1016/S2213-8587(20)30310-7 [DOI] [PubMed] [Google Scholar]
- 5.Murphy HR, Howgate C, O’Keefe J, Myers J, Morgan M, Coleman MA, et al. Characteristics and outcomes of pregnant women with type 1 or type 2 diabetes: a 5-year national population-based cohort study. Lancet Diabetes Endocrinol 2021;9:153–64. doi: 10.1016/S2213-8587(20)30406-X [DOI] [PubMed] [Google Scholar]
- 6.Feig DS, Murphy HR. Continuous glucose monitoring in pregnant women with type 1 diabetes: benefits for mothers, using pumps or pens, and their babies. Diabet Med 2018;35:430–5. doi: 10.1111/dme.13585 [DOI] [PubMed] [Google Scholar]
- 7.Wilkie G, Melnik V, Brainard L, Antonioli S, Baltich Nelson B, Leung K, et al. Continuous glucose monitor use in type 2 diabetes mellitus in pregnancy and perinatal outcomes: a systematic review and meta-analysis. Am J Obstet Gynecol MFM 2023;5:100969. doi: 10.1016/j.ajogmf.2023.100969 [DOI] [PubMed] [Google Scholar]
- 8.Gyamfi-Bannerman C, Thom EA, Blackwell SC, Tita ATN, Reddy UM, Saade GR, et al. Antenatal betamethasone for women at risk for late preterm delivery. N Engl J Med 2016;374:1311–20. doi: 10.1056/NEJMoa1516783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American Diabetes Association Professional Practice Committee. 15. Management of diabetes in pregnancy: Standards of Care in Diabetes–2024. Diabetes Care 2024;47:S282–94. doi: 10.2337/dc24-S015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Battelino T, Danne T, Bergenstal RM, Amiel SA, Beck R, Biester T, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care 2019;42:1593–603. doi: 10.2337/dci19-0028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grantz KL, Grewal J, Kim S, Grobman WA, Newman RB, Owen J, et al. Unified standard for fetal growth velocity: the Eunice Kennedy Shriver National Institute of Child Health and Human Development Fetal Growth Studies. Am J Obstet Gynecol 2022;227:916–22.e1. doi: 10.1016/J.AJOG.2022.07.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feig DS, Donovan LE, Corcoy R,Murphy KE, Amiel SA, Hunt KF, et al. Continuous glucose monitoring in pregnant women with type 1 diabetes (CONCEPTT): a multicentre international randomised controlled trial. Lancet 2017;390:2347–59. doi: 10.1016/S0140-6736(17)32400-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Secher AL, Ringholm L, Andersen HU, Damm P, Mathiesen ER. The effect of real-time continuous glucose monitoring in pregnant women with diabetes: a randomized controlled trial. Diabetes Care 2013;36:1877–83. doi: 10.2337/dc12-2360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Voormolen DN, DeVries JH, Sanson RME, Heringa MP, de Valk HW, Kok M, et al. Continuous Glucose Monitoring During Diabetic Pregnancy (GlucoMOMS): a multicentre randomized controlled trial. Diabetes Obes Metab 2018;20:1894–902. doi: 10.1111/dom.13310 [DOI] [PubMed] [Google Scholar]
- 15.Murphy HR, Rayman G, Lewis K, Kelly S, Johal B, Duffield K, et al. Effectiveness of continuous glucose monitoring in pregnant women with diabetes: randomised clinical trial. BMJ 2008;337:a1680. doi: 10.1136/bmj.a1680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.7. Diabetes technology: standards of care in diabetes–2024. Diabetes Care 2024;47:S126–44. doi: 10.2337/dc24-S007 [DOI] [PMC free article] [PubMed] [Google Scholar]


