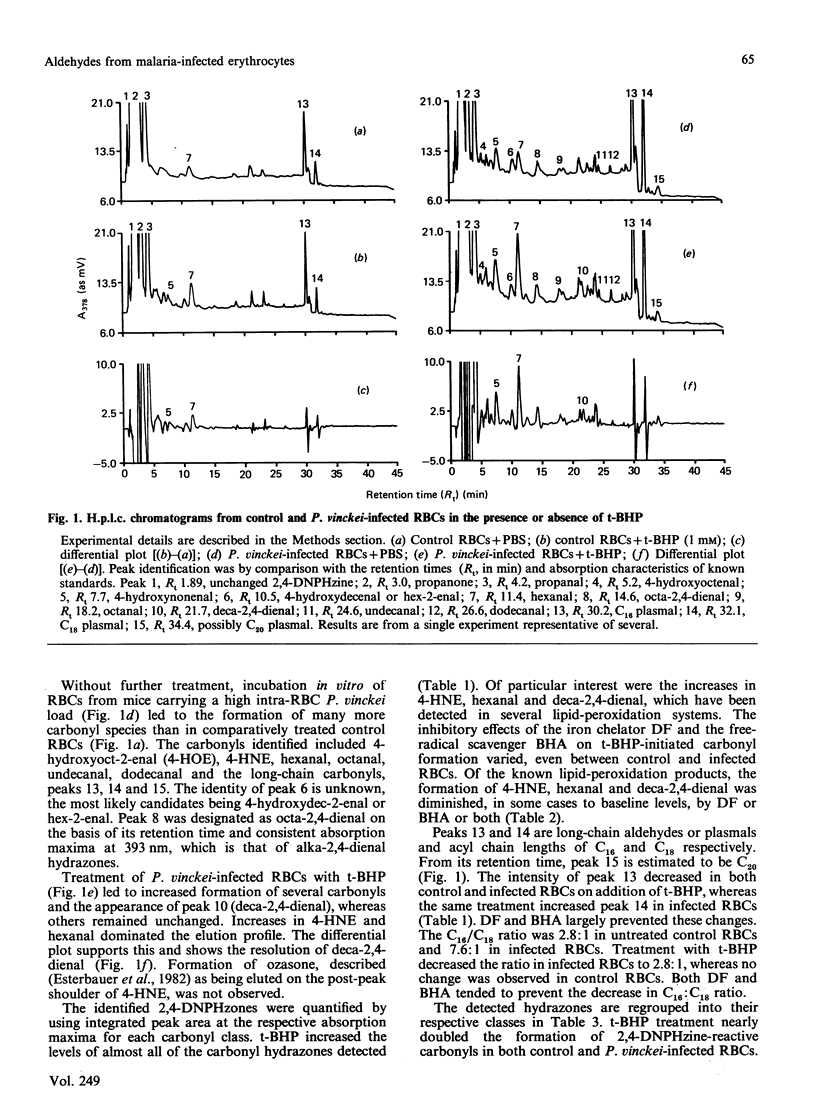
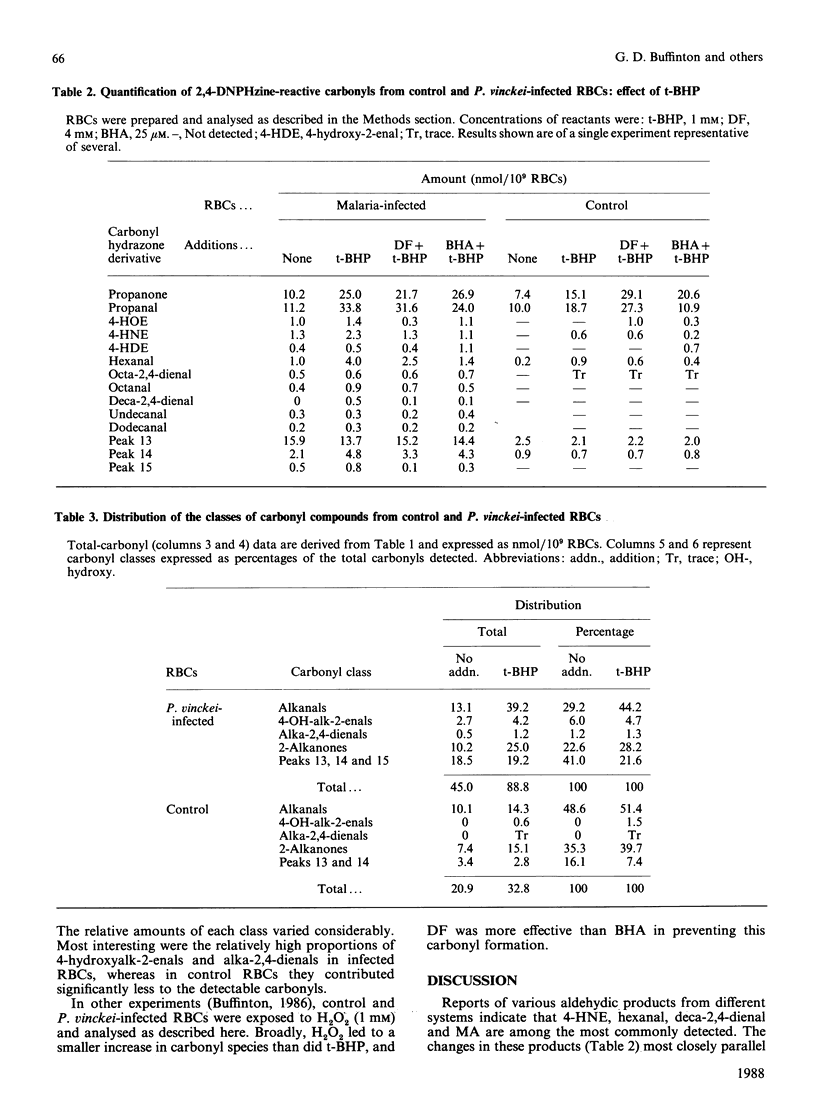
Abstract
Reversed-phase h.p.l.c. was used to detect 2,4-dinitrophenylhydrazine-reactive carbonyl products, which excludes malonaldehyde, in malaria-parasite (Plasmodium vinckei)-infected murine red blood cells (RBCs). A number of alkanals, 4-hydroxyalk-2-enals and alka-2,4-dienals were tentatively identified by comparison with authentic standards. The formation of 4-hydroxynon-2-enal, deca-2,4-dienal and hexanal was greater in P. vinckei-infected RBCs than in their uninfected counterparts and was increased by the presence of t-butyl hydroperoxide. Several of these aldehydes have previously been shown to be toxic to various types of cells, including P. falciparum, in vitro. The iron chelator desferrioxamine and the free-radical scavenger butylated hydroxyanisole inhibited the formation of these aldehydes. These experiments demonstrate that products of lipid peroxidation other than malonaldehyde are formed during the exposure of malaria-infected RBCs in vitro to drugs that generate reactive oxygen species and have anti-parasitic activity. The formation of products of this type during the natural course of malaria infection may have implications for the mechanisms underlying intra-RBC parasite death and the tissue damage associated with the disease.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beppu M., Murakami K., Kikugawa K. Fluorescent and cross-linked proteins of human erythrocyte ghosts formed by reaction with hydroperoxylinoleic acid, malonaldehyde and monofunctional aldehydes. Chem Pharm Bull (Tokyo) 1986 Feb;34(2):781–788. doi: 10.1248/cpb.34.781. [DOI] [PubMed] [Google Scholar]
- Clark I. A., Allison A. C., Cox F. E. Protection of mice against Babesia and Plasmodium with BCG. Nature. 1976 Jan 29;259(5541):309–311. doi: 10.1038/259309a0. [DOI] [PubMed] [Google Scholar]
- Clark I. A., Butcher G. A., Buffinton G. D., Hunt N. H., Cowden W. B. Toxicity of certain products of lipid peroxidation to the human malaria parasite Plasmodium falciparum. Biochem Pharmacol. 1987 Feb 15;36(4):543–546. doi: 10.1016/0006-2952(87)90364-9. [DOI] [PubMed] [Google Scholar]
- Clark I. A., Cowden W. B., Butcher G. A. Free oxygen radical generators as antimalarial drugs. Lancet. 1983 Jan 29;1(8318):234–234. doi: 10.1016/s0140-6736(83)92603-x. [DOI] [PubMed] [Google Scholar]
- Clark I. A., Cowden W. B., Hunt N. H., Maxwell L. E., Mackie E. J. Activity of divicine in Plasmodium vinckei-infected mice has implications for treatment of favism and epidemiology of G-6-PD deficiency. Br J Haematol. 1984 Jul;57(3):479–487. doi: 10.1111/j.1365-2141.1984.tb02922.x. [DOI] [PubMed] [Google Scholar]
- Clark I. A., Hunt N. H., Cowden W. B., Maxwell L. E., Mackie E. J. Radical-mediated damage to parasites and erythrocytes in Plasmodium vinckei infected mice after injection of t-butyl hydroperoxide. Clin Exp Immunol. 1984 Jun;56(3):524–530. [PMC free article] [PubMed] [Google Scholar]
- Clark I. A., Hunt N. H., Cowden W. B. Oxygen-derived free radicals in the pathogenesis of parasitic disease. Adv Parasitol. 1986;25:1–44. doi: 10.1016/s0065-308x(08)60341-3. [DOI] [PubMed] [Google Scholar]
- Clark I. A., Hunt N. H. Evidence for reactive oxygen intermediates causing hemolysis and parasite death in malaria. Infect Immun. 1983 Jan;39(1):1–6. doi: 10.1128/iai.39.1.1-6.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark I. A., Virelizier J. L., Carswell E. A., Wood P. R. Possible importance of macrophage-derived mediators in acute malaria. Infect Immun. 1981 Jun;32(3):1058–1066. doi: 10.1128/iai.32.3.1058-1066.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterbauer H., Cheeseman K. H., Dianzani M. U., Poli G., Slater T. F. Separation and characterization of the aldehydic products of lipid peroxidation stimulated by ADP-Fe2+ in rat liver microsomes. Biochem J. 1982 Oct 15;208(1):129–140. doi: 10.1042/bj2080129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterbauer H., Zollner H., Scholz N. Reaction of glutathione with conjugated carbonyls. Z Naturforsch C. 1975 Jul-Aug;30(4):466–473. doi: 10.1515/znc-1975-7-808. [DOI] [PubMed] [Google Scholar]
- Greenwood B. M., Stratton D., Williamson W. A., Mohammed I. A study of the role of immunological factors in the pathogenesis of the anaemia of acute malaria. Trans R Soc Trop Med Hyg. 1978;72(4):378–385. doi: 10.1016/0035-9203(78)90131-1. [DOI] [PubMed] [Google Scholar]
- Gupta C. M., Alam A., Mathur P. N., Dutta G. P. A new look at nonparasitized red cells of malaria-infected monkeys. Nature. 1982 Sep 16;299(5880):259–261. doi: 10.1038/299259a0. [DOI] [PubMed] [Google Scholar]
- Howard R. J., Mitchell G. F. Accelerated clearance of uninfected red cells from Plasmodium berghei-infected mouse blood in normal mice. Aust J Exp Biol Med Sci. 1979 Oct;57(5):455–457. doi: 10.1038/icb.1979.46. [DOI] [PubMed] [Google Scholar]
- Jain S. K., Hochstein P. Polymerization of membrane components in aging red blood cells. Biochem Biophys Res Commun. 1980 Jan 15;92(1):247–254. doi: 10.1016/0006-291x(80)91545-4. [DOI] [PubMed] [Google Scholar]
- Jain S. K., Mohandas N., Clark M. R., Shohet S. B. The effect of malonyldialdehyde, a product of lipid peroxidation, on the deformability, dehydration and 51Cr-survival of erythrocytes. Br J Haematol. 1983 Feb;53(2):247–255. doi: 10.1111/j.1365-2141.1983.tb02018.x. [DOI] [PubMed] [Google Scholar]
- Jain S. K. The accumulation of malonyldialdehyde, a product of fatty acid peroxidation, can disturb aminophospholipid organization in the membrane bilayer of human erythrocytes. J Biol Chem. 1984 Mar 25;259(6):3391–3394. [PubMed] [Google Scholar]
- Jürgens G., Lang J., Esterbauer H. Modification of human low-density lipoprotein by the lipid peroxidation product 4-hydroxynonenal. Biochim Biophys Acta. 1986 Jan 3;875(1):103–114. doi: 10.1016/0005-2760(86)90016-0. [DOI] [PubMed] [Google Scholar]
- O'Leary D. S., Barr C. F., Wellde B. T., Conrad M. E. Experimental infection with Plasmodium falciparum in Aotus monkeys. 3. The development of disseminated intravascular coagulation. Am J Trop Med Hyg. 1972 May;21(3):282–287. doi: 10.4269/ajtmh.1972.21.282. [DOI] [PubMed] [Google Scholar]
- Ockenhouse C. F., Schulman S., Shear H. L. Induction of crisis forms in the human malaria parasite Plasmodium falciparum by gamma-interferon-activated, monocyte-derived macrophages. J Immunol. 1984 Sep;133(3):1601–1608. [PubMed] [Google Scholar]
- Ockenhouse C. F., Shear H. L. Oxidative killing of the intraerythrocytic malaria parasite Plasmodium yoelii by activated macrophages. J Immunol. 1984 Jan;132(1):424–431. [PubMed] [Google Scholar]
- Poli G., Dianzani M. U., Cheeseman K. H., Slater T. F., Lang J., Esterbauer H. Separation and characterization of the aldehydic products of lipid peroxidation stimulated by carbon tetrachloride or ADP-iron in isolated rat hepatocytes and rat liver microsomal suspensions. Biochem J. 1985 Apr 15;227(2):629–638. doi: 10.1042/bj2270629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauenstein E., Esterbauer H. Formation and properties of reactive aldehydes. Ciba Found Symp. 1978;(67):225–244. doi: 10.1002/9780470720493.ch15. [DOI] [PubMed] [Google Scholar]
- Schroit A. J., Tanaka Y., Madsen J., Fidler I. J. The recognition of red blood cells by macrophages: role of phosphatidylserine and possible implications of membrane phospholipid asymmetry. Biol Cell. 1984;51(2):227–238. doi: 10.1111/j.1768-322x.1984.tb00303.x. [DOI] [PubMed] [Google Scholar]
- Schwartz R. S., Chiu D. T., Lubin B. Plasma membrane phospholipid organization in human erythrocytes. Curr Top Hematol. 1985;5:63–112. [PubMed] [Google Scholar]
- Stocker R., Cowden W. B., Tellam R. L., Weidemann M. J., Hunt N. H. Lipids from Plasmodium vinckei-infected erythrocytes and their susceptibility to oxidative damage. Lipids. 1987 Jan;22(1):51–57. doi: 10.1007/BF02534875. [DOI] [PubMed] [Google Scholar]
- Stocker R., Hunt N. H., Buffinton G. D., Weidemann M. J., Lewis-Hughes P. H., Clark I. A. Oxidative stress and protective mechanisms in erythrocytes in relation to Plasmodium vinckei load. Proc Natl Acad Sci U S A. 1985 Jan;82(2):548–551. doi: 10.1073/pnas.82.2.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozencraft A. O., Croft S. L., Sayers G. Oxygen radical release by adherent cell populations during the initial stages of a lethal rodent malarial infection. Immunology. 1985 Nov;56(3):523–531. [PMC free article] [PubMed] [Google Scholar]


