Abstract
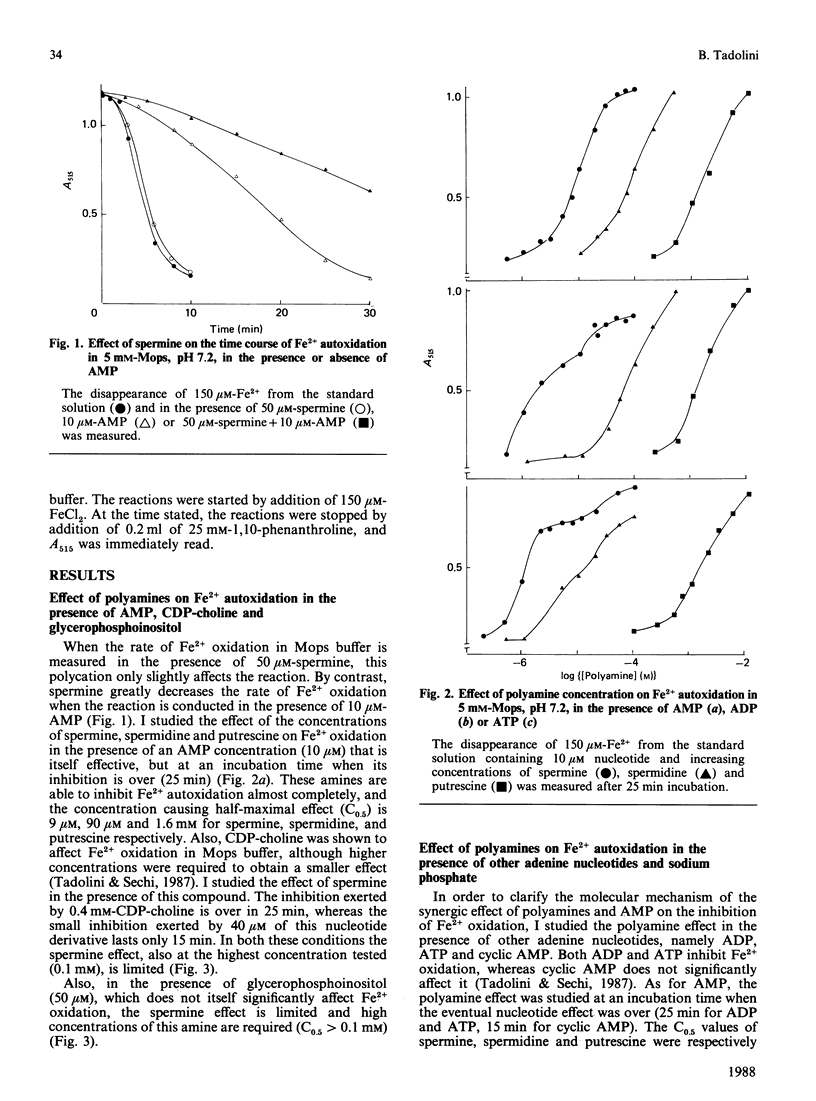
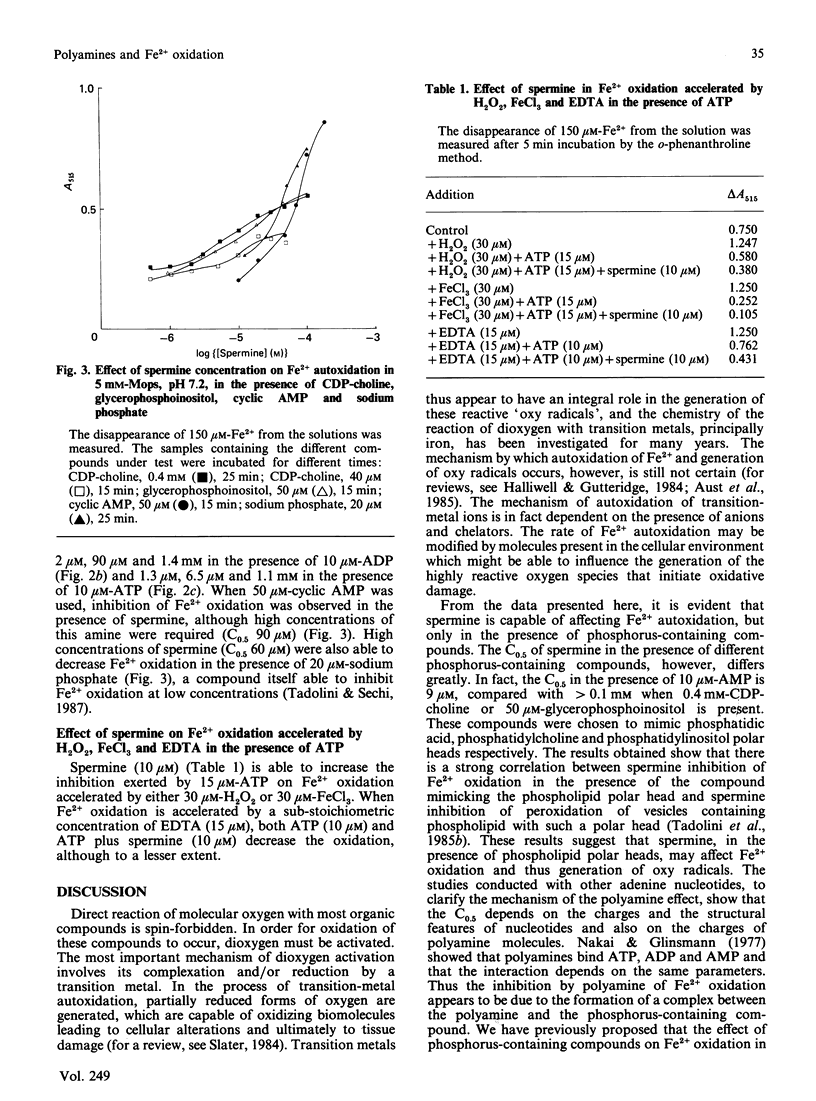
Polyamines appear to inhibit peroxidation of vesicles containing acidic phospholipids. A correlation exists between polyamine binding to phospholipid vesicles and its protective effect. However, phosphatidylinositol-containing vesicles which bind spermine are not protected by the polyamine [Tadolini, Cabrini, Landi, Varani & Pasquali (1985) Biogenic Amines 3, 97-106]. In the present paper I tested the hypothesis that polyamines, in particular spermine, by forming a ternary complex with iron and the phospholipid polar head may change the susceptibility of Fe2+ to autoxidation and thus its ability to generate free oxygen radicals. Different compounds mimicking phospholipid polar heads were studied, namely AMP, mimicking phosphatidic acid, CDP-choline, mimicking phosphatidylcholine, and glycerophosphoinositol, mimicking phosphatidylinositol. The results support the proposed hypothesis. In the presence of CDP-choline or of glycerophosphoinositol, spermine poorly affects Fe2+ autoxidation, whereas a considerable inhibition is observed in the presence of AMP. The ability of other phosphorus-containing compounds (ATP, ADP, cyclic AMP, sodium phosphate) to affect Fe2+ autoxidation in the presence of polyamines was also evaluated to understand the molecular mechanism of this phenomenon. It is proposed that polyamines may be part of the passive cellular defence mechanism against the oxidative damage caused by Fe2+.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aust S. D., Morehouse L. A., Thomas C. E. Role of metals in oxygen radical reactions. J Free Radic Biol Med. 1985;1(1):3–25. doi: 10.1016/0748-5514(85)90025-x. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J. 1984 Apr 1;219(1):1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson E. K., Fridovich I. The interaction of bovine erythrocyte superoxide dismutase with hydrogen peroxide: inactivation of the enzyme. Biochemistry. 1975 Dec 2;14(24):5294–5299. doi: 10.1021/bi00695a010. [DOI] [PubMed] [Google Scholar]
- Jellinck P. H., Perry G. Effect of polyamines on the metabolism of [16-14C]estradiol by rat-liver microsomes. Biochim Biophys Acta. 1967 Apr 4;137(2):367–374. doi: 10.1016/0005-2760(67)90112-9. [DOI] [PubMed] [Google Scholar]
- Kitada M., Igarashi K., Hirose S., Kitagawa H. Inhibition by polyamines of lipid peroxide formation in rat liver microsomes. Biochem Biophys Res Commun. 1979 Mar 30;87(2):388–394. doi: 10.1016/0006-291x(79)91808-4. [DOI] [PubMed] [Google Scholar]
- Kitada M., Naito Y., Igarashi K., Hirose S., Kanakubo Y., Kitagawa H. Possible mechanism of inhibition by polyamines of lipid peroxidation in rat liver microsomes. Res Commun Chem Pathol Pharmacol. 1981 Sep;33(3):487–497. [PubMed] [Google Scholar]
- Lambeth D. O., Ericson G. R., Yorek M. A., Ray P. D. Implications for in vitro studies of the autoxidation of ferrous ion and the iron-catalyzed autoxidation of dithiothreitol. Biochim Biophys Acta. 1982 Dec 17;719(3):501–508. doi: 10.1016/0304-4165(82)90239-2. [DOI] [PubMed] [Google Scholar]
- MAHLER H. R., ELOWE D. G. Studies on metalloflavoproteins. II. The rôle of iron in diphosphopyridine nucleotide cytochrome c reductase. J Biol Chem. 1954 Sep;210(1):165–179. [PubMed] [Google Scholar]
- Nakai C., Glinsmann W. Interactions between polyamines and nucleotides. Biochemistry. 1977 Dec 13;16(25):5636–5641. doi: 10.1021/bi00644a039. [DOI] [PubMed] [Google Scholar]
- Samuni A., Chevion M., Czapski G. Unusual copper-induced sensitization of the biological damage due to superoxide radicals. J Biol Chem. 1981 Dec 25;256(24):12632–12635. [PubMed] [Google Scholar]
- Schuber F., Hong K., Düzgünes N., Papahadjopoulos D. Polyamines as modulators of membrane fusion: aggregation and fusion of liposomes. Biochemistry. 1983 Dec 20;22(26):6134–6140. doi: 10.1021/bi00295a015. [DOI] [PubMed] [Google Scholar]
- Slater T. F. Free-radical mechanisms in tissue injury. Biochem J. 1984 Aug 15;222(1):1–15. doi: 10.1042/bj2220001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadolini B., Cabrini L., Landi L., Varani E., Pasquali P. Polyamine binding to phospholipid vesicles and inhibition of lipid peroxidation. Biochem Biophys Res Commun. 1984 Jul 31;122(2):550–555. doi: 10.1016/s0006-291x(84)80068-6. [DOI] [PubMed] [Google Scholar]
- Tadolini B. Polyamines effect on subcellular fractionation of rat liver homogenate. Biochem Biophys Res Commun. 1980 Jan 29;92(2):598–605. doi: 10.1016/0006-291x(80)90375-7. [DOI] [PubMed] [Google Scholar]
- van Hemmen J. J., Meuling W. J. Inactivation of Escherichia coli by superoxide radicals and their dismutation products. Arch Biochem Biophys. 1977 Aug;182(2):743–748. doi: 10.1016/0003-9861(77)90556-2. [DOI] [PubMed] [Google Scholar]


