Abstract
Recognising sex differences in disease prevalence can lead to clues as to its pathogenesis, for example the role of hormonal factors and related influences such as body composition, as well as forming the basis for new treatments. However, if different methods are used to define the disorder it can be difficult to explore differences in prevalence, making it necessary to draw on multiple sources of evidence. This narrative review addresses sex differences in the prevalence of knee and hip osteoarthritis, which are the most common forms of large joint osteoarthritis. Females appear to have a higher prevalence of knee osteoarthritis across a wide range of disease definitions, while findings for the hip vary depending on how the disease is defined. Clinically or symptomatically defined hip osteoarthritis is more common in females, whereas radiographically defined hip osteoarthritis is more common in males. Therefore, understanding sex differences in large joint arthritis requires consideration that osteoarthritis, as defined structurally, more commonly affects females at the knee, whereas the opposite is true at the hip. Furthermore, despite structural changes in hip osteoarthritis being more common in males, symptomatic hip osteoarthritis is more common in females. The basis for these disparities is currently unclear, but may reflect a combination of hormonal, biomechanical and behavioural factors.
Keywords: osteoarthritis, epidemiology, knee, hip, sex difference
Introduction
Knee and hip osteoarthritis, the most common forms of large joint osteoarthritis, are chronic, disabling, and highly prevalent conditions (1, 2). In the United Kingdom, large joint osteoarthritis has contributed to a significant increase in individuals leaving the workforce, with associated healthcare costs estimated to reach approximately £5 billion per year (3). During the early stages of the disease, primary treatments involve lifestyle interventions, exercise therapy and analgesia (1). In end-stage disease, the definitive treatment is costly joint replacement. Unfortunately, roughly 10-30% of individuals still suffer from chronic pain and loss of function after joint replacement, justifying research into novel treatments (4, 5). One consequence of the increasing prevalence of this condition is the increased demand for joint replacement procedures, placing strain on healthcare services, thus contributing substantially to the societal impact of this disease (6).
Recognising sex differences in disease prevalence can help in understanding pathogenesis and guiding treatment. However, this may be complicated if different methods are used to define the disorder. Previous research has indicated there are large sex differences in knee and hip osteoarthritis, and several comprehensive reviews have explored this topic (7–11). However, these reviews do not address the sex differences in disease prevalence according to disease definitions. Osteoarthritis can be defined clinically, symptomatically, and radiographically for the purpose of epidemiological studies (12). A clinical definition is usually obtained from healthcare records, where an individual has been diagnosed as having osteoarthritis by a healthcare professional. It is generally assumed that these individuals have symptoms, as asymptomatic cases are unlikely to receive a diagnosis. In some studies, osteoarthritis is defined by the concurrent presence of symptoms (such as pain, stiffness, or swelling) and observable radiographic changes (such as joint space narrowing or osteophyte formation) (13). Conversely, other studies adopt a purely radiographic definition (14), relying on established diagnostic criteria (e.g. Kellgren-Lawrence Scoring) (15). Additionally, as total knee and hip replacements are primarily used for the treatment of end stage symptomatic osteoarthritis, these procedures can serve as proxies for disease presence (16). When considering the prevalence of knee and hip osteoarthritis, these definitions should be considered individually as they measure different disease characteristics and are not necessarily interchangeable (17).
Females are reported to have an increased prevalence of osteoarthritis across all joints (18), although conflicting research has indicated that when defined radiographically, hip osteoarthritis is more common in males (19). In addition, prior work has suggested that females often receive fewer healthcare interventions for osteoarthritis, despite their higher prevalence (20, 21). Therefore, this narrative review was initiated to review the current literature regarding sex differences in knee and hip osteoarthritis prevalence according to different disease definitions, to review sex differences in rates of joint replacement, and to consider the possible reasons for the differences found. The focus of this paper will be on knee and hip osteoarthritis, given they are the most common large joints affected by osteoarthritis (2).
Clinical and symptomatic differences in knee and hip osteoarthritis
Knee osteoarthritis
Studies have consistently shown that symptomatic and clinical knee osteoarthritis are more prevalent in females than males ( Table 1 ). For instance, large scale European studies from Spain and the UK, which are based on healthcare records, reveal a prevalence of clinical knee osteoarthritis ranging from 3.2-3.6% in females, compared to 2.2-2.5% in males (22, 23). In a sizeable meta-analysis of prevalence estimates from Chinese populations, aged predominantly over 40 years old, symptomatic knee osteoarthritis was found to be roughly twice as prevalent in females than males (19.1% females vs 10.9% males). These higher prevalence figures are likely attributed to the advanced ages of the populations studied and to differences in disease definition (24). Prospective cohort studies (25–27) tend to show higher prevalence estimates of symptomatic osteoarthritis compared to population-wide prevalence estimates (22, 23) ( Table 1 ). Many diverse smaller studies have also found an increased prevalence of symptomatic knee osteoarthritis in females (20, 21, 28–39). Only one study found the prevalence of clinical knee osteoarthritis to be broadly similar between the sexes (F: 2.4% and M: 2.5%) (40).
Table 1.
Prevalence of sex-stratified clinical and symptomatic knee osteoarthritis.
| Author | Country | Population | Age (years) | OA type | Diagnosis method | OA prevalence (%) | |||
|---|---|---|---|---|---|---|---|---|---|
| Male (%) |
Female (%) |
Mean (Range/SD) | Total | Male | Female | ||||
| Prieto-Alhambra et al., 2014 (22) | Spain | 1,529,595 (46.8) | 1,737,231 (53.2) | 67.3 (SD11.0) | Clinical knee | ICD code | 96,222 (2.9) | 34,243 (2.2) | 61,979 (3.6) |
| Swain et al., 2022 (23) | UK | 1,690,618 | Inclusion ≥20 (NA) | Clinical knee | Coded diagnosis (2017) | NA (2.3) | NA (2.5) | NA (3.2) | |
| Li et al., 2020 (24) | China | 74,908 | Inclusion ≥15 (NA) | Symptomatic knee | Assorted | 10,937 (14.6) | NA (10.9) | NA (19.1) | |
| Mork et al., 2012 (28) | Norway | 14,766 (49.3) | 15,191 (50.7) | 43.79 (SD 14.0) | Symptomatic knee | Questionnaire | 351 (1.2) | 132 (0.9) | 219 (1.4) |
| Hawker et al., 2000 (20) | Canada | 11,930 (42.0) | 16,521 (58.0) | M: 69.4 (SD 8.7) F: 70.9 (SD 9.6) |
Symptomatic hip and knee | WOMAC score ≥39 | 1,325 (4.7) | 332 (2.8) | 993 (6.0) |
| Tang et al., 2015 (35) | China | 8,367 (48.8) | 8,761 (51.2) | M: 59.8 (SD 0.2) F: 59.8 (0.2) |
Symptomatic knee | Questionnaire (CHARLS) | 1,360 (8.1) | 478 (5.7) | 902 (10.3) |
| Park et al., 2017 (34) | Korea | 3,830 (42.7) | 5,146 (57.3) | Inclusion ≥50 (NA) | Symptomatic knee | Questionnaire | 754 (8.4) | 260 (6.8) | 494 (9.6) |
| Welling et al., 2017 (40) | Finland | 3,904 (47.3) | 4,344 (52.7) | Inclusion ≥46 (NA) | Clinical knee | ICD code | 202 (2.5) | 98 (2.5) | 104 (2.4) |
| Zhang et al., 2016 (43) | China | 3,609 (50.7) | 3,517 (49.4) | 43.9 (SD 16.6) | Clinical knee | ACR criteria | 983 (13.8) | 446 (12.4) | 537 (15.3) |
| Haq et al., 2005 (36) | Bangladesh | 2,582 (50.0) | 2,578 (50.0) | Inclusion ≥15 (NA) | Symptomatic knee | Questionnaire and examination | 451 (8.7) | 190 (7.4) | 261 (10.1) |
| Plotnikoff et al., 2015 (31) | Canada | 1,542 (32.6) | 3,189 (67.4) | 52.5 (SD 16.5) | Symptomatic knee | Questionnaire | 352 (7.4) | 146 (9.5) | 206 (6.5) |
| Ji et al., 2023 (37) | China | 1,950 (49.7) | 1,974 (50.3) | 58.44 (SD 9.2) | Symptomatic knee | ACR criteria | 404 (10.3) | 126 (6.5) | 278 (14.1) |
| Tukker et al., 2009 (25) | The Netherlands | 1,640 (44.8) | 2,024 (55.2) | 54.6 (NA) | Symptomatic knee | Questionnaire | 547 (14.9) | 213 (13.0) | 334 (16.5) |
| Picavet et al., 2003 (41) | The Netherlands | 1,641 (44.8) | 2,023 (55.2) | Inclusion ≥25 (NA) | Self-reported knee | Questionnaire | 441 (12.0) | 166 (10.1) | 275 (13.6) |
| Grotle et al., 2008 (33) | Norway | 1,480 (45.3) | 1,786 (54.7) | 60.44 (IQR 21) | Symptomatic knee | Questionnaire | 233 (7.1) | 92 (6.2) | 141 (7.9) |
| Felson et al., 1987 (39) | USA | 587 (41.4) | 831 (58.6) | 73 (Range 63-94) | Symptomatic knee | X-rays and questionnaire | 135 (9.5) | 40 (6.8) | 95 (11.4) |
| Carmona et al., 2001 (30) | Spain | 1,014 (46.3) | 1,178 (53.7) | Inclusion ≥20 (NA) | Symptomatic knee | ACR criteria | 223 (10.2) | 58 (5.7) | 165 (14.0) |
| Macias-Hernandez SI et al., 2020 (32) | Mexico | 80 (39.2) | 124 (60.8) | 57.4 (SD 10.9) | Clinical knee | Questionnaire | 40 (19.6) | 10 (12.5) | 30 (24.2) |
ACR, American College of Rheumatology; ICD, International Classification of Diseases; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index; UK, United Kingdom; USA, United States of America.
Hip osteoarthritis
In a large prospective study of adults over the age of 40 years old in the UK, hip osteoarthritis, defined by both prolonged hip pain and hospital diagnosis, has been found to be more common in females than males (hip pain lasting ≥3 months: Female 9.8% vs male 6.2% & hospital diagnosed: Female 1.5% vs male 1.1%) (19). Similarly, a comprehensive population study conducted in Spain also revealed a slightly higher prevalence of clinical hip osteoarthritis among females compared to males (1.0% vs 0.8%) (22). In a Korean study examining the prevalence of hip pain, deemed to be osteoarthritic in origin, females exhibited nearly three times higher rates (13.9%) than males (4.6%) (34). A large Canadian study looking at self-reported hip osteoarthritis found females to have a more modest increased risk of symptomatic hip osteoarthritis compared to males (6.6% vs 5.5%) (31). Likewise, several smaller studies also concluded that symptomatic and clinical hip osteoarthritis is more common in females than males (25, 26, 32, 33, 41) ( Table 2 ). In contrast, studies that have defined symptomatic hip osteoarthritis using pain with co-existent ipsilateral radiographic osteoarthritis suggest either no sex difference (42) or even a slightly higher prevalence in males than females (27, 43).
Table 2.
Prevalence of sex-stratified clinical and symptomatic hip osteoarthritis.
| Author | Country | Population | Age (years) | OA type | Diagnosis method | OA prevalence (%) | |||
|---|---|---|---|---|---|---|---|---|---|
| Male (%) |
Female (%) |
Mean (Range/SD) | Total | Male | Female | ||||
| Prieto-Alhambra et al., 2014 (22) | Spain | 1,529,595 (46.8) | 1,737,231 (53.2) | 67.3 (SD11.0) | Clinical hip | ICD code | 30,349 (0.9) | 12,698 (0.8) | 17,652 (1.0) |
| Swain et al., 2022 (23) | UK | 1,690,618 | Inclusion ≥20 (NA) | Clinical hip | Coded diagnosis (2017) | NA (1.1) | NA (1.2) | NA (1.7) | |
| Faber et al., 2022 (19) | UK | 19,294 (47.8) | 21,046 (52.2) | 63.7 (Range 44-82) | Symptomatic hip | Hip pain >3 months Hospital diagnosed |
3251 (8.1) 527 (1.3) |
1193 (6.2) 220 (1.1) |
2058 (9.8) 307 (1.5) |
| Mork et al., 2012 (28) | Norway | 14,766 (49.3) | 15,191 (50.7) | 43.79 (SD 14.0) | Symptomatic hip | Questionnaire | 322 (1.1) | 102 (0.7) | 220 (1.4) |
| Hawker et al., 2000 (20) | Canada | 11,930 (41.9) | 16,521 (58.1) | M: 69.4 (SD 8.7) F: 70.9 (SD 9.6) |
Symptomatic hip and knee | WOMAC score ≥39 | 1,325 (4.7) | 332 (2.8) | 993 (6.0) |
| Jüni et al., 2010 (21) | UK | 12,078 (46.4) | 13,968 (53.6) | 62.44 (SD 12.0) | Symptomatic hip | New Zealand Score ≥43 | 256 (1.0) | 81 (0.7) | 175 (1.3) |
| Park et al., 2017 (34) | Korea | 3,830 (42.7) | 5,146 (57.3) | Inclusion ≥50 (NA) | Symptomatic hip | Questionnaire | 891 (9.9) | 176 (4.6) | 715 (13.9) |
| Welling et al., 2017 (40) | Finland | 3,904 (47.3) | 4,344 (52.7) | Inclusion ≥46 (NA) | Clinical hip | ICD code | 40 (0.5) | 22 (0.6) | 18 (0.4) |
| Zhang et al., 2016 (43) | China | 3,609 (50.6) | 3,517 (49.4) | 43.9 (SD 16.6) | Clinical hip | ACR criteria | 42 (0.6) | 23 (0.6) | 19 (0.5) |
| Plotnikoff et al., 2015 (31) | Canada | 1,542 (32.6) | 3,189 (67.4) | 52.5 (SD 16.5) | Symptomatic hip | Questionnaire | 278 (5.9) | 102 (6.6) | 176 (5.5) |
| Tukker et al., 2009 (25) | The Netherlands | 1,640 (44.8) | 2,024 (55.2) | 54.6 (NA) | Symptomatic hip | Questionnaire | 356 (9.7) | 107 (6.5) | 249 (12.3) |
| Picavet et al., 2003 (41) | The Netherlands | 1,641 (44.8) | 2,023 (55.2) | Inclusion ≥25 (NA) | Self-reported hip | Questionnaire | 258 (7.0) | 64 (3.9) | 194 (9.6) |
| Grotle et al., 2008 (33) | Norway | 1,480 (45.3) | 1,786 (54.7) | 60.44 (IQR 21) | Symptomatic hip | Questionnaire | 179 (5.5) | 68 (4.6) | 111 (6.2) |
| Jordan et al., 2009 (26) | USA | 1,162 (37.9) | 1,906 (61.1) | Inclusion ≥45 (NA) | Symptomatic hip | Questionnaire | 1,123 (36.6) | 370 (31.8) | 753 (39.5) |
| Kim et al., 2014 (27) | USA | 434 (44.4) | 544 (55.6) | 63.5 (SD 9.0) | Symptomatic hip | X-ray and symptoms | 39 (4.0) | 23 (5.3) | 16 (3.0) |
| Macias-Hernandez SI et al., 2020 (32) | Mexico | 80 (39.2) | 124 (60.8) | 57.4 (SD 10.9) | Clinical hip | Questionnaire | 37 (18.1) | 6 (7.5) | 31 (25.0) |
ACR, American College of Rheumatology; ICD, International Classification of Diseases; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index; UK, United Kingdom; USA, United States of America.
Radiographic osteoarthritis
Radiographic osteoarthritis is a composite diagnosis made on the basis of changes observed in joint imaging. Kellgren and Lawrence described the radiographic features of osteoarthritis as joint space narrowing, osteophytosis, subchondral sclerosis and cyst formation (15).
Studies consistently report higher prevalence figures for radiographic knee osteoarthritis in females over males (32, 34, 38, 44–46) ( Table 3 ). In the large prospective Rotterdam Study, radiographic knee osteoarthritis was found to be twice as common in females as males (20.0% vs 9.0%, respectively) (47). A study based in the USA with a high proportion of African Americans found higher prevalence estimates for radiographic knee osteoarthritis, but again a higher prevalence was seen in females (F: 31.0% vs M: 23.7%) (48). The prevalence estimates for radiographic knee osteoarthritis tend to be higher than estimates for symptomatic and clinical osteoarthritis but the sex differences are equivalent ( Tables 1 , 3 ), and this is likely due to the structural joint changes (e.g. osteophytes) often being asymptomatic (27). In the aforementioned studies, radiographs (X-rays) were used to define disease, but it is worth noting that high-resolution dual-energy X-ray absorptiometry (DXA) scans are increasingly being used for this purpose (49, 50).
Table 3.
Prevalence of sex-stratified radiographic knee osteoarthritis.
| Author | Country | Population (%) | Age | OA type | Diagnosis method | OA prevalence (%) | |||
|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Mean (Range) | Total | Male | Female | ||||
| Park et al., 2017 (34) | Korea | 3,830 (46.7) |
5,146 (57.3) |
Inclusion ≥ 50 (NA) |
Radiographic knee | KL grade≥2 | 1,858 (20.7) | 628 (16.4) | 1,230 (23.9) |
| Hoeven et al., 2012 (47) | Netherland (Rotterdam) | 2,372 (42.0) | 3,278 (58.0) | M: 67.5 (SD 7.6) F: 68.6 (SD 8.3) |
Radiographic knee | KL grade≥2 | 868 (15.0) | 213 (9.0) | 655 (20.0) |
| Jordan et al., 2007 (48) | USA (Johnston County) | 1,162 (37.9) | 1,906 (62.1) | Inclusion ≥45 (NA) | Radiographic knee | KL grade≥2 | 866 (27.8) | 275 (23.7) | 591 (31.0) |
| Muraki et al., 2014 (45) | Japan (ROAD study) | 553 (35.5) | 1,005 (64.5) | M: 68.1 (SD 10.7) F: 66.5 (SD 11.0) |
Radiographic knee | KL grade≥2 | 769 (49.3) | 214 (38.7) | 555 (55.2) |
| Felson et al., 1995 (38) | USA | 313 (36.0) | 556 (64.0) | 70.8 (SD 5.0) (Range 63-91) | Radiographic knee | KL grade≥2 | 322 (37.1) | NA (11.1) | NA (18.1) |
| Cho et al., 2015 (46) | Korea | 298 (42.8) |
398 (57.2) |
72.0 (SD 5.0) (Range 65-91) |
Radiographic knee | KL grade≥2 | 265 (38.1) | 51 (17.1) | 214 (53.8) |
| Ho-Pham et al., 2014 (44) | Vietnam | 170 (25.8) | 488 (74.2) | M: 55.1 (SD 15.8) F: 55.9 (SD 12.6) (Range 40-98) |
Radiographic knee | KL grade≥2 | 225 (34.2) | 53 (31.2) | 172 (35.3) |
| Macías-Hernández et al., 2020 (32) | Mexico | 80 (39.2) |
124 (60.8) |
57.4 (SD 10.9) (Range 42-86) |
Radiographic knee | KL grade≥2 | 52 (25.5) | 14 (17.5) | 38 (30.6) |
KL, Kellgren-Lawrence grade; UK, United Kingdom; USA, United States of America.
At the hip, the opposite association is seen in the majority of studies ( Table 4 ), with radiographic osteoarthritis appearing to be more common in males as compared to females (27, 34, 46, 51–53). The largest study to date, looking at data from 40,340 individuals over 40 years old in the UK, estimated the prevalence of radiographic osteoarthritis (defined as grade ≥2 on high-resolution DXA scans) to be 8.2% in males compared to 3.5% in females (19). There were three studies that reported a higher prevalence of radiographic hip osteoarthritis in females (26, 32, 47). One of these studies, involving the Johnston County Osteoarthritis Project, was specifically designed to investigate racial differences in osteoarthritis. As a result, Caucasian women over 65 years old were intentionally under-sampled, while African Americans of both sexes were oversampled. This may have led to biased sex-based prevalence estimates, particularly given that osteoarthritis is more common in African Americans (26, 48). Another of these studies, a very small Mexican study, sampled only 204 individuals (32). Whilst radiographic hip osteoarthritis has been shown to be more common in males, this is not always the case for symptomatic radiographic hip osteoarthritis as previously mentioned (27, 42, 43).
Table 4.
Prevalence of sex-stratified radiographic hip osteoarthritis.
| Author | Country | Population (%) | Age | OA type | Diagnosis method | OA prevalence (%) | |||
|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Mean (Range) | Total | Male | Female | ||||
| Faber et al., 2022 (19) | UK (UK Biobank) |
19,294 (47.8) | 21,046 (52.2) | M: 64.4 (Range 44-81) F: 63.0 (Range 45-82) |
Radiographic hip | KL grade≥2 | 3,017 (7.5) | 2,086 (10.8) | 931 (4.4) |
| Park et al., 2017 (34) | Korea | 3,830 (46.7) | 5,146 (57.3) | Inclusion ≥ 50 (NA) |
Radiographic hip | KL grade≥2 | 57 (0.6) | 42 (1.1) | 15 (0.3) |
| Hoeven et al., 2012 (47) | Netherland (Rotterdam) | 2,372 (42.0) | 3,278 (58.0) | M: 67.5 (SD 7.6) F: 68.6 (SD 8.3) |
Radiographic hip | KL grade≥2 | 348 (6.0) | 119 (5.0) | 229 (7.0) |
| Jordan et al., 2009 (26) | USA (Johnston County) | 1,162 (37.9) | 1,906 (62.1) | Inclusion ≥45 (NA) | Radiographic hip | KL grade≥2 | 857 (27.6) | 295 (25.4) | 562 (29.5) |
| Iidaka et al., 2016 (52) | Japan (ROAD study) |
1,043 (35.1) | 1,932 (64.9) | M: 71.0 (SD 10.7) F: 69.8 (SD 11.3) (Range 23-94) |
Radiographic hip | KL grade≥2 | 467 (15.7) | 190 (18.2) | 277 (14.3) |
| Tepper and Hochberg, 1993 (53) | US (NHANES-I) |
2,358 | NA (Range 55-74) | Radiographic hip | KL grade≥2 | 73 (3.1) | NA (3.2) | NA (3.0) | |
| Kim et al., 2014 (27) | USA | 434 (44.4) | 544 (55.6) | 63.5 (SD 9.0) (Range 51-92) |
Radiographic hip | KL grade≥2 | 181 (18.5) | 107 (24.7) | 74 (13.6) |
| Hirsch et al., 1998 (51) | US (Pima Indians) |
294 (38.9) | 461 (61.1) | M: 58.1 (SD 9.9) F: 58.4 (SD 9.1) (Range 45-93) |
Radiographic hip | KL grade≥2 | 27 (3.6) | 14 (4.8) | 13 (2.8) |
| Cho et al., 2015 (46) | Korea | 298 (42.8) | 398 (57.2) | 72.0 (SD 5.0) (Range 65-91) |
Radiographic hip | KL grade≥2 | 15 (2.2) | 8 (2.7) | 7 (1.8) |
| Macías-Hernández et al., 2020 (32) | Mexico | 80 (39.2) | 124 (60.8) | 57.4 (SD 10.9) (Range 42-86) |
Radiographic hip | KL grade≥2 | 54 (26.5) | 15 (18.7) | 39 (31.4) |
Although it is known that radiographic features do not perfectly correlate with symptoms (17), they are strongly associated in both sexes (19, 49, 54, 55). However, it has been shown that females tend to experience more severe pain than males with equivalent radiographic changes (56). In addition, at the hip, females show a stronger association between radiographic changes and both symptoms and total hip replacement (19). Any tendency for females to experience worse pain for a given degree of structural change could explain their higher prevalence of clinical/symptomatic as opposed to radiographic hip osteoarthritis. Such differences might also be expected to translate into sex differences in the rate of interventions such as joint replacement.
Sex differences in rates of hip and knee joint replacement
Based on the data presented, it would be reasonable to anticipate that females would constitute a larger proportion of total joint replacements given their higher disease burden. The National Joint Registry collates information on all joint replacements conducted in England and Wales from both public and private healthcare providers. In 2022, 100,095 total knee replacements were performed, with a higher number of cases in females (n=54,731- 55%) than male (n=45,364 – 45%), despite similar mean ages seen between the groups (females: 70.0 vs males: 69.7 years) (16). Regarding hip replacements, during the same time period, 95,880 primary hip replacements were conducted in England and Wales (Female 60,687 [63.3%] vs Male 31,308 [36.7%], mean age F: 70.22 vs M: 67.8 years) (57). Data from the American Joint Replacement Registry suggests, between 2012-2022, roughly 62% of primary total knee replacements and 57% of primary total hip replacements were done in females (58). These data show that in both the UK and USA, females receive a greater proportion of joint replacements. The largest observational studies already presented ( Tables 1 , 2 ) suggest that symptomatic knee osteoarthritis is roughly 30% more common in females (22–24), and symptomatic hip osteoarthritis is 40% more common in females (19, 22, 23). Therefore, the allocation of joint replacements reported in the UK and USA would appear to be in line with other measures reflecting sex-differences in symptomatic knee and hip osteoarthritis prevalence.
As well as sex differences in the rate of joint replacements, it has been shown that females often have worse symptoms prior to joint replacement than males (59). This is highlighted by data that showed females in the twelve months before surgery were more likely to use analgesia and seek healthcare than males (60). Qualitative studies suggest that females might delay joint replacement for several reasons: they often exhibit greater apprehension about surgery (61), tend to have more questions prior to surgery (62) and prioritise avoiding surgery more than males do (63). Clinicians caring for patients with large joint osteoarthritis should consider these differences in patient-level factors, particularly regarding decisions about proceeding to surgery. In addition, there is evidence to suggest that clinician-related factors can lead to sex-based discrepancies in care. Studies have shown that females are less likely to receive a referral to an orthopaedic surgeon when presenting with the same symptom levels as males (64). In the case of hip osteoarthritis, this may be attributed to the greater prevalence of structural disease in males, leading to surgeons prioritising them for treatment.
Following joint replacement surgery, it has been found that females report worse outcomes in pain and function (59). This might be because they are at a more advanced disease stage when their surgery takes place (11). Alternatively, it might be due to sex differences in the experience of musculoskeletal pain (8). We will now consider the potential aetiological reasons for the observed sex differences described so far.
Potential aetiologic mechanisms
Studies comparing sex differences in the prevalence of knee and hip osteoarthritis show a greater prevalence of symptomatic and clinically defined osteoarthritis in females. A similar female predominance is also seen in relation to rates of joint replacement. Consistent with these findings, when examining sex differences in structural changes of osteoarthritis, as reflected by radiographic osteoarthritis, females show a higher prevalence of radiographic knee osteoarthritis. On the other hand, radiographic hip osteoarthritis is more prevalent in males. These somewhat discrepant findings could be explained by the existence of both sex-based effects on structure which are joint specific, and more generalised sex differences in pain perception. Understanding the underlying mechanism of these differences could shed light on the pathogenesis of osteoarthritis.
Hormonal
The influence of sex hormones on large joint osteoarthritis has been extensively studied (8). Most studies showing sex differences in the prevalence of knee and hip osteoarthritis are focused on older populations ( Tables 1 , 2 ), where the females are likely to be post-menopausal (65). There are established links between menopause and increased rates of hip and knee replacement (66). Indeed, one study found that females whose age at menopause was 50–54 years had a hazard ratio of 0.89 (95% CI 0.84-0.94) for undergoing total knee replacement when compared to females whose age at menopause was 40 years or younger (66). Genetic factors have also been implicated in the association between sex steroid levels and osteoarthritis. The Genetics of Osteoarthritis consortium found 3 genetic risk loci that were female specific. Two of these were associated with total hip replacement and one was associated with osteoarthritis at all sites (67). One of these loci (FANCL) was associated with early menopause.
In addition to the association between endogenous sex steroids and osteoarthritis, attempts have been made to elucidate whether exogenous sex steroids affect osteoarthritis. Preclinical studies indicate that selective oestrogen receptor modulators (SERMs) treatment has consistently positive effects on osteoarthritis, especially for postmenopausal patients with early-stage osteoarthritis (68). Despite these promising data, the use of exogenous sex hormones has not yet shown efficacy at a clinical level for osteoarthritis, although trials investigating the use of exogenous oestrogen for knee osteoarthritis in females are ongoing (69).
In terms of the mechanisms by which sex hormones might influence osteoarthritis, oestrogen has been found to protect against articular cartilage and subchondral bone degradation, likely through the sex hormone receptors expressed by osteoblasts, osteoclasts and chondrocytes (9, 70, 71). However, since any such chondroprotective effect would be expected to be generalised, it is difficult to understand how this might underlie the higher risk of structural deterioration of knee joints in females, but not hip joints. On the other hand, sex hormones might contribute to sex differences in pain perception.
Sex differences in pain perception and related behaviours
Pain is a subjective experience that encompasses sensory, emotional and cognitive components (72). Important differences are thought to exist in how pain is felt between males and females, which may help to explain why symptomatic hip osteoarthritis is more common in females, despite radiographic hip osteoarthritis being more prevalent in males. It has been shown in animal models that both central and peripheral neuronal signalling of pain is highly sexually dimorphic due to greater peripheral nociceptor plasticity (73), altered dopaminergic signally within the spinal column (74), and reduced downregulation of pain within the midbrain (periaqueductal gray) (72) in females. In human studies, females have been shown to have higher activation of their prefrontal cortex in response to painful stimuli and this is thought to lead to an increased perception of pain (75, 76). High concentrations of oestradiol have been found to carry an anti-nociceptive effect, whereas it may be pronociceptive at lower concentrations (8). In addition, oestrogens have been found to decrease proinflammatory cytokine production in the synovial membrane (77). Although the precise causal mechanisms for this are unknown, females have been shown to have a tendency to upregulate pain pathways through increased transcriptional activity of pain related genes (72). Taken together, these mechanisms likely contribute to the increased burden of symptomatic osteoarthritis in females.
Another potential reason why clinically defined osteoarthritis is more common in females is that they have been shown to seek more healthcare input from primary care, which could lead to more recorded diagnoses (78). Recent studies have looked at dispositional traits and their association with knee osteoarthritis. Dispositional traits, which are neurobiologically based, can be divided into groups comprising ‘protective’ or ‘vulnerable’ dispositional traits. Whilst there is no association between sex and dispositional traits, a strong association has been found between ‘vulnerable’ dispositional traits and pain threshold (79). Therefore, the observed sex differences in healthcare utilisation and clinical knee and hip osteoarthritis likely reflect a combination of sex-related differences in disease course and sociocultural differences in healthcare access (80).
Body composition
Obesity is a risk factor for the development of knee and hip osteoarthritis, particularly knee osteoarthritis, and differences in relative weight seen between the sexes might explain some of the variance in disease prevalence. For example, the association between knee osteoarthritis and obesity has frequently been found to be stronger in females (81). The reasons for this are likely multifactorial. High body mass results in excess joint loading and this phenomenon might be exacerbated by sex differences in fat distribution (82). Moreover, the problem of increased load is further compounded in females due to their relatively reduced cartilage volume (83). This combination may contribute to the increased rate of cartilage loss observed in females compared to males (9). In addition, obesity is a key component of metabolic syndrome, which further includes hypertension, dyslipidaemia, hyperglycaemia, and insulin resistance that are also thought to independently contribute to increased osteoarthritis risk (84). Metabolic syndrome has been shown to be more common in females so this may contribute to the increased prevalence of osteoarthritis in females (85, 86).
Females have decreased lean mass, a proxy for muscle strength, relative to men and increased fat mass (87). This may confer an additional sex-related risk factor for osteoarthritis, as low skeletal muscle mass has been found to be associated with knee osteoarthritis (88). Mechanistic pathways by which lower-limb skeletal muscle effects a reduction in knee osteoarthritis have been proposed (89). Skeletal muscle, known to be more abundant in males, has been suggested to mediate an anti-inflammatory effect and increase resistance of chondrocytes to cytokine induced cartilage damage (90). Furthermore, skeletal muscle may exert a direct positive effect on cartilage by enhancing expression of the dominant (type II) and stabilising (type IX) collagen in cartilage (91).
Sex differences in obesity and body composition are unlikely to account for the higher prevalence of radiographic hip osteoarthritis in males. Interestingly, the association between weight and osteoarthritis at the hip is relatively weak compared with the knee (92). Whilst height is a strong risk factor for radiographic osteoarthritis at the hip (93, 94). Therefore, it is probable that sex differences in biomechanics play a greater role at the hip than obesity, which we will now discuss.
Biomechanical
Sex-related biomechanical differences at the hip may contribute to the increased prevalence of radiographic osteoarthritis in men (27). This could be partly explained by the increased prevalence of cam morphology of the hip (a bulging aspherical femoral head) in males compared to females (95), given the strong association between cam and hip osteoarthritis (96). In addition, larger lesser trochanters have been implicated as a risk factor for hip osteoarthritis (97). As males have larger lesser trochanters, this risk factor may contribute to the increased rate of radiographic hip osteoarthritis observed in men (98). Though the mechanism by which this risk is conferred is unclear, it has been suggested that aberrant joint forces conferred by the iliopsoas through the lesser trochanter may contribute to the development of hip osteoarthritis (97).
Acetabular dysplasia is a reported risk factor for the development of hip osteoarthritis, as it increases joint load and accelerates cartilage wear (99). Acetabular dysplasia has been reported to have a general prevalence of 3.4%, making it a common risk factor for the development of osteoarthritis (100). While some studies report that acetabular dysplasia is more common in females (99), this finding lacks consistency, with other studies suggesting that the sex-related differences in acetabular dysplasia are unlikely to be of clinical significance (100).
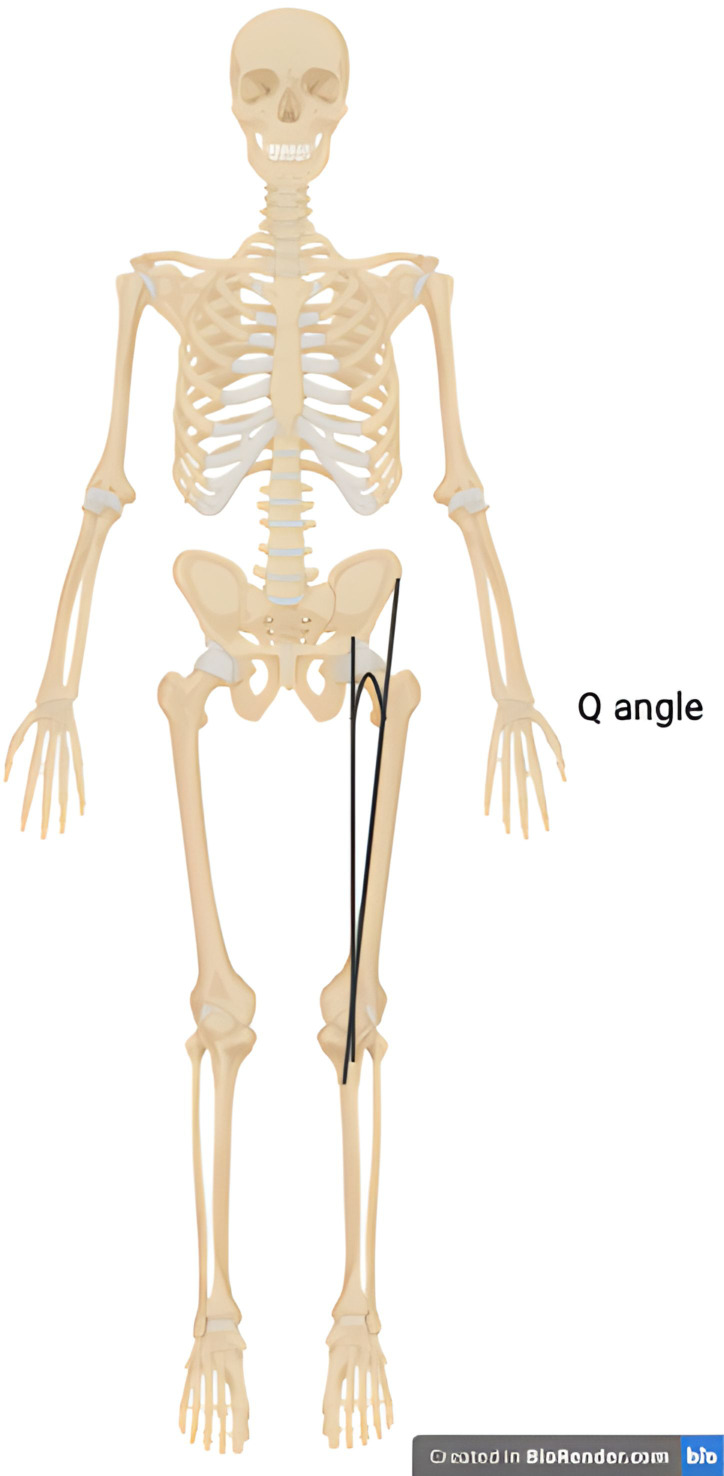
Sex-related biomechanical risk factors for both medial and lateral tibiofemoral osteoarthritis have been proposed (101, 102). Whilst varus (bow-legged) deformity of the knee is significantly more common in knee osteoarthritis overall, the incidence of valgus (knock-knee) deformity in females with osteoarthritis is elevated relative to men with osteoarthritis (103). The Q angle is the angle of pull on the patella and is defined by the angle between the centre of the patella and the tibial tubercle and a line between the centre of the patella and the anterior superior iliac spine ( Figure 1 ). As females have wider pelvises, this angle is greater and may result in load shifting to the lateral compartment and increased risk of lateral tibiofemoral osteoarthritis (101). In addition, a magnetic resonance imaging-based evaluation of knees throughout the stages of osteoarthritis found that females have increased medial tibiofemoral contact area and a reduced congruity index at all stages of osteoarthritis. This joint configuration may predispose females to the development of both symptomatic and radiographic knee osteoarthritis (102).
Figure 1.
Illustration of the Q angle. The Q angle is defined as the angle between a line that passes through the centre of the patella and the tibial tubercle and a line that pass through the centre of the patella and the anterior superior iliac spine.
Conclusion
Our literature review shows that knee and hip osteoarthritis is more common in females than males when defined clinically or symptomatically, irrespective of country or ethnicity. Similar female predominance is also seen in relation to rates of joint replacement. When large joint osteoarthritis is defined radiographically, knee osteoarthritis remains more common in females than males, while the opposite holds true for the hip, where male disease predominates. The underlying reasons for these sex differences in knee and hip osteoarthritis prevalence are currently unclear, yet factors such as differing hormone levels, pain perception, body composition and pelvic architecture between the sexes may contribute. Developing a better understanding of the biological mechanisms that lead to the observed sex differences in large joint osteoarthritis could offer opportunities to develop therapies that could benefit both sexes, highlighting the need for further investigation.
Funding Statement
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. BF is supported by an NIHR Academic Clinical Lectureship. BZ is supported by an NIHR Academic Clinical Fellowship. RB and MJ are supported by a Wellcome Trust collaborative award (209233/Z/17/Z).
Author contributions
BF: Conceptualization, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. FM: Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. MJ: Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. BZ: Conceptualization, Data curation, Formal analysis, Investigation, Writing – review & editing. RB: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. JT: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet. (2019) 393:1745–59. doi: 10.1016/S0140-6736(19)30417-9 [DOI] [PubMed] [Google Scholar]
- 2. Allen KD, Thoma LM, Golightly YM. Epidemiology of osteoarthritis. Osteoarthritis Cartilage. (2022) 30:184–95. doi: 10.1016/j.joca.2021.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Versus Arthritis . THE STATE OF MUSCULOSKELETAL HEALTH 2023: Arthritis and other musculoskeletal conditions in numbers. UK: Versus Arthritis. (2023). p. 6. [Google Scholar]
- 4. Wylde V, Beswick A, Bruce J, Blom A, Howells N, Gooberman-Hill R. Chronic pain after total knee arthroplasty. EFORT Open Rev. (2018) 3:461–70. doi: 10.1302/2058-5241.3.180004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. George SZ, Bolognesi MP, Bhavsar NA, Penrose CT, Horn ME. Chronic pain prevalence and factors associated with high impact chronic pain following total joint arthroplasty: an observational study. J Pain. (2022) 23:450–8. doi: 10.1016/j.jpain.2021.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Matharu GS, Culliford DJ, Blom AW, Judge A. Projections for primary hip and knee replacement surgery up to the year 2060: an analysis based on data from The National Joint Registry for England, Wales, Northern Ireland and the Isle of Man. Ann R Coll Surg Engl. (2022) 104:443–8. doi: 10.1308/rcsann.2021.0206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. O’Connor MI. Sex differences in osteoarthritis of the hip and knee. J Am Acad Orthop Surg. (2007) 15 Suppl 1:S22–5. doi: 10.5435/00124635-200700001-00007 [DOI] [PubMed] [Google Scholar]
- 8. Gulati M, Dursun E, Vincent K, Watt FE. The influence of sex hormones on musculoskeletal pain and osteoarthritis. Lancet Rheumatol. (2023) 5:e225–e38. doi: 10.1016/S2665-9913(23)00060-7 [DOI] [PubMed] [Google Scholar]
- 9. Segal NA, Nilges JM, Oo WM. Sex differences in osteoarthritis prevalence, pain perception, physical function and therapeutics. Osteoarthritis Cartilage. (2024) 32:1045–53. doi: 10.1016/j.joca.2024.04.002 [DOI] [PubMed] [Google Scholar]
- 10. Peshkova M, Lychagin A, Lipina M, Di Matteo B, Anzillotti G, Ronzoni F, et al. Gender-related aspects in osteoarthritis development and progression: A review. Int J Mol Sci. (2022) 23:2767. doi: 10.3390/ijms23052767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Choong ALC, Shadbolt C, Dowsey MM, Choong PFM. Sex-based differences in the outcomes of total hip and knee arthroplasty: a narrative review. ANZ J Surg. (2021) 91:553–7. doi: 10.1111/ans.16299 [DOI] [PubMed] [Google Scholar]
- 12. Faber BG, Frysz M, Tobias JH. Unpicking observational relationships between hip shape and osteoarthritis: hype or hope? Curr Opin Rheumatol. (2020) 32:110–8. doi: 10.1097/BOR.0000000000000673 [DOI] [PubMed] [Google Scholar]
- 13. Nelson AE, Liu F, Lynch JA, Renner JB, Schwartz TA, Lane NE, et al. Association of incident symptomatic hip osteoarthritis with differences in hip shape by active shape modeling: the Johnston county osteoarthritis project. Arthritis Care Res. (2014) 66:74–81. doi: 10.1002/acr.22094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lane NE, Nevitt MC, Hochberg MC, Hung YY, Palermo L. Progression of radiographic hip osteoarthritis over eight years in a community sample of elderly white women. Arthritis Rheumatol. (2004) 50:1477–86. doi: 10.1002/art.20213 [DOI] [PubMed] [Google Scholar]
- 15. Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. (1957) 16:494–502. doi: 10.1136/ard.16.4.494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Reed M Brittain R Howard P Lawrence S Stonadge J Wilkinson M et al. Age and gender for primary knee replacement procedures in 2022, according to procedure type. UK: National Joint Registry; (2023). Available at: https://reports.njrcentre.org.uk/knees-primary-procedures-patient-characteristics/K07v1NJR?reportid=351A3B2E-F983-4D53-BC1E-D9A90DC93AAA&defaults=DC:Reporting_Period:Date_Range=%22MAX%22,J:Filter:Calendar_Year=%22MAX%22,H:Filter:Joint=%22Knee%22. [Google Scholar]
- 17. Kim C, Nevitt MC, Niu J, Clancy MM, Lane NE, Link TM, et al. Association of hip pain with radiographic evidence of hip osteoarthritis: diagnostic test study. BMJ. (2015) 351:h5983. doi: 10.1136/bmj.h5983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Srikanth VK, Fryer JL, Zhai G, Winzenberg TM, Hosmer D, Jones G. A meta-analysis of sex differences prevalence, incidence and severity of osteoarthritis. Osteoarthritis Cartilage. (2005) 13:769–81. doi: 10.1016/j.joca.2005.04.014 [DOI] [PubMed] [Google Scholar]
- 19. Faber BG, Ebsim R, Saunders FR, Frysz M, Lindner C, Gregory JS, et al. A novel semi-automated classifier of hip osteoarthritis on DXA images shows expected relationships with clinical outcomes in UK Biobank. Rheumatol (Oxford). (2021) 61:3586–95. doi: 10.1093/rheumatology/keab927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hawker GA, Wright JG, Coyte PC, Williams JI, Harvey B, Glazier R, et al. Differences between men and women in the rate of use of hip and knee arthroplasty. N Engl J Med. (2000) 342:1016–22. doi: 10.1056/NEJM200004063421405 [DOI] [PubMed] [Google Scholar]
- 21. Juni P, Low N, Reichenbach S, Villiger PM, Williams S, Dieppe PA. Gender inequity in the provision of care for hip disease: population-based cross-sectional study. Osteoarthritis Cartilage. (2010) 18:640–5. doi: 10.1016/j.joca.2009.12.010 [DOI] [PubMed] [Google Scholar]
- 22. Prieto-Alhambra D, Judge A, Javaid MK, Cooper C, Diez-Perez A, Arden NK. Incidence and risk factors for clinically diagnosed knee, hip and hand osteoarthritis: influences of age, gender and osteoarthritis affecting other joints. Ann Rheum Dis. (2014) 73:1659–64. doi: 10.1136/annrheumdis-2013-203355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Swain S, Sarmanova A, Mallen C, Kuo CF, Coupland C, Doherty M, et al. Trends in incidence and prevalence of osteoarthritis in the United Kingdom: findings from the Clinical Practice Research Datalink (CPRD). Osteoarthritis Cartilage. (2020) 28:792–801. doi: 10.1016/j.joca.2020.03.004 [DOI] [PubMed] [Google Scholar]
- 24. Li D, Li S, Chen Q, Xie X. The prevalence of symptomatic knee osteoarthritis in relation to age, sex, area, region, and body mass index in China: A systematic review and meta-analysis. Front Med (Lausanne). (2020) 7:304. doi: 10.3389/fmed.2020.00304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tukker A, Visscher TL, Picavet HS. Overweight and health problems of the lower extremities: osteoarthritis, pain and disability. Public Health Nutr. (2009) 12:359–68. doi: 10.1017/S1368980008002103 [DOI] [PubMed] [Google Scholar]
- 26. Jordan JM, Helmick CG, Renner JB, Luta G, Dragomir AD, Woodard J, et al. Prevalence of hip symptoms and radiographic and symptomatic hip osteoarthritis in African Americans and Caucasians: the Johnston County Osteoarthritis Project. J Rheumatol. (2009) 36:809–15. doi: 10.3899/jrheum.080677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kim C, Linsenmeyer KD, Vlad SC, Guermazi A, Clancy MM, Niu J, et al. Prevalence of radiographic and symptomatic hip osteoarthritis in an urban United States community: the Framingham osteoarthritis study. Arthritis Rheumatol. (2014) 66:3013–7. doi: 10.1002/art.v66.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mork PJ, Holtermann A, Nilsen TI. Effect of body mass index and physical exercise on risk of knee and hip osteoarthritis: longitudinal data from the Norwegian HUNT Study. J Epidemiol Community Health. (2012) 66:678–83. doi: 10.1136/jech-2011-200834 [DOI] [PubMed] [Google Scholar]
- 29. Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. (2008) 58:26–35. doi: 10.1002/art.23176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Carmona L, Ballina J, Gabriel R, Laffon A, Group ES. The burden of musculoskeletal diseases in the general population of Spain: results from a national survey. Ann Rheum Dis. (2001) 60:1040–5. doi: 10.1136/ard.60.11.1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Plotnikoff R, Karunamuni N, Lytvyak E, Penfold C, Schopflocher D, Imayama I, et al. Osteoarthritis prevalence and modifiable factors: a population study. BMC Public Health. (2015) 15:1195. doi: 10.1186/s12889-015-2529-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Macias-Hernandez SI, Zepeda-Borbon ER, Lara-Vazquez BI, Cuevas-Quintero NM, Morones-Alba JD, Cruz-Medina E, et al. Prevalence of clinical and radiological osteoarthritis in knee, hip, and hand in an urban adult population of Mexico City. Reumatol Clin (Engl Ed). (2020) 16:156–60. doi: 10.1016/j.reuma.2018.06.001 [DOI] [PubMed] [Google Scholar]
- 33. Grotle M, Hagen KB, Natvig B, Dahl FA, Kvien TK. Prevalence and burden of osteoarthritis: results from a population survey in Norway. J Rheumatol. (2008) 35:677–84. [PubMed] [Google Scholar]
- 34. Park JH, Hong JY, Han K, Suh SW, Park SY, Yang JH, et al. Prevalence of symptomatic hip, knee, and spine osteoarthritis nationwide health survey analysis of an elderly Korean population. Med (Baltimore). (2017) 96:e6372. doi: 10.1097/MD.0000000000006372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tang X, Wang S, Zhan S, Niu J, Tao K, Zhang Y, et al. The prevalence of symptomatic knee osteoarthritis in China: results from the China health and retirement longitudinal study. Arthritis Rheumatol. (2016) 68:648–53. doi: 10.1002/art.39465 [DOI] [PubMed] [Google Scholar]
- 36. Haq SA, Darmawan J, Islam MN, Uddin MZ, Das BB, Rahman F, et al. Prevalence of rheumatic diseases and associated outcomes in rural and urban communities in Bangladesh: a COPCORD study. J Rheumatol. (2005) 32:348–53. [PubMed] [Google Scholar]
- 37. Ji S, Liu L, Li J, Zhao G, Cai Y, Dong Y, et al. Prevalence and factors associated with knee osteoarthritis among middle-aged and elderly individuals in rural Tianjin: a population-based cross-sectional study. J Orthop Surg Res. (2023) 18:266. doi: 10.1186/s13018-023-03742-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Felson DT, Zhang Y, Hannan MT, Naimark A, Weissman BN, Aliabadi P, et al. The incidence and natural history of knee osteoarthritis in the elderly. Framingham Osteoarthritis Study Arthritis Rheumatol. (1995) 38:1500–5. doi: 10.1002/art.1780381017 [DOI] [PubMed] [Google Scholar]
- 39. Felson DT, Naimark A, Anderson J, Kazis L, Castelli W, Meenan RF. The prevalence of knee osteoarthritis in the elderly. the framingham osteoarthritis study. Arthritis Rheumatism. (1987) 30:914–8. doi: 10.1002/art.1780300811 [DOI] [PubMed] [Google Scholar]
- 40. Welling M, Auvinen J, Lehenkari P, Mannikko M, Karppinen J, Eskola PJ. Association between height and osteoarthritis of the knee and hip: The Northern Finland Birth Cohort 1966 Study. Int J Rheum Dis. (2017) 20:1095–104. doi: 10.1111/apl.2017.20.issue-9 [DOI] [PubMed] [Google Scholar]
- 41. Picavet HS, Hazes JM. Prevalence of self reported musculoskeletal diseases is high. Ann Rheum Dis. (2003) 62:644–50. doi: 10.1136/ard.62.7.644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Collaborators GBDO. Global, regional, and national burden of osteoarthritis, 1990-2020 and projections to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet Rheumatol. (2023) 5:e508–e22. doi: 10.1016/S2665-9913(23)00163-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhang JF, Song LH, Wei JN, Zhang AL, Dong HY, Wen HY, et al. Prevalence of and risk factors for the occurrence of symptomatic osteoarthritis in rural regions of Shanxi Province, China. Int J Rheum Dis. (2016) 19:781–9. doi: 10.1111/apl.2016.19.issue-8 [DOI] [PubMed] [Google Scholar]
- 44. Ho-Pham LT, Lai TQ, Mai LD, Doan MC, Pham HN, Nguyen TV. Prevalence of radiographic osteoarthritis of the knee and its relationship to self-reported pain. PloS One. (2014) 9:e94563. doi: 10.1371/journal.pone.0094563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Muraki S, Akune T, Nagata K, Ishimoto Y, Yoshida M, Tokimura F, et al. Association of knee osteoarthritis with onset and resolution of pain and physical functional disability: the ROAD study. Mod Rheumatol. (2014) 24:966–73. doi: 10.3109/14397595.2014.883055 [DOI] [PubMed] [Google Scholar]
- 46. Cho HJ, Morey V, Kang JY, Kim KW, Kim TK. Prevalence and risk factors of spine, shoulder, hand, hip, and knee osteoarthritis in community-dwelling Koreans older than age 65 years. Clin Orthop Relat Res. (2015) 473:3307–14. doi: 10.1007/s11999-015-4450-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hoeven TA, Kavousi M, Clockaerts S, Kerkhof HJ, van Meurs JB, Franco O, et al. Association of atherosclerosis with presence and progression of osteoarthritis: the Rotterdam Study. Ann Rheum Dis. (2013) 72:646–51. doi: 10.1136/annrheumdis-2011-201178 [DOI] [PubMed] [Google Scholar]
- 48. Jordan JM, Helmick CG, Renner JB, Luta G, Dragomir AD, Woodard J, et al. Prevalence of knee symptoms and radiographic and symptomatic knee osteoarthritis in African Americans and Caucasians: the Johnston County Osteoarthritis Project. J Rheumatol. (2007) 34:172–80. [PubMed] [Google Scholar]
- 49. Faber BG, Ebsim R, Saunders FR, Frysz M, Lindner C, Gregory JS, et al. Osteophyte size and location on hip DXA scans are associated with hip pain: findings from a cross sectional study in UK Biobank. Bone. (2021) 153:116146. doi: 10.1016/j.bone.2021.116146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zheng J, Frysz M, Faber BG, Lin H, Ebsim R, Ge J, et al. Comparison between UK Biobank and Shanghai Changfeng suggests distinct hip morphology may contribute to ethnic differences in the prevalence of hip osteoarthritis. Osteoarthritis Cartilage. (2023) 5:S1063–4584(23)00958-5. doi: 10.1016/j.joca.2023.10.006 [DOI] [PubMed] [Google Scholar]
- 51. Hirsch R, Fernandes RJ, Pillemer SR, Hochberg MC, Lane NE, Altman RD, et al. Hip osteoarthritis prevalence estimates by three radiographic scoring systems. Arthritis Rheumatol. (1998) 41:361–8. doi: [DOI] [PubMed] [Google Scholar]
- 52. Iidaka T, Muraki S, Akune T, Oka H, Kodama R, Tanaka S, et al. Prevalence of radiographic hip osteoarthritis and its association with hip pain in Japanese men and women: the ROAD study. Osteoarthritis Cartilage. (2016) 24:117–23. doi: 10.1016/j.joca.2015.07.017 [DOI] [PubMed] [Google Scholar]
- 53. Tepper S, Hochberg MC. Factors associated with hip osteoarthritis: data from the First National Health and Nutrition Examination Survey (NHANES-I). Am J Epidemiol. (1993) 137:1081–8. doi: 10.1093/oxfordjournals.aje.a116611 [DOI] [PubMed] [Google Scholar]
- 54. Arden NK, Lane NE, Parimi N, Javaid KM, Lui LY, Hochberg MC, et al. Defining incident radiographic hip osteoarthritis for epidemiologic studies in women. Arthritis Rheumatol. (2009) 60:1052–9. doi: 10.1002/art.24382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Beynon RA, Saunders FR, Ebsim R, Frysz M, Faber BG, Gregory JS, et al. Dual-energy X-ray absorptiometry derived knee shape may provide a useful imaging biomarker for predicting total knee replacement: Findings from a study of 37,843 people in UK Biobank. Osteoarthr Cartil Open. (2024) 6:100468. doi: 10.1016/j.ocarto.2024.100468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cho HJ, Chang CB, Yoo JH, Kim SJ, Kim TK. Gender differences in the correlation between symptom and radiographic severity in patients with knee osteoarthritis. Clin Orthop Relat Res. (2010) 468:1749–58. doi: 10.1007/s11999-010-1282-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Reed M, Brittain R, Howard P, Lawrence S, Stonadge J, Wilkinson M, et al. Age and gender for primary hip replacement procedures in 2022, according to procedure type. UK: National Joint Registry; (2023). Available at: https://reports.njrcentre.org.uk/hips-primary-procedures-patient-characteristics/H05v1NJR?reportid=351A3B2E-F983-4D53-BC1E-D9A90DC93AAA&defaults=DC:Reporting_Period:Date_Range=%22MAX%22,J:Filter:Calendar_Year=%22MAX%22,H:Filter:Joint=%22Hip%22. Accesed 31/05/2024. (Accesed May 31, 2024). [Google Scholar]
- 58. Registry AJR . Annual report 2022 (2022). Available online at: https://connect.registryapps.net/hubfs/PDFs%20and%20PPTs/2022%20AJRR%20Annual%20Report.pdf?hsCtaTracking=e22b6617-7eba-4a95-8113-7aa80eb589d1%7C9509b20f-338c-45c0-aeb2-16a46cffd1d2 (Accesed May 31, 2024).
- 59. Mehta SP, Perruccio AV, Palaganas M, Davis AM. Do women have poorer outcomes following total knee replacement? Osteoarthritis Cartilage. (2015) 23:1476–82. doi: 10.1016/j.joca.2015.05.007 [DOI] [PubMed] [Google Scholar]
- 60. Bawa HS, Weick JW, Dirschl DR. Gender disparities in osteoarthritis-related health care utilization before total knee arthroplasty. J Arthroplasty. (2016) 31:2115–8 e1. doi: 10.1016/j.arth.2016.03.044 [DOI] [PubMed] [Google Scholar]
- 61. Karlson EW, Daltroy LH, Liang MH, Eaton HE, Katz JN. Gender differences in patient preferences may underlie differential utilization of elective surgery. Am J Med. (1997) 102:524–30. doi: 10.1016/S0002-9343(97)00050-8 [DOI] [PubMed] [Google Scholar]
- 62. Mora M, Shell JE, Thomas CS, Ortiguera CJ, O’Connor MI. Gender differences in questions asked in an online preoperative patient education program. Gend Med. (2012) 9:457–62. doi: 10.1016/j.genm.2012.10.002 [DOI] [PubMed] [Google Scholar]
- 63. Torrente-Jimenez RS, Feijoo-Cid M, Rivero-Santana AJ, Perestelo-Perez L, Torres-Castano A, Ramos-Garcia V, et al. Gender differences in the decision-making process for undergoing total knee replacement. Patient Educ Couns. (2022) 105:3459–65. doi: 10.1016/j.pec.2022.08.014 [DOI] [PubMed] [Google Scholar]
- 64. Borkhoff CM, Hawker GA, Wright JG. Patient gender affects the referral and recommendation for total joint arthroplasty. Clin Orthop Relat Res. (2011) 469:1829–37. doi: 10.1007/s11999-011-1879-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Gold EB. The timing of the age at which natural menopause occurs. Obstet Gynecol Clin North Am. (2011) 38:425–40. doi: 10.1016/j.ogc.2011.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Eun Y, Yoo JE, Han K, Kim D, Lee KN, Lee J, et al. Female reproductive factors and risk of joint replacement arthroplasty of the knee and hip due to osteoarthritis in postmenopausal women: a nationwide cohort study of 1.13 million women. Osteoarthritis Cartilage. (2022) 30:69–80. doi: 10.1016/j.joca.2021.10.012 [DOI] [PubMed] [Google Scholar]
- 67. Boer CG, Hatzikotoulas K, Southam L, Stefansdottir L, Zhang Y, Coutinho de Almeida R, et al. Deciphering osteoarthritis genetics across 826,690 individuals from 9 populations. Cell. (2021) 184:4784–818 e17. doi: 10.1016/j.cell.2021.07.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Xiao YP, Tian FM, Dai MW, Wang WY, Shao LT, Zhang L. Are estrogen-related drugs new alternatives for the management of osteoarthritis? Arthritis Res Ther. (2016) 18:151. doi: 10.1186/s13075-016-1045-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Mitoma T, Maki J, Ooba H, Eto E, Takahashi K, Kondo T, et al. Protocol for a randomised, placebo-controlled, double-blinded clinical trial on the effect of oestrogen replacement on physical performance to muscle resistance exercise for older women with osteoarthritis of knee joint: the EPOK trial. BMC Geriatr. (2023) 23:104. doi: 10.1186/s12877-023-03828-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Schicht M, Ernst J, Nielitz A, Fester L, Tsokos M, Guddat SS, et al. Articular cartilage chondrocytes express aromatase and use enzymes involved in estrogen metabolism. Arthritis Res Ther. (2014) 16:R93. doi: 10.1186/ar4539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Xu X, Li X, Liang Y, Ou Y, Huang J, Xiong J, et al. Estrogen modulates cartilage and subchondral bone remodeling in an ovariectomized rat model of postmenopausal osteoarthritis. Med Sci Monit. (2019) 25:3146–53. doi: 10.12659/MSM.916254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Osborne NR, Davis KD. Chapter Eight - Sex and gender differences in pain. In: Moro E, Arabia G, Tartaglia MC, Ferretti MT, editors. International Review of Neurobiology, vol. 164. UK: Academic Press; (2022). p. 277–307. [DOI] [PubMed] [Google Scholar]
- 73. Ferrari LF, Khomula EV, Araldi D, Levine JD. Marked sexual dimorphism in the role of the ryanodine receptor in a model of pain chronification in the rat. Sci Rep. (2016) 6:31221. doi: 10.1038/srep31221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Presto P, Mazzitelli M, Junell R, Griffin Z, Neugebauer V. Sex differences in pain along the neuraxis. Neuropharmacology. (2022) 210:109030. doi: 10.1016/j.neuropharm.2022.109030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Straube T, Schmidt S, Weiss T, Mentzel H-J, Miltner WHR. Sex differences in brain activation to anticipated and experienced pain in the medial prefrontal cortex. Hum Brain Mapping. (2009) 30:689–98. doi: 10.1002/hbm.20536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Henderson LA, Gandevia SC, Macefield VG. Gender differences in brain activity evoked by muscle and cutaneous pain: A retrospective study of single-trial fMRI data. NeuroImage. (2008) 39:1867–76. doi: 10.1016/j.neuroimage.2007.10.045 [DOI] [PubMed] [Google Scholar]
- 77. Martín-Millán M, Castañeda S. Estrogens, osteoarthritis and inflammation. Joint Bone Spine. (2013) 80:368–73. doi: 10.1016/j.jbspin.2012.11.008 [DOI] [PubMed] [Google Scholar]
- 78. Thompson AE, Anisimowicz Y, Miedema B, Hogg W, Wodchis WP, Aubrey-Bassler K. The influence of gender and other patient characteristics on health care-seeking behaviour: a QUALICOPC study. BMC Fam Pract. (2016) 17:38. doi: 10.1186/s12875-016-0440-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Mickle AM, Staud R, Garvan CS, Kusko DA, Sambuco N, Addison BR, et al. Dispositional traits help explain individual differences in relationships between a radiographic knee osteoarthritis measure, pain, and physical function. Ther Adv Musculoskelet Dis. (2024) 16:1759720X241235805. doi: 10.1177/1759720X241235805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ferre IM, Roof MA, Anoushiravani AA, Wasterlain AS, Lajam CM. Understanding the observed sex discrepancy in the prevalence of osteoarthritis. JBJS Rev. (2019) 7:e8. doi: 10.2106/JBJS.RVW.18.00182 [DOI] [PubMed] [Google Scholar]
- 81. Felson DT, Anderson JJ, Naimark A, Walker AM, Meenan RF. Obesity and knee osteoarthritis. The Framingham Study. Ann Intern Med. (1988) 109:18–24. doi: 10.7326/0003-4819-109-1-18 [DOI] [PubMed] [Google Scholar]
- 82. Wen L, Kang J-H, Yim Y-R, Kim J-E, Lee J-W, Lee K-E, et al. Associations between body composition measurements of obesity and radiographic osteoarthritis in older adults: Data from the Dong-gu Study. BMC Musculoskeletal Disord. (2016) 17:192. doi: 10.1186/s12891-016-1040-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Cicuttini F, Forbes A, Morris K, Darling S, Bailey M, Stuckey S. Gender differences in knee cartilage volume as measured by magnetic resonance imaging. Osteoarthritis Cartilage. (1999) 7:265–71. doi: 10.1053/joca.1998.0200 [DOI] [PubMed] [Google Scholar]
- 84. Wei G, Lu K, Umar M, Zhu Z, Lu WW, Speakman JR, et al. Risk of metabolic abnormalities in osteoarthritis: a new perspective to understand its pathological mechanisms. Bone Res. (2023) 11:63. doi: 10.1038/s41413-023-00301-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Li F-E, Zhang F-L, Zhang P, Liu D, Liu H-Y, Guo Z-N, et al. Sex-based differences in and risk factors for metabolic syndrome in adults aged 40 years and above in Northeast China: Results from the cross-sectional China national stroke screening survey. BMJ Open. (2021) 11:e038671. doi: 10.1136/bmjopen-2020-038671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Beigh SH, Jain S. Prevalence of metabolic syndrome and gender differences. Bioinformation. (2012) 8:613–6. doi: 10.6026/97320630008613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Bredella MA. Sex differences in body composition. Adv Exp Med Biol. (2017) 1043:9–27. doi: 10.1007/978-3-319-70178-3_2 [DOI] [PubMed] [Google Scholar]
- 88. Lee SY, Ro HJ, Chung SG, Kang SH, Seo KM, Kim DK. Low skeletal muscle mass in the lower limbs is independently associated to knee osteoarthritis. PloS One. (2016) 11:e0166385. doi: 10.1371/journal.pone.0166385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Krishnasamy P, Hall M, Robbins SR. The role of skeletal muscle in the pathophysiology and management of knee osteoarthritis. Rheumatol (Oxford). (2018) 57:iv22–33. doi: 10.1093/rheumatology/kex515 [DOI] [PubMed] [Google Scholar]
- 90. Cairns DM, Uchimura T, Kwon H, Lee PG, Seufert CR, Matzkin E, et al. Muscle cells enhance resistance to pro-inflammatory cytokine-induced cartilage destruction. Biochem Biophys Res Commun. (2010) 392:22–8. doi: 10.1016/j.bbrc.2009.12.138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Cairns DM, Lee PG, Uchimura T, Seufert CR, Kwon H, Zeng L. The role of muscle cells in regulating cartilage matrix production. J Orthop Res. (2010) 28:529–36. doi: 10.1002/jor.21014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Reyes C, Leyland KM, Peat G, Cooper C, Arden NK, Prieto-Alhambra D. Association between overweight and obesity and risk of clinically diagnosed knee, hip, and hand osteoarthritis: A population-based cohort study. Arthritis Rheumatol. (2016) 68:1869–75. doi: 10.1002/art.39707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Faber BG, Frysz M, Boer CG, Evans D, Ebsim R, Flynn K, et al. The identification of distinct protective and susceptibility mechanisms for hip osteoarthritis: findings from a genome-wide association study meta-analysis of minimum joint space width and Mendelian randomisation cluster analyses. eBioMedicine. (2023) 95:104759. doi: 10.1016/j.ebiom.2023.104759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Croft P, Coggon D, Cruddas M, Cooper C. Osteoarthritis of the hip: an occupational disease in farmers. BMJ. (1992) 304:1269–72. doi: 10.1136/bmj.304.6837.1269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Zucker BE, Ebsim R, Lindner C, Hardcastle S, Cootes T, Tobias JH, et al. High bone mass and cam morphology are independently related to hip osteoarthritis: findings from the High Bone Mass cohort. BMC Musculoskelet Disord. (2022) 23:757. doi: 10.1186/s12891-022-05603-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Faber BG, Ebsim R, Saunders FR, Frysz M, Gregory JS, Aspden RM, et al. Cam morphology but neither acetabular dysplasia nor pincer morphology is associated with osteophytosis throughout the hip: findings from a cross-sectional study in UK Biobank. Osteoarthritis Cartilage. (2021) 29:1521–9. doi: 10.1016/j.joca.2021.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Faber BG, Bredbenner TL, Baird D, Gregory J, Saunders F, Giuraniuc CV, et al. Subregional statistical shape modelling identifies lesser trochanter size as a possible risk factor for radiographic hip osteoarthritis, a cross-sectional analysis from the Osteoporotic Fractures in Men Study. Osteoarthritis Cartilage. (2020) 28:1071–8. doi: 10.1016/j.joca.2020.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Marchand LS, Todd DC, Kellam P, Adeyemi TF, Rothberg DL, Maak TG. Is the lesser trochanter profile a reliable means of restoring anatomic rotation after femur fracture fixation? Clin Orthop Relat Res. (2018) 476:1253–61. doi: 10.1007/s11999.0000000000000226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Riedstra NS, Vinge R, Herfkens J, Eygendaal D, Bierma-Zeinstra SMA, Runhaar J, et al. Acetabular dysplasia and the risk of developing hip osteoarthritis at 2,5,8, and 10 years follow-up in a prospective nationwide cohort study (CHECK). Semin Arthritis Rheumatol. (2023) 60:152194. doi: 10.1016/j.semarthrit.2023.152194 [DOI] [PubMed] [Google Scholar]
- 100. Jacobsen S, Sonne-Holm S, Soballe K, Gebuhr P, Lund B. Hip dysplasia and osteoarthrosis: a survey of 4151 subjects from the Osteoarthrosis Substudy of the Copenhagen City Heart Study. Acta Orthop. (2005) 76:149–58. doi: 10.1080/00016470510030517 [DOI] [PubMed] [Google Scholar]
- 101. Wei J, Gross D, Lane NE, Lu N, Wang M, Zeng C, et al. Risk factor heterogeneity for medial and lateral compartment knee osteoarthritis: analysis of two prospective cohorts. Osteoarthritis Cartilage. (2019) 27:603–10. doi: 10.1016/j.joca.2018.12.013 [DOI] [PubMed] [Google Scholar]
- 102. Tummala S, Schiphof D, Byrjalsen I, Dam EB. Gender differences in knee joint congruity quantified from MRI: A validation study with data from center for clinical and basic research and osteoarthritis initiative. Cartilage. (2018) 9:38–45. doi: 10.1177/1947603516684590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Huber S, Mitterer JA, Vallant SM, Simon S, Hanak-Hammerl F, Schwarz GM, et al. Gender-specific distribution of knee morphology according to CPAK and functional phenotype classification: analysis of 8739 osteoarthritic knees prior to total knee arthroplasty using artificial intelligence. Knee Surg Sports Traumatol Arthrosc. (2023) 31:4220–30. doi: 10.1007/s00167-023-07459-z [DOI] [PubMed] [Google Scholar]