Abstract
Background and Objective
The biomarkers of hand function may differ based on level of motor impairment after stroke. The objective of this study was to determine the relationship between resting state functional connectivity (RsFC) and unimanual contralesional hand function after stroke and whether brain-behavior relationships differ based on level of grasp function.
Methods
Sixty-two individuals with chronic, left-hemisphere stroke were separated into three functional levels based on Box and Blocks Test performance with the contralesional hand: Low (moved 0 blocks), Moderate (moved >0% but <90% of blocks relative to the ipsilesional hand), and High (moved ≥90% of blocks relative to the ipsilesional hand).
Results
RsFC in the ipsilesional and interhemispheric motor networks was reduced in the Low group compared to the Moderate and High groups. While interhemispheric RsFC correlated with hand function (grip strength and Stroke Impact Scale Hand) across the sample, contralesional RsFC correlated with hand function in the Low group and no measures of connectivity correlated with hand function in the Moderate and High groups. Linear regression modeling found that contralesional RsFC significantly predicted hand function in the Low group, while no measure correlated with hand function in the High group. Corticospinal tract integrity was the only predictor of hand function for the Moderate group and in an analysis across the entire sample.
Conclusions
Differences in brain-hand function relationships based on level of motor impairment may have implications for predictive models of treatment response and the development of intervention protocols aimed at improving hand function after stroke.
Keywords: stroke, hand function, functional connectivity, corticospinal tract
Introduction
After stroke, changes in brain function and brain structure occur both in the area of the lesion and at remote sites, impacting motor function. 1 Brain-behavior relationships are important in not only determining the neural correlates of motor deficits after stroke but also in defining the mechanisms of treatment-induced changes and developing targets for restorative intervention approaches. 2 The integrity of both functional and structural connections in the motor system have been shown to support skilled movement after stroke3 -7; however, the biomarkers of motor function may differ based on subgroups. 8
Recent studies have suggested that the neural correlates of arm and hand function in individuals post-stroke may differ based on level of motor capacity.6,9,10 While ipsilesional and interhemispheric pathways are thought to support upper extremity movement after stroke,3 -5,11,12 the contralesional hemisphere may play a unique role in movement in individuals with relatively limited movement ability.9,13 -15 Resting state functional connectivity (RsFC) provides an avenue through which brain function-motor behavior relationships can be explored in individuals post-stroke with a range of motor abilities without the confound of movement performance on activation 16 and has been suggested as a measure for development as a possible motor system biomarker after stroke. 17
Several studies have found that interhemispheric RsFC correlates with measures that assess a combination of arm and hand impairment or function after stroke.4,18 -20 However, these studies examined brain-behavior relationships in the study population as a whole and not in functional subgroups. Recovery of hand function is of particular interest after stroke as the hand is the primary way an individual interacts with the environment. Measures of unimanual paretic hand impairment and function have shown a relationship with interhemispheric RsFC across the study population after stroke, 4 however, brain-behavior relationships based on level of grasp function were not explored. While previous studies have shown differences in RsFC based on level of hand function, the measure used in those studies to define hand function and stratify individuals combined both unimanual and bimanual tasks, limiting translation to our understanding of unimanual hand function.21 -23 The relationship between RsFC and unimanual paretic hand function based on level of grasp ability has not been explored.
The purpose of this study was to examine the relationship between RsFC and hand function after stroke and to determine whether brain-behavior relationships differed based on level of grasp function. We hypothesized that RsFC between motor regions would correlate with hand function across the sample but that the relationship between RsFC and hand function would differ between functional subgroups. Specifically, we expected RsFC in interhemispheric motor regions to correlate with hand function in individuals with relatively higher levels of grasp function but RsFC in contralesional motor regions to correlate with hand function in individuals with relatively lower levels of grasp function. Finally, given previous work showing relationships between motor function and the integrity of the corticospinal tract and interhemispheric motor pathways after stroke,3,6,24,25 we explored whether structural measures of these key motor pathways added to or were better than RsFC for the prediction of current hand function.
Methods
Participants
Sixty-two individuals were recruited as part of a larger multi-lab study on brain-behavior relationships in chronic stroke.26 -28 Participants were included if they were monolingual speakers of English, experienced a left-hemisphere ischemic or hemorrhagic stroke ≥6 months prior to study inclusion, could follow simple instructions, and could walk 8 m with or without an assistive device but with no physical assistance. Exclusion criteria consisted of contraindications for MRI, clinical history of dementia, alcohol abuse, psychiatric disorder, traumatic brain injury, or extensive visual acuity or visual-spatial problems. All participants provided written informed consent through a protocol approved by the Institutional Review Board at the University of South Carolina that followed the standards of the World Medical Association Declaration of Helsinki.
Hand Function Assessments
Each participant completed three measures of hand function: Box and Blocks Test (BBT), grip strength, and Stroke Impact Scale (SIS) hand domain. The BBT is a measure of gross hand dexterity with the score representing the number of blocks moved in 1 minute. 29 Three functional subgroups (Low, Moderate, and High) were created based on BBT performance asymmetry scores of the more-impaired, contralesional hand relative to the less-impaired, ipsilesional hand. We chose to use the BBT to create functional subgroups because it focuses on unimanual grasp function, is quick and simple to administer, and has been suggested as a useful tool for stratification after stroke. 30 The Low group consisted of participants who could not move a single block with the contralesional hand (0% with the contralesional hand relative to the ipsilesional hand). The Moderate group consisted of participants who were able to move >0% but <90% of blocks with the contralesional hand compared to the ipsilesional hand. The High group included participants who were able to move ≥90% of blocks with the contralesional hand compared to the ipsilesional hand consistent with data showing that older adults without stroke tend to have symmetrical performance on the BBT. 29 Grip strength was assessed using a handheld dynamometer (mean of three trials). The SIS hand domain is a patient-reported outcome measure that indicates perceived difficulty in completing functional tasks with the more impaired hand with scores that range from 0 (could not do) to 100 (not difficult). 31
Due to well-described floor and ceiling effects,32 -34 no individual clinical measure provides an index of motor status across a stroke population with a range of motor abilities. In the current study, we included two measures, grip strength and SIS hand domain score, to capture hand function. To reduce the dimensionality of the measures (which were highly correlated: r = .801, P < .001) and to minimize the number of statistical tests, a principal component analysis was performed that included contralesional grip strength and SIS hand domain score to produce a single hand function score for each participant. The first component accounted for 88.5% of the variance in hand function scores and was highly correlated with the individual variables (r = .941 for both grip strength and SIS hand); this component score was used in subsequent analyses.
MRI Acquisition
MRI data was collected within 2 days of motor behavioral testing on a 3T Siemens scanner; 48 participants completed the MRI session on a Trio and 14 participants completed the MRI after update to the Prisma configuration. Data collected on the Prisma was distributed across subgroups with 3 participants (25%), 8 participants (31%), and 3 participants (13%) in the Low, Moderate, and High groups, respectively. Resting state fMRI was collected with eyes closed using a 12-channel head coil and echo planar imaging sequence for 6 minutes on the Trio (N = 48; TR = 1850 milliseconds, TE = 30 milliseconds, 34 axial slices, 3 mm thick with 20% gap, 3.25 × 3.25 mm in plane, 196 volumes, 208 × 208 mm FOV, 64 × 64 mm matrix size), while the Prisma acquisition used a 20-channel head/neck coil and multiband sequence (MB factor = 2) for 10 or 11 minutes (N = 14; TR = 1650 milliseconds, TE = 35 milliseconds, 50 axial slices, 2 mm thick with 20% gap, 2.4 × 2.4 mm in plane, 370 or 427 volumes, 216 × 216 mm FOV, 90 × 90 mm matrix size). Diffusion weighted images were acquired using echo planar imaging to estimate the fractional anisotropy (FA) of motor pathways; two sequences were acquired in opposite phase encoding directions (for 47 participants: Trio, TR = 4987 milliseconds, TE = 79.2 milliseconds, 50 axial slices, 2.3 mm3 voxel size; for 1 participant: Trio, TR = 6100 milliseconds, TE = 101 milliseconds, 45 axial slices, 2.7 mm3 voxel size; for 14 participants: Prisma, TR = 5250 milliseconds, TE = 80 milliseconds, 80 axial slices, 1.5 mm3 voxel size). Each sequence included 36 volumes with noncollinear diffusion directions at a b-value of 1000 seconds/mm2 and 5 or 7 volumes with b-value of 0. Finally, high resolution T1-weighted (TR = 2250 milliseconds, TE = 4.52/4.15 milliseconds, 1 mm3 voxel size) and T2-weighted images (TR = 2800 milliseconds, TE = 402 milliseconds, 1 mm3 voxel size) were acquired for each participant.
MRI Processing
Stroke lesions were manually outlined on the T2 image which was then coregistered to the T1 image. T1-weighted images were normalized into standard MNI space utilizing enantiomorphic unified segmentation-normalization routines as part of SPM 1235 (Wellcome Department of Cognitive Neurology, London, UK), which also applied a lesion-mask cost function. 36 Resting state fMRI data was pre-processed using a published, custom approach (NiiStat; https://github.com/neurolabusc/NiiStat) that utilizes processes from SPM 12 and FSL (FMRIB Center, Oxford, UK). 37 Briefly, images were realigned and unwarped, slice time corrected (except for participants with multiband sequence images), normalized and smoothed (Gaussian kernel FWHM = 6 mm). Regressors were added for the mean signal from white matter and six head motion parameters, and linear, quadratic, and cubic trends, followed by the application of a bandpass filter (0.01-0.1 Hz). Next, an independent component analysis was performed to identify and remove lesion-driven artifacts. 37 After pre-processing, the brain was segmented utilizing a probabilistic John Hopkins University (JHU) atlas 38 included in the Niistat package, and the mean BOLD signal was correlated between a set of a priori regions of interest (ROIs) in both the lesioned and nonlesioned hemispheres: primary motor cortex (M1, precentral gyrus), primary sensory cortex (S1, postcentral gyrus), premotor cortex (PM, posterior section of the middle frontal gyrus), and supplementary motor area (SMA, posterior section of the superior frontal gyrus) (Supplemental Figure 1). Connectivity between these cortical ROIs have been shown to be related to hand movement after stroke during task-based fMRI.39 -41 Three motor networks were investigated: ipsilesional (connections between all ipsilesional regions), contralesional (connections between all contralesional regions), and interhemispheric (connections between homologous regions). Correlation coefficients (r) between each ROI pair were calculated and transformed to Fisher’s Z scores for statistical analysis.
Diffusion images were undistorted using FSL’s TOPUP and Eddy tools42,43 and excess skull was removed. Voxelwise maps of FA were created using the FSL dtifit tool. The skull-stripped (based on segmentation estimates) T1 image was nonlinearly normalized (using SPM12’s “old normalization” function) to match the undistorted FA images. FA was extracted from two JHU ROIs that represent pathways shown to support movement after stroke: the corticospinal tract (CST; ROI in the brainstem) and the body of the corpus callosum.3,6,10,24,25 For the CST, an FA ratio (FA lesioned hemisphere/FA nonlesioned hemisphere) was calculated to determine relative integrity of the lesioned side compared to the nonlesioned side for each individual.
Statistical Analyses
All statistical analyses were performed in SPSS 28 (IBM Corp., Armonk, NY). ROIs were grouped into an interhemispheric network (connections between four homologous pairs), ipsilesional network (six connections between ROIs in the ipsilesional hemisphere), and contralesional network (six connections between ROIs in the contralesional hemisphere). Separate one-way multiple analysis of variances were performed on each of these networks to determine if connectivity between motor regions differed between subgroups (Low, Moderate, High). Total lesion volume (mm3) was found to significantly differ across subgroups (Table 1) and significantly correlate with individual ROI lesion volume across the population (r = .271-.806; all P < .034); thus, lesion volume was included as a covariate. Effects size was reported using partial eta squared (ƞ2; .01-.059 = small effect; .06-.139 = medium effect; ≥.14 = large effect). 44 Significant differences between groups (P < .05) were investigated further to determine the location of differences with a least significant difference post-hoc test using a corrected P-value (.05/number of connections in the model: P < .0125 for the interhemispheric network, P < .008 for the ipsilesional and contralesional networks).
Table 1.
Participant Demographics by Subgroup.
| Low | Moderate | High | |
|---|---|---|---|
| N | 12 | 26 | 24 |
| Age (y) | 59.4 (43-77) | 58.9 (37-76) | 61.6 (48-80) |
| Sex | 10M/2F | 18M/8F | 14M/10F |
| Hand dominance (prior to stroke) | 11R/1L | 24R/2L | 22R/2L |
| Months post-stroke | 80.4 (10-284) | 49.2 (9-185) | 45.8 (10-201) |
| BBT paretic hand | 0.00 (0-0) | 31.3 (2-66) | 53.3 (36-83) |
| BBT non-paretic hand | 46.3 (18-66) | 52.7 (36-74) | 53.5 (29-80) |
| Grip strength (kg) | 3.2 ‡ (0.0-15.3) | 25.9 ‡ (3.0-61.3) | 36.2 ‡ (12.0-58.3) |
| SIS hand domain | 6.5 ‡ (0-15) | 60.6 ‡ (0-100) | 84.4 ‡ (35-100) |
| Lesion volume (cc) | 189.2*, † (0.8-365.4) | 87.4* (0.4-340.2) | 88.3 † (0.2-234.6) |
| CST FA ratio | 0.67*, † (0.46-0.88) | 0.87* (0.57-1.10) | 0.92 † (0.70-1.03) |
| CC body FA | 0.257 (0.058-0.477) | 0.347 (0.060-0.531) | 0.368 (0.127-0.556) |
| Interhemispheric network connectivity | 0.304*, † (−0.297-.912) | 0.679* (0.216-0.862) | 0.764 † (0.423-0.928) |
| Ipsilesional network connectivity | 0.438 (0.103-0.765) | 0.486 (0.103-0.731) | 0.564 (0.229-0.796) |
| Contralesional network connectivity | 0.591 (0.325-0.778) | 0.523 (0.060-0.709) | 0.573 (0.327-0.806) |
Values represent subgroup mean (range).
BBT, Box and Blocks Test; SIS, Stroke Impact Scale; CC, corpus callosum; CST, corticospinal tract; FA, fractional anisotropy; M, male; F, female; R, right; L, left. CST FA ratio, lesioned hemisphere FA/non-lesioned hemisphere FA.
P < .01 for difference between Low and Moderate subgroups.
P < .01 for difference between Low and High subgroups.
P < .01 for difference between all subgroups.
To examine the relationship between RsFC and hand function score, RsFC was averaged between the ROI pairs to produce a mean interhemispheric, ipsilesional, and contralesional value for each participant, similar to previous studies in stroke.4,19,45 Pearson’s correlations with 95% confidence intervals (CI) were run to examine the relationship between mean interhemispheric, ipsilesional, and contralesional RsFC and the hand function score for the Low, Moderate, and High subgroups, as well as across the whole population; a corrected P < .0167 was used to determine significance (.05/3 networks).
Lastly, linear regression modeling was performed to examine which variable or variables best predicted current hand function across all participants as well as for each functional subgroup. Possible predictors included demographic variables (age, months post-stroke), total lesion volume, functional connectivity (interhemispheric, ipsilesional, contralesional RsFC), and motor pathway FA (CST FA ratio, corpus callosum body FA). Variables that at least weakly correlated with hand function (P < .05) were entered into a forward stepwise model (P < .05 to enter, P > .1 to leave); all final models were inspected for collinearity (variance inflation factor <10).
Results
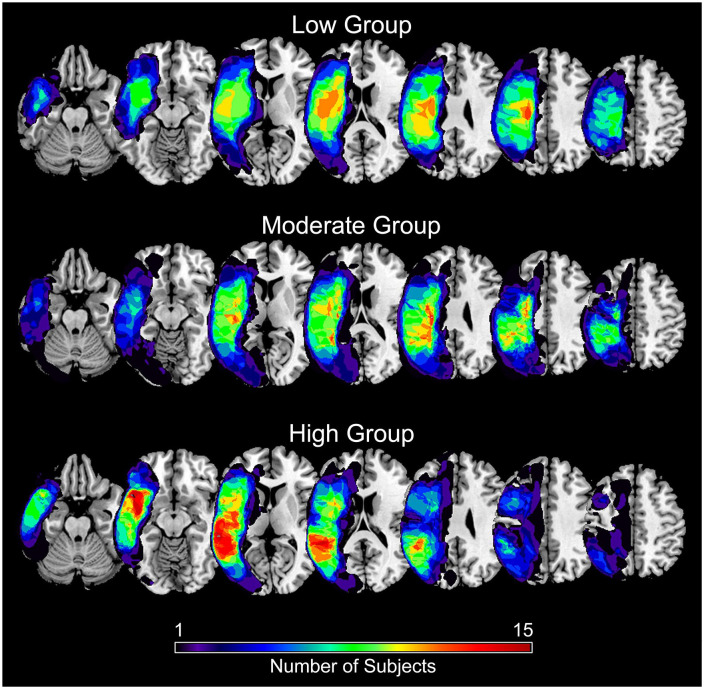
Sixty-two individuals with chronic stroke were included: 12 in the Low group, 26 in the Moderate group, and 24 in the High group (Table 1). The three groups did not significantly differ in age or months-post stroke but did differ in lesion volume, CST FA ratio, grip strength, and SIS hand domain score. Stroke lesions were cortical and subcortical along the middle cerebral artery territory in the left hemisphere (Figure 1). Resting-state functional connectivity (RsFC) between all regions in the interhemispheric, ipsilesional, and contralesional motor networks across all participants is shown in Supplemental Figure 2.
Figure 1.
Summary mask of stroke lesions by functional subgroup (Low, Moderate, High). Color represents the number of participants with a lesion in that voxel.
Interhemispheric and Intrahemispheric RsFC Based on Level of Grasp Function
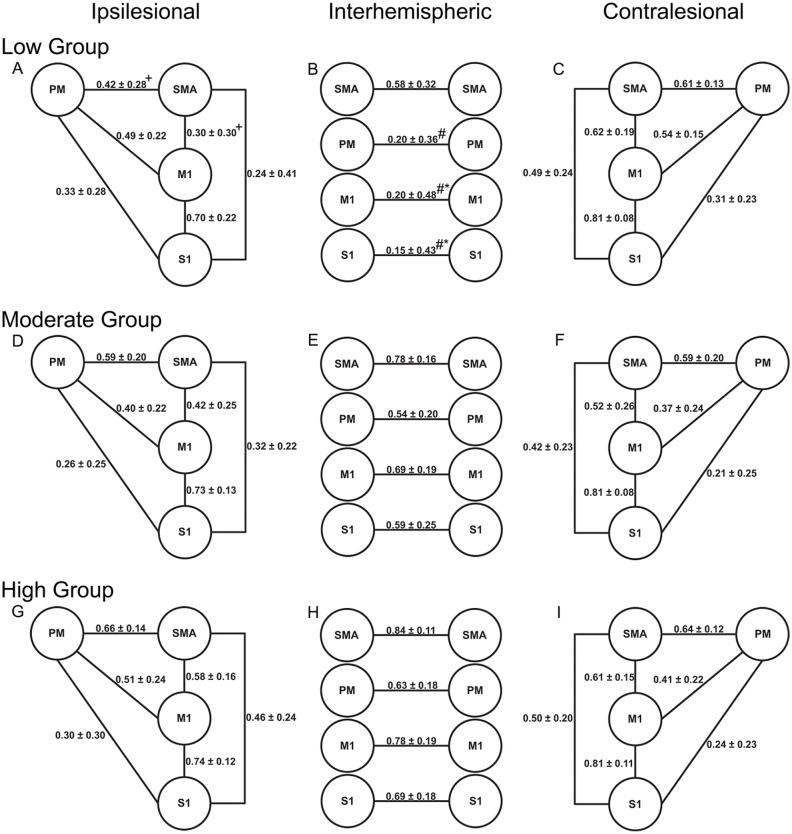
Interhemispheric RsFC between homologous motor regions differed between functional subgroups (P = .016, ƞ2 = .154; Figure 2). Specifically, the Low group had significantly lower interhemispheric RsFC between homologous M1 and S1 compared to the Moderate group (P < .008). The Low group also had significantly lower interhemispheric RsFC between homologous M1, S1, and PM ROIs compared to the High group (P < .002). No significant differences were found between the Moderate and High groups for any interhemispheric connections. Ipsilesional RsFC also differed between functional subgroups (P = .004, ƞ2 = .228). Specifically, the Low group had significantly lower RsFC than the High group for two connections (P < .006): PM-SMA and M1-SMA. Contralesional RsFC did not significantly differ between functional subgroups (P = .288, ƞ2 = .120).
Figure 2.
Mean resting state functional connectivity for the ipsilesional, interhemispheric, and contralesional motor networks for the Low Group (A-C), Moderate Group (D-F) and High Group (H-I).
Values represent mean r values ± standard deviation (before Fisher’s Z transformation).
Abbreviations: M1, primary motor cortex; PM, premotor cortex; SMA, supplementary motor area; S1, primary sensory cortex.
+Significantly different from the High group (P < .006).
#Signifcantly different from the High Group (P < .002).
*Significantly different from the Moderate group (P < .008).
Relationship Between RsFC and Hand Function Based on Level of Grasp Function
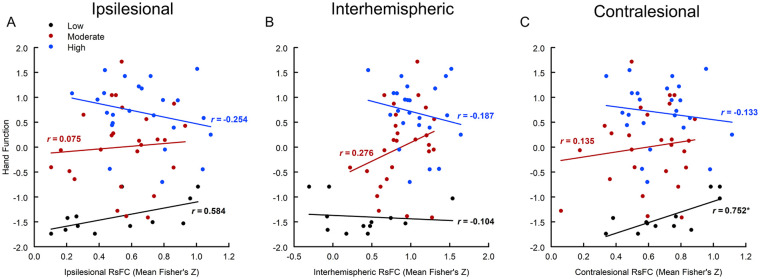
Across all participants, mean interhemispheric connectivity was found to significantly correlate with the hand function score (r = .466, 95% CI: 0.245-0.641; P < .001) but ipsilesional (r = .179, 95% CI: −0.074-0.411; P = .163) and contralesional (r = .018, 95% CI: −0.233-0.267; P = .889) connectivity did not. In the Low group, mean contralesional connectivity significantly correlated with the hand function score (r = .752, 95% CI: 0.314-0.926; P = .005); mean ipsilesional connectivity also showed a positive relationship with hand function (r = .584, 95% CI: 0.015-0.867; P = .046) but this relationship did not reach the corrected significance level (Figure 3). In the Moderate group, interhemispheric connectivity showed a positive correlation with hand function score (r = .276, 95% CI: −0.125-0.599; P = .172) but this relationship was not statistically significant; ipsilesional and contralesional connectivity did not correlate with hand function in this group. In the High group, hand function did not significantly correlate with mean functional connectivity in the interhemispheric, ipsilesional or contralesional network (|r|≤.254, P ≥ .231). Exploratory correlations between individual measures and RsFC are reported in Supplemental Table 1.
Figure 3.
Relationship between hand function score (first principal component of contralesional grip strength and Stroke Impact Scale Hand domain) and mean ipsilesional (A), interhemispheric (B), and contralesional (C) resting state functional connectivity (Fisher’s Z score) by functional subgroups (Low, Moderate, High).
r = correlation coefficient in each subgroup.
*P < .0167.
Predictors of Cross-Sectional Hand Function Based on Level of Grasp Function
Across all participants, mean interhemispheric RsFC, CST FA ratio (r = .595, 95% CI: 0.405-0.735; P < .001) and months post-stroke (r = −.265, 95% CI: −0.483-0.017; P = .037) were entered as variables into the stepwise regression model. Only CST FA ratio was found to be a significant predictor of hand function in this model (Table 2; R2 = .343, P < .001). For the Low group, both ipsilesional and contralesional RsFC were entered into the model; no other variables showed a correlation with hand function. Only contralesional RsFC remained in the model and was found to be a significant predictor of hand function (R2 = .523, P = .005). For the Moderate group, CST FA ratio (r = .437, 95% CI: 0.060-0.705; P = .026) was the only variable entered into the regression model and was a significant predictor of hand function (R2 = .157). For the High group, no variable was found to show a correlation with hand function (all P > .05); therefore, no regression model was generated for this subgroup.
Table 2.
Significant Predictors of Hand Function Across all Groups and by Subgroup.
| Group | Predictors | Beta value | Adjusted R2 | F statistic | P value for F |
|---|---|---|---|---|---|
| All groups | CST FA ratio | .595* | .343 | 32.854 | <.001 |
| Low group | Mean contralesional RsFC | .752* | .523 | 13.048 | .005 |
| Moderate group | CST FA ratio | .437* | .157 | 5.661 | .026 |
| High group | None |
Abbreviations: RsFC, resting state functional connectivity; CST FA, corticospinal tract fractional anisotropy.
P < .05.
Discussion
This study investigated brain-hand function relationships based on level of grasp function in individuals with left-hemisphere stroke. Overall, individuals with less functional grasp ability had lower RsFC in ipsilesional and interhemispheric motor networks. While interhemispheric RsFC correlated with hand function across the whole sample similar to previous studies,4,18 -20 differences in the relationship between RsFC and hand function were found based on level of grasp function. While the Low subgroup showed a positive relationship between contralesional RsFC and hand function, no measure of motor system RsFC signifcantly correlated with hand function in the Moderate or High subgroups. CST FA ratio also contributed to the prediction of cross-sectional hand function for the whole sample and in the Moderate subgroup. Differences in brain-hand function relationships based on level of motor function may have implications for predictive models of treatment response and the development of intervention protocols aimed at improving hand function after stroke.
To our knowledge, this is the first study to investigate the relationship between RsFC and unimanual hand function based on level of functional grasp ability. In the current study, the Low function group that had very limited grasp function (could not pick up and move one block) had lower functional connectivity in ipsilesional and interhemispheric motor connections but not contralesional connections compared to groups with some remaining unimanual ability to grasp and transport a block. Previous studies have shown both increased and decreased functional connectivity between groups of individuals post-stroke with varied levels of hand function.21 -23 However, in those studies, the stratification of subjects into functional levels included only two categories based on both unimanual and bimanual hand function: a “completely paralyzed” group that was unable to use the more-impaired hand even as a stabilizer during a bimanual task (eg, not able to stabilize a paper to allow the less-impaired hand to use scissors) and a “partially paralyzed” group that included individuals with a wide range of hand ability levels (from only being able to use the more-impaired hand as a stabilizer to being able to manipulate objects with the more-impaired hand). Our three subgroups were created based on the ability to grip, transport, and release a block with the more-impaired hand only, and were therefore focused solely on unimanual grasp function. As such, the current study provides novel data on functional brain connectivity across a range of levels of unimanual hand motor function in individuals with chronic stroke.
Relationships between upper extremity motor function and RsFC have been shown in previous studies, with interhemispheric connectivity often showing a positive correlation with motor function.4,18 -20 We found a similar relationship between interhemispheric RsFC and unimanual hand function when analyzing participants across groups. However, the relationship betweeen RsFC and hand function differed when subgroups were investigated. In the Low group, contralesional RsFC positively correlated with hand function (higher functional connectivity corresponded to greater hand function). This finding is in line with research suggesting that the contralesional hemisphere plays an important role in supporting movement in individuals with relatively severe motor impairment.9,14,15,46 Engagement of contralesional motor regions may support movement through alternative pathways (eg, reticulospinal) in individuals with severe motor impairment who have significant damage to the ipsilesional CST.47,48 In the High group, motor system RsFC did not correlate with unimanual hand function. Individuals in this group showed little deficit on performance measures (BBT, grip strength) but reported continued difficulty in completing functional tasks with the more impaired hand (SIS). In previous studies in older adults without stroke, higher sensorimotor network RsFC has been associated with better hand performance (ie, faster motor speed, stronger grip strength).49,50 While it is not entirely clear why we did not find a relationship between RsFC and hand function in this subgroup, hand function in individuals with mild impairment may be related to connectivity in other networks, such as frontal-parietal or prefrontal networks,51,52 or require measurement of hand dexterity (eg, precision grip) to examine brain-hand function relationships. The current study did not include an age-matched control group without stroke which could inform the brain-behavior relationships found, especially in the High group. Overall, the results of the current study suggest that level of hand function should be considered in future studies on brain-motor behavior relationships and how these relationships are predictive of or change in response to treatment after stroke.
Previous studies have investigated whether measures of RsFC or white matter pathway integrity best predict cross-sectional arm motor function after stroke with conflicting results; some studies reported that brain structure was a more robust biomarker of motor status post-stroke, while others have found that both structural pathway integrity and RsFC are distinct but important predictors.45,53,54 These studies primarily focused on interhemispheric RsFC and CST integrity and did not look at subgroups based on level of grasp function. In the current study, CST FA ratio best predicted unimanual hand function across all groups, despite the fact that interhemispheric RsFC was also found to correlate with hand function across all groups.
Residual integrity of the descending CST has frequently been reported to correlate with upper extremity impairment and function after stroke.3,5,25,55 The findings of the current study are consistent with this previous work and suggest that some level of structural integrity of the CST is needed for motor cortical regions to drive hand movement. Two previous studies that included both a measure of CST integrity and a measure of interhemispheric RsFC also found that CST integrity was a stronger predictor of upper extremity impairment when assessed in individuals with chronic stroke with a range of severity levels.45,54 In the Moderate group, only CST FA ratio was a significant predictor of hand function, while in the Low group, only contralesional RsFC was a significant predictor, suggesting that the ability of measures of brain function and brain structure to predict motor function may vary based on functional level. Previous work has suggested that biomarkers of arm motor impairment and function may vary based on functional subgroups.6,10,56 The results of the current study suggest that level of grasp function may be an important factor in determining the appropriate biomarker(s) for unimanual hand function in chronic stroke.
Level of functional grasp ability was determined based on performance on the BBT test with the contralesional hand relative to the ipsilesional hand. We chose to use the BBT because it focused on grasp function, is quick and simple to administer, and has been suggested as a useful tool in defining hand function level after stroke. 30 Contralesional grip strength (a measure of hand impairment) was then combined with a patient-reported measure of perceived difficulty (SIS Hand) to provide an overall measure of hand function for examination of brain-behavior relationships. Performance on the BBT was correlated with both grip strength (r = .786, P < .001) and SIS Hand score (r = .875, P < .001), suggesting these two measures provided some insight into overall grasp function. Using a combination of these measures allowed us to assess hand function across a sample with a range of functional levels. This approach did lead to overlap in the combined measure of hand function (first principal component) between severity groups that were based on grasp function alone (BBT asymmetry performance) (see Figure 3). While positively correlated, grip strength and self-reported perceived difficulty of hand function still provide some degree of unique information from hand grasp ability, which may have contributed to the observed overlap. For example, an individual who moved a relatively small number of blocks on the BBT (eg, <10) might have similar grip strength and SIS Hand score to an individual who was unable to move one block. While the best approach for stratification of individuals in stroke studies (ie, clinical presentation vs measures of brain function or structure) remains unknown, our approach provided a way to categorize individuals based on unimanual hand function using a measure (BBT) that is accessible in a variety of clinical environments. Future larger studies should explore optimal measures and approaches for stratification based on hand function after stroke.
Differences in brain-behavior relationships based on level of grasp function may have implications for stroke rehabilitation trials. Previous studies have reported differences in the neural correlates of upper extremity function based on motor severity using measures that include both arm and hand movement6,56; our findings extend this work by showing differences in the neural correlates of hand function based on level of unimanual hand movement ability. The choice of a brain-based biomarker to understand arm motor status, arm motor recovery, or treatment response may be impacted by level of motor ability. 17 Additionally, degree of motor impairment may be an appropriate stratification approach when designing rehabilitation trials, especially those aimed at brain repair. 8 The development, implementation, and assessment of intervention approaches aimed at improving hand function should consider differences in brain-behavior relationships based on functional subgroups.
A limitation of this work is that the data was acquired with two scanners. Tools such as ComBat 57 can harmonize data to minimize variance and potential bias. However, the method requires at least two scans per scanner to estimate scanner effects, and these tools require that that sample size and covariates are balanced and controlled for across scanners to develop an unbiased estimate of scanner effects. 57 Given our small sample size, we did not harmonize our data. As we note, groups were relatively well distributed across instruments, minimizing bias concerns. However, these differences are likely to have increased some of the variance in our data, reducing our statistical power. Therefore, while we have confidence in the positive effects we report, caution should be exercised in interpreting null results.
This was a cross-sectional study in individuals with chronic, left-hemisphere stroke who were mostly right-hand dominant. Due to differences in the role each hemisphere plays in the control of skilled upper extremity motor tasks,58 -60 the results of the current study may not generalize to persons with right-hemisphere stroke. Also, given the small number of individuals who were left-hand dominant (N = 5) and our overall subgroup sample size, we were not able to examine the effect of pre-stroke hand dominance. Additionally, RsFC has been shown to change over time after the stroke event61,62; the brain-behavior relationships shown in the current study may be different in the acute and subacute stages of recovery. Future studies could investigate the neural correlates of hand function based on level of grasp ability in earlier stages of recovery, pre-stroke hand dominance, and in individuals with right-hemisphere stroke. Second, recent studies have suggested that stroke lesion topography may impact structural and functional connectivity and their interaction. 63 However, our sample size did not provide sufficient power to investigate lesion factors within the hand function subgroups. Future studies could investigate this factor with a larger sample in each subgroup. Third, a small number of participants reported experiencing a previous stroke or transient ischemic attack (N = 6; Low group = 1, Moderate group = 2, High group = 3). Individual variation in conditions such as previous stroke frequency and presence of diffuse white matter disease could affect the brain-behavior relationships reported here. Further studies could investigate how these factors influence the relationship between hand function and RsFC post-stroke. Finally, our approach to the creation of functional subgroups was based on BBT asymmetry performance with the contralesional hand relative to the ipsilesional hand. This approach defined a functional group with a small sample size (Low Group: N = 12) which may have limited our correlation and regression analyses. Further investigation is needed to define brain function-motor behavior relationships in individuals with this level of hand function post-stroke. Furthermore, the number of subgroups (ie, 3), clinical measure (BBT), and cut-off values used to determine subgroups in the current study may not be optimal. The best approach for defining subgroups based on level of grasp function should be investigated in future studies.
In conclusion, motor system RsFC in individuals with chronic left hemisphere stroke varied between groups based on level of unimanual grasp function; individuals with low levels of hand function had reduced ipsilesional and interhemispheric motor system connectivity when compared to individuals with greater residual hand motor ability. While CST FA ratio predicted cross-sectional hand function across the whole population, the neural correlates of hand function varied by functional subgroups. CST FA ratio correlated with hand function in individuals with moderate levels of hand function whereas contralesional RsFC correlated with hand function in individuals with limited hand function. Differences in brain-hand function relationships based on level of motor impairment may have implications for predictive models of treatment response and the development of intervention protocols aimed at improving hand function after stroke.
Supplemental Material
Supplemental material, sj-docx-1-nnr-10.1177_15459683241270080 for Brain-Hand Function Relationships Based on Level of Grasp Function in Chronic Left-Hemisphere Stroke by Elizabeth Rizor, Julius Fridriksson, Denise M. Peters, Chris Rorden, Leonardo Bonilha, Grigori Yourganov, Stacy L. Fritz and Jill Campbell Stewart in Neurorehabilitation and Neural Repair
Footnotes
Supplementary material for this article is available on the Neurorehabilitation & Neural Repair website along with the online version of this article.
Author Contributions: Elizabeth Rizor: Conceptualization; Formal analysis; Methodology; Visualization; Writing – original draft.
Julius Fridriksson: Funding acquisition; Investigation; Methodology; Resources; Supervision; Writing – review & editing.
Denise Peters: Formal analysis; Investigation; Methodology; Writing – review & editing.
Chris Rorden: Data curation; Methodology; Software; Writing – review & editing.
Leonardo Bonilha: Methodology; Software; Writing – review & editing.
Grigori Yourganov: Methodology; Software; Writing – review & editing.
Stacy Fritz: Investigation; Methodology; Resources; Writing – review & editing.
Jill Stewart: Conceptualization; Formal analysis; Methodology; Visualization; Writing – original draft.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by grants P50 DC014664 from the National Institute on Deafness and other Communication Disorders, P20 GM135007 from the National Institute of General Medical Sciences, 15SDG24970011 from the American Heart Association, and a South Carolina Honors College Science Undergraduate Research Fellowship.
ORCID iDs: Denise M. Peters  https://orcid.org/0000-0003-2066-4624
https://orcid.org/0000-0003-2066-4624
Jill Campbell Stewart  https://orcid.org/0000-0002-3275-5729
https://orcid.org/0000-0002-3275-5729
References
- 1. Guggisberg AG, Koch PJ, Hummel FC, Buetefisch CM. Brain networks and their relevance for stroke rehabilitation. Clin Neurophysiol. 2019;130(7):1098-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cramer SC. Repairing the human brain after stroke. II. Restorative therapies. Ann Neurol. 2008;63(5):549-560. [DOI] [PubMed] [Google Scholar]
- 3. Burke E, Dodakian L, See J, et al. A multimodal approach to understanding motor impairment and disability after stroke. J Neurol. 2014;261(6):1178-1186. [DOI] [PubMed] [Google Scholar]
- 4. Carter AR, Astafiev SV, Lang CE, et al. Resting interhemispheric functional magnetic resonance imaging connectivity predicts performance after stroke. Ann Neurol. 2010;67(3):365-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stinear CM, Barber PA, Smale PR, Coxon JP, Fleming MK, Byblow WD. Functional potential in chronic stroke patients depends on corticospinal tract integrity. Brain. 2007;130(Pt 1):170-180. [DOI] [PubMed] [Google Scholar]
- 6. Stewart JC, Dewanjee P, Tran G, et al. Role of corpus callosum integrity in arm function differs based on motor severity after stroke. Neuroimage Clin. 2017;14:641-647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wu J, Quinlan EB, Dodakian L, et al. Connectivity measures are robust biomarkers of cortical function and plasticity after stroke. Brain. 2015;138(Pt 8):2359-2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cramer SC. Stratifying patients with stroke in trials that target brain repair. Stroke. 2010;41(10 Suppl):S114-S116. [DOI] [PubMed] [Google Scholar]
- 9. Bradnam LV, Stinear CM, Barber PA, Byblow WD. Contralesional hemisphere control of the proximal paretic upper limb following stroke. Cereb Cortex. 2012;22(11):2662-2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hayward K, Ferris JK, Lohse KR, et al. Observational study of neuroimaging biomarkers of severe upper limb impairment after stroke. Neurology. 2022;99(4):e402-e413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Murase N, Duque J, Mazzocchio R, Cohen LG. Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol. 2004;55(3):400-409. [DOI] [PubMed] [Google Scholar]
- 12. Plantin J, Verneau M, Godbolt AK, et al. Recovery and prediction of bimanual hand use after stroke. Neurology. 2021;97(7):e706-e719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mohapatra S, Harrington R, Chan E, Dromerick AW, Breceda EY, Harris-Love M. Role of contralesional hemisphere in paretic arm reaching in patients with severe arm paresis due to stroke: a preliminary report. Neurosci Lett. 2016;617:52-58. [DOI] [PubMed] [Google Scholar]
- 14. Sankarasubramanian V, Machado AG, Conforto AB, et al. Inhibition versus facilitation of contralesional motor cortices in stroke: deriving a model to tailor brain stimulation. Clin Neurophysiol. 2017;128(6):892-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bestmann S, Swayne O, Blankenburg F, et al. The role of contralesional dorsal premotor cortex after stroke as studied with concurrent TMS-fMRI. J Neurosci. 2010;30(36):11926-11937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Carter AR, Shulman GL, Corbetta M. Why use a connectivity-based approach to study stroke and recovery of function? Neuroimage. 2012;62(4):2271-2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Boyd LA, Hayward KS, Ward NS, et al. Biomarkers of stroke recovery: consensus-based core recommendations from the stroke recovery and rehabilitation roundtable. Neurorehabil Neural Repair. 2017;31(10-11):864-876. [DOI] [PubMed] [Google Scholar]
- 18. Baldassarre A, Ramsey L, Rengachary J, et al. Dissociated functional connectivity profiles for motor and attention deficits in acute right-hemisphere stroke. Brain. 2016;139(Pt 7):2024-2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Urbin MA, Hong X, Lang CE, Carter AR. Resting-state functional connectivity and its association with multiple domains of upper-extremity function in chronic stroke. Neurorehabil Neural Repair. 2014;28(8):761-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen JL, Schlaug G. Resting state interhemispheric motor connectivity and white matter integrity correlate with motor impairment in chronic stroke. Front Neurol. 2013;4:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hong W, Lin Q, Cui Z, Liu F, Xu R, Tang C. Diverse functional connectivity patterns of resting-state brain networks associated with good and poor hand outcomes following stroke. Neuroimage Clin. 2019;24:102065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yin D, Song F, Xu D, et al. Patterns in cortical connectivity for determining outcomes in hand function after subcortical stroke. PLoS One. 2012;7(12):e52727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhao Z, Wang X, Fan M, et al. Altered effective connectivity of the primary motor cortex in stroke: a resting-state fMRI study with granger causality analysis. PLoS One. 2016;11(11):e0166210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang LE, Tittgemeyer M, Imperati D, et al. Degeneration of corpus callosum and recovery of motor function after stroke: a multimodal magnetic resonance imaging study. Hum Brain Mapp. 2012;33(12):2941-2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lindenberg R, Renga V, Zhu LL, Betzler F, Alsop D, Schlaug G. Structural integrity of corticospinal motor fibers predicts motor impairment in chronic stroke. Neurology. 2010;74(4):280-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Peters DM, Fridriksson J, Stewart JC, et al. Cortical disconnection of the ipsilesional primary motor cortex is associated with gait speed and upper extremity motor impairment in chronic left hemispheric stroke. Hum Brain Mapp. 2018;39(1):120-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fridriksson J, Yourganov G, Bonilha L, Basilakos A, Den Ouden DB, Rorden C. Revealing the dual streams of speech processing. Proc Natl Acad Sci U S A. 2016;113(52):15108-15113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stark BC, Basilakos A, Hickok G, Rorden C, Bonilha L, Fridriksson J. Neural organization of speech production: a lesion-based study of error patterns in connected speech. Cortex. 2019;117:228-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Desrosiers J, Bravo G, Hebert R, Dutil E, Mercier L. Validation of the Box and Block Test as a measure of dexterity of elderly people: reliability, validity, and norms studies. Arch Phys Med Rehabil. 1994;75(7):751-755. [PubMed] [Google Scholar]
- 30. Thompson-Butel AG, Lin GG, Shiner CT, McNulty PA. Two common tests of dexterity can stratify upper limb motor function after stroke. Neurorehabil Neural Repair. 2014;28(8):788-796. [DOI] [PubMed] [Google Scholar]
- 31. Duncan PW, Wallace D, Lai SM, Johnson D, Embretson S, Laster LJ. The Stroke Impact Scale version 2.0. Evaluation of reliability, validity, and sensitivity to change. Stroke. 1999;30(10):2131-2140. [DOI] [PubMed] [Google Scholar]
- 32. Stewart JC, Cramer SC. Patient-reported measures provide unique insights into motor function after stroke. Stroke. 2013;44(4):1111-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Thompson-Butel AG, Lin G, Shiner CT, McNulty PA. Comparison of three tools to measure improvements in upper-limb function with poststroke therapy. Neurorehabil Neural Repair. 2015;29(4):341-348. [DOI] [PubMed] [Google Scholar]
- 34. Lin JH, Hsu MJ, Sheu CF, et al. Psychometric comparisons of 4 measures for assessing upper-extremity function in people with stroke. Phys Ther. 2009;89(8):840-850. [DOI] [PubMed] [Google Scholar]
- 35. Rorden C, Bonilha L, Fridriksson J, Bender B, Karnath HO. Age-specific CT and MRI templates for spatial normalization. Neuroimage. 2012;61(4):957-965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Brett M, Leff AP, Rorden C, Ashburner J. Spatial normalization of brain images with focal lesions using cost function masking. Neuroimage. 2001;14(2):486-500. [DOI] [PubMed] [Google Scholar]
- 37. Yourganov G, Fridriksson J, Stark B, Rorden C. Removal of artifacts from resting-state fMRI data in stroke. Neuroimage Clin. 2018;17:297-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Faria AV, Joel SE, Zhang Y, et al. Atlas-based analysis of resting-state functional connectivity: evaluation for reproducibility and multi-modal anatomy-function correlation studies. Neuroimage. 2012;61(3):613-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Grefkes C, Nowak DA, Eickhoff SB, et al. Cortical connectivity after subcortical stroke assessed with functional magnetic resonance imaging. Ann Neurol. 2008;63(2):236-246. [DOI] [PubMed] [Google Scholar]
- 40. Rehme AK, Eickhoff SB, Wang LE, Fink GR, Grefkes C. Dynamic causal modeling of cortical activity from the acute to the chronic stage after stroke. Neuroimage. 2011;55:1147-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Grefkes C, Eickhoff SB, Nowak DA, Dafotakis M, Fink GR. Dynamic intra- and interhemispheric interactions during unilateral and bilateral hand movements assessed with fMRI and DCM. Neuroimage. 2008;41(4):1382-1394. [DOI] [PubMed] [Google Scholar]
- 42. Andersson JL, Skare S, Ashburner J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage. 2003;20(2):870-888. [DOI] [PubMed] [Google Scholar]
- 43. Andersson JLR, Sotiropoulos SN. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage. 2016;125:1063-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cohen J. Statistical Power Analysis for the Behaivoral Sciences. 2nd ed. Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 45. Carter AR, Patel KR, Astafiev SV, et al. Upstream dysfunction of somatomotor functional connectivity after corticospinal damage in stroke. Neurorehabil Neural Repair. 2012;26(1):7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Johansen-Berg H, Rushworth MF, Bogdanovic MD, Kischka U, Wimalaratna S, Matthews PM. The role of ipsilateral premotor cortex in hand movement after stroke. Proc Natl Acad Sci U S A. 2002;99(22):14518-14523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bradnam LV, Stinear CM, Byblow WD. Ipsilateral motor pathways after stroke: implications for non-invasive brain stimulation. Front Hum Neurosci. 2013;7:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Plow EB, Sankarasubramanian V, Cunningham DA, et al. Models to tailor brain stimulation therapies in stroke. Neural Plast. 2016;2016:4071620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hirsiger S, Koppelmans V, Mérillat S, et al. Structural and functional connectivity in healthy aging: associations for cognition and motor behavior. Hum Brain Mapp. 2016;37(3):855-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Seidler R, Erdeniz B, Koppelmans V, Hirsiger S, Merillat S, Jancke L. Associations between age, motor function, and resting state sensorimotor network connectivity in healthy older adults. Neuroimage. 2015;108:47-59. [DOI] [PubMed] [Google Scholar]
- 51. Schulz R, Buchholz A, Frey BM, et al. Enhanced effective connectivity between primary motor cortex and intraparietal sulcus in well-recovered stroke patients. Stroke. 2016;47(2):482-489. [DOI] [PubMed] [Google Scholar]
- 52. Schulz R, Runge CG, Bönstrup M, et al. Prefrontal-premotor pathways and motor output in well-recovered stroke patients. Front Neurol. 2019;10:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lam TK, Binns MA, Honjo K, et al. Variability in stroke motor outcome is explained by structural and functional integrity of the motor system. Sci Rep. 2018;8(1):9480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lin LY, Ramsey L, Metcalf NV, et al. Stronger prediction of motor recovery and outcome post-stroke by cortico-spinal tract integrity than functional connectivity. PLoS One. 2018;13(8):e0202504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lewis AF, Stewart JC. Comparison of corticospinal tract integrity measures extracted from standard versus native space in chronic stroke. J Neurosci Methods. 2021;359:109216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Quinlan EB, Dodakian L, See J, McKenzie A, Stewart JC, Cramer SC. Biomarkers of rehabilitation therapy vary according to stroke severity. Neural Plast. 2018;2018:9867196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Beer JC, Tustison NJ, Cook PA, et al. Longitudinal ComBat: a method for harmonizing longitudinal multi-scanner imaging data. Neuroimage. 2020;220:117129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Haaland KY, Elsinger CL, Mayer AR, Durgerian S, Rao SM. Motor sequence complexity and performing hand produce differential patterns of hemispheric lateralization. J Cogn Neurosci. 2004;16(4):621-636. [DOI] [PubMed] [Google Scholar]
- 59. Mani S, Mutha PK, Przybyla A, Haaland KY, Good DC, Sainburg RL. Contralesional motor deficits after unilateral stroke reflect hemisphere-specific control mechanisms. Brain. 2013;136(Pt 4):1288-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Stewart JC, Gordon J, Winstein CJ. Control of reach extent with the paretic and nonparetic arms after unilateral sensorimotor stroke: kinematic differences based on side of brain damage. Exp Brain Res. 2014;232(7):2407-2419. [DOI] [PubMed] [Google Scholar]
- 61. Wang L, Yu C, Chen H, et al. Dynamic functional reorganization of the motor execution network after stroke. Brain. 2010;133(Pt 4):1224-1238. [DOI] [PubMed] [Google Scholar]
- 62. Park CH, Chang WH, Ohn SH, et al. Longitudinal changes of resting-state functional connectivity during motor recovery after stroke. Stroke. 2011;42(5):1357-1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Griffis JC, Metcalf NV, Corbetta M, Shulman GL. Structural disconnections explain brain network dysfunction after stroke. Cell Rep. 2019;28(10):2527-2540.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-nnr-10.1177_15459683241270080 for Brain-Hand Function Relationships Based on Level of Grasp Function in Chronic Left-Hemisphere Stroke by Elizabeth Rizor, Julius Fridriksson, Denise M. Peters, Chris Rorden, Leonardo Bonilha, Grigori Yourganov, Stacy L. Fritz and Jill Campbell Stewart in Neurorehabilitation and Neural Repair