Abstract
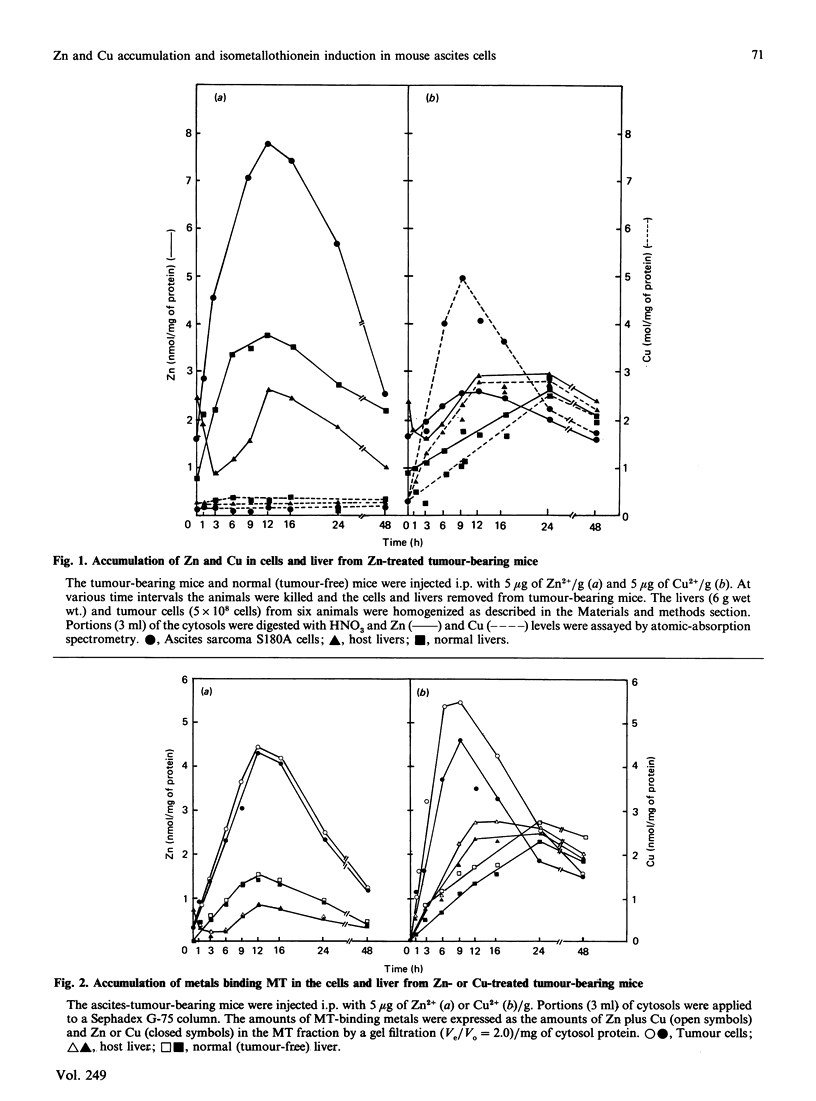
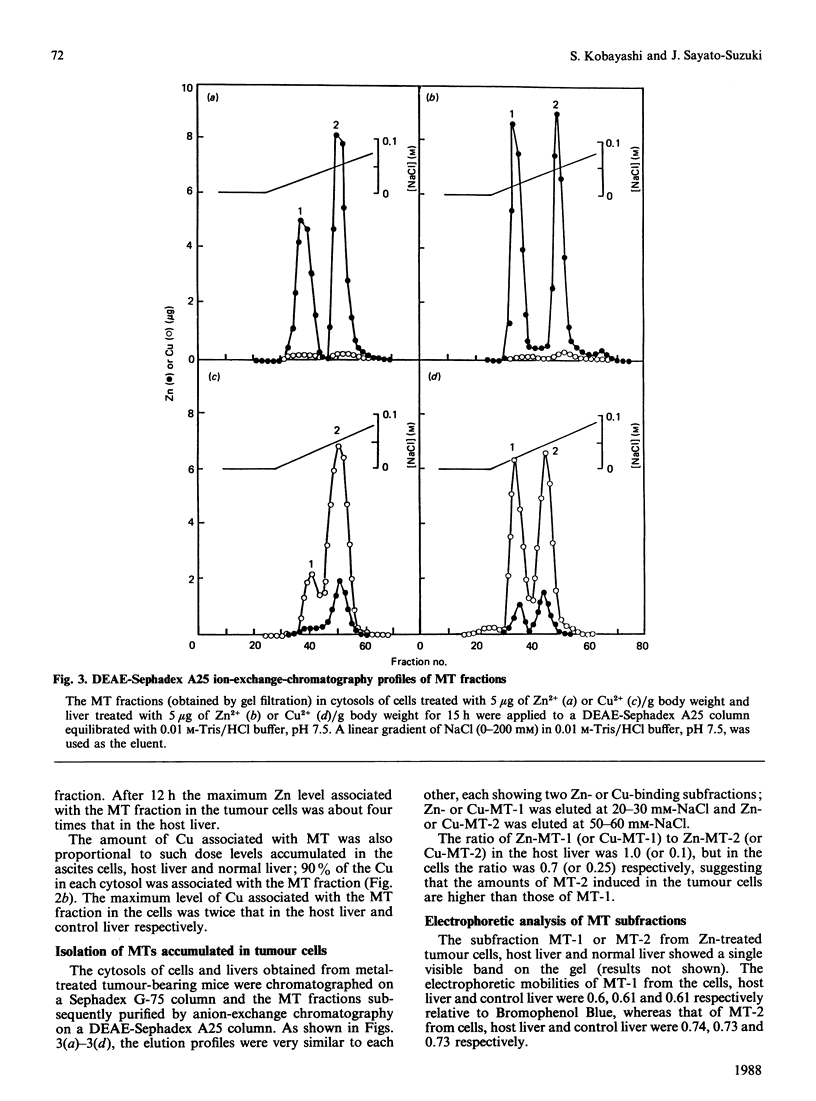
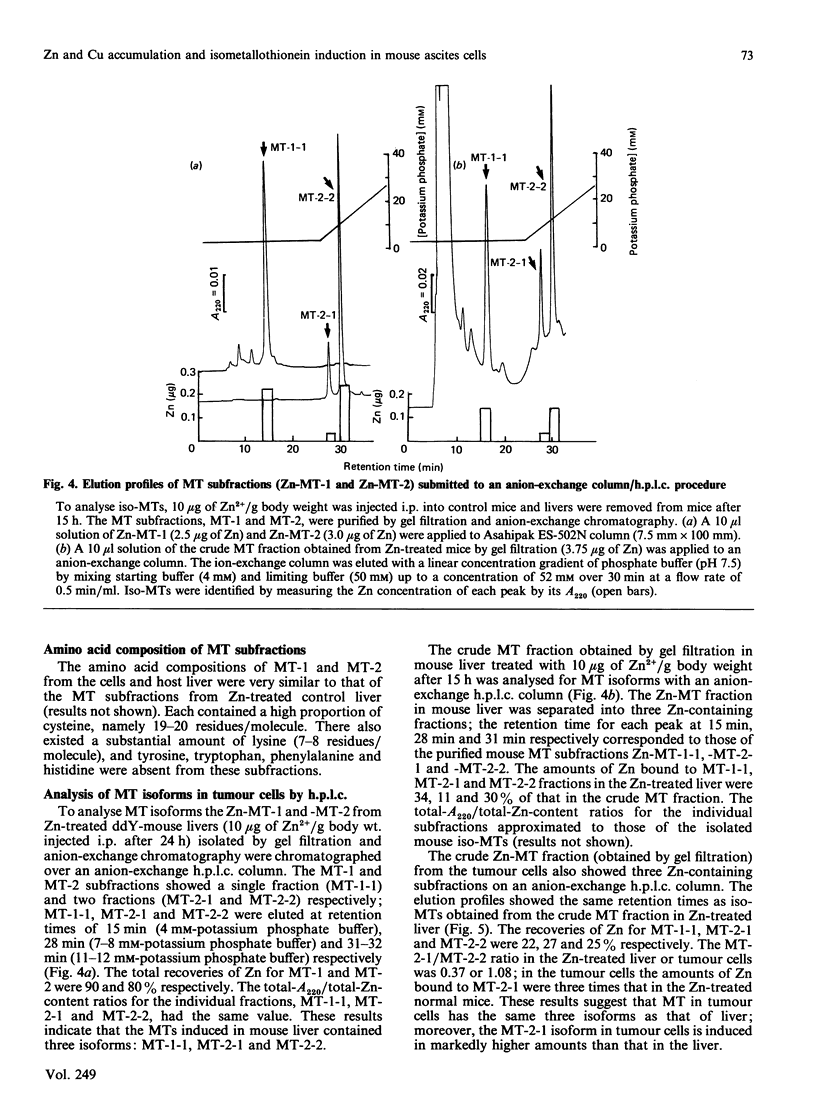
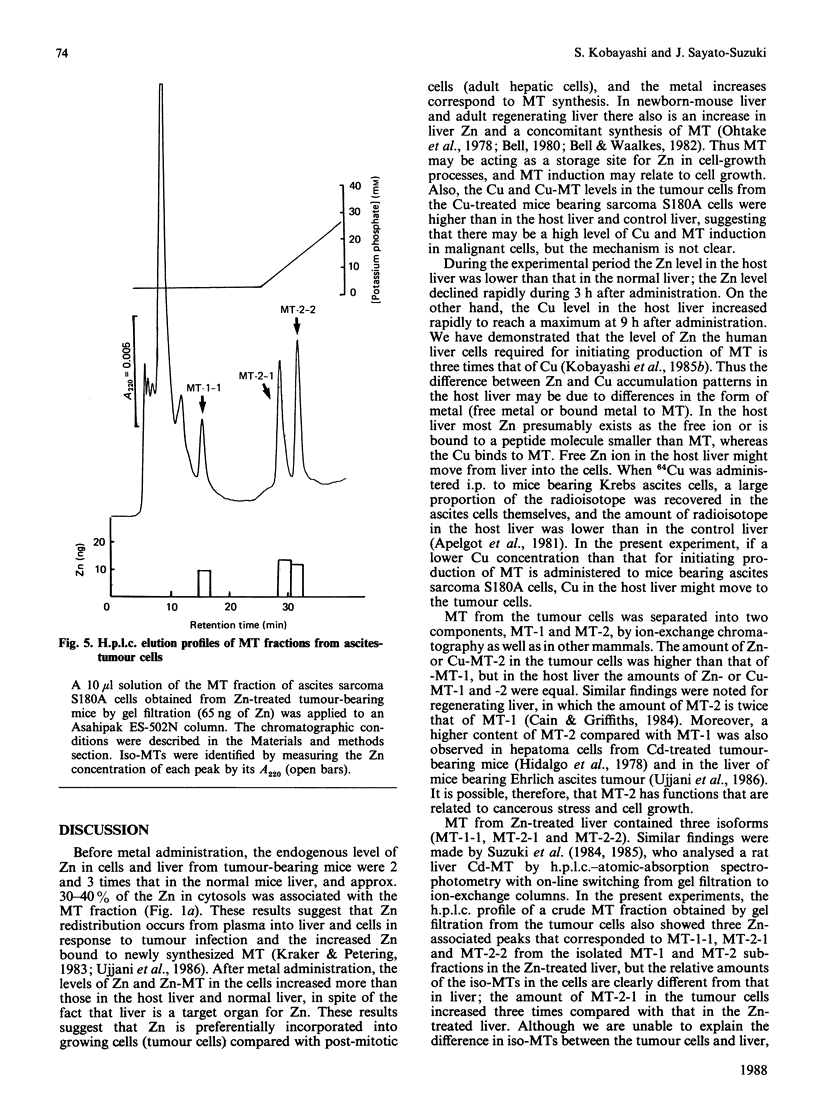
To investigate Zn and Cu accumulation and isometallothionein (iso-MT) induction in ascites-sarcoma S180A cells, 5 micrograms of Zn2+ or Cu2+/g body weight was administered to tumour-bearing mice intraperitoneally. In the tumour cells the Zn or Cu concentration increased more than in the host liver, which is the target organ for those metals; the maximum Zn or Cu level was about 2-3 times that in the host liver. The amounts of Zn-MT or Cu-MT accumulated in the tumour cells and host liver were proportional to such dose accumulation levels in the each cytosol; the maximum level of Zn-MT or Cu-MT was 4 or 2 times higher than in the host liver. MT accumulated in the tumour cells showed two subfractions (MT-1 and MT-2); the ratio of Zn (or Cu) bound to MT-1 to that bound to MT-2 in the host liver and tumour cells was 1.0 (or 1.0) and 0.7 (or 0.25) respectively, suggesting that the induction level of MT-2 in the tumour cells is more than that of MT-1. The h.p.l.c. profiles (using an anion-exchange column) of the isolated MT-1 and MT-2 subfractions from Zn-treated normal-mouse liver showed a single peak (MT-1-1) and two peaks (MT-2-1 and MT-2-2) respectively; mouse MTs were separated into three isoforms. In the ascites cells, the MT fraction obtained by a gel filtration was also separated into three isoforms; however, the amount of MT-2-1 isoform was 3 times that in the Zn-treated normal-mouse liver.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apelgot S., Coppey J., Grisvard J., Guillé E., Sissoëff I. Distribution of copper-64 in control mice and in mice bearing ascitic Krebs tumor cells. Cancer Res. 1981 Apr;41(4):1502–1507. [PubMed] [Google Scholar]
- Bell J. U. Induction of hepatic metallothionein in the immature rat following administration of cadmium. Toxicol Appl Pharmacol. 1980 Jun 15;54(1):148–155. doi: 10.1016/0041-008x(80)90015-0. [DOI] [PubMed] [Google Scholar]
- Bell J. U., Waalkes M. P. Role of hepatic metallothionein during perinatal development in the rat. Dev Toxicol Environ Sci. 1982;9:99–111. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Cain K., Griffiths B. L. A comparison of isometallothionein synthesis in rat liver after partial hepatectomy and parenteral zinc injection. Biochem J. 1984 Jan 1;217(1):85–92. doi: 10.1042/bj2170085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpentieri U., Myers J., Thorpe L., Daeschner C. W., 3rd, Haggard M. E. Copper, zinc, and iron in normal and leukemic lymphocytes from children. Cancer Res. 1986 Feb;46(2):981–984. [PubMed] [Google Scholar]
- Cohen Y., Epelbaum R., Haim N., McShan D., Zinder O. The value of serum copper levels in non-Hodgkin's lymphoma. Cancer. 1984 Jan 15;53(2):296–300. doi: 10.1002/1097-0142(19840115)53:2<296::aid-cncr2820530219>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Fisher G. L., Spitler L. E., McNeill K. L., Rosenblatt L. S. Serum copper and zinc levels in melanoma patients. Cancer. 1981 Apr 1;47(7):1838–1844. doi: 10.1002/1097-0142(19810401)47:7<1838::aid-cncr2820470720>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Hidalgo H. A., Koppa V., Bryan S. E. Induction of cadmium-thionein in mouse tumor cells. Toxicol Appl Pharmacol. 1978 Aug;45(2):521–530. doi: 10.1016/0041-008x(78)90114-x. [DOI] [PubMed] [Google Scholar]
- Hrgovcic M., Tessmer C. F., Thomas F. B., Ong P. S., Gamble J. F., Shullenberger C. C. Serum copper observations in patients with malignant lymphoma. Cancer. 1973 Dec;32(6):1512–1524. doi: 10.1002/1097-0142(197312)32:6<1512::aid-cncr2820320631>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Inutsuka S., Araki S. Plasma copper and zinc levels in patients with malignant tumors of digestive organs: clinical evaluation of the C1/Zn ratio. Cancer. 1978 Aug;42(2):626–631. doi: 10.1002/1097-0142(197808)42:2<626::aid-cncr2820420232>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Karin M., Slater E. P., Herschman H. R. Regulation of metallothionein synthesis in HeLa cells by heavy metals and glucocorticoids. J Cell Physiol. 1981 Jan;106(1):63–74. doi: 10.1002/jcp.1041060108. [DOI] [PubMed] [Google Scholar]
- Kobayashi S., Imano M., Kimura M. Induction and degradation of Zn-, Cu- and Cd-thionein in Chang liver cells. Chem Biol Interact. 1985 Jan;52(3):319–334. doi: 10.1016/0009-2797(85)90027-4. [DOI] [PubMed] [Google Scholar]
- Kobayashi S., Okada T., Kimura M. Effects of dexamethasone on metallothionein induction by Zn, Cu, and Cd in Chang liver cells. Chem Biol Interact. 1985 Nov;55(3):347–356. doi: 10.1016/s0009-2797(85)80141-1. [DOI] [PubMed] [Google Scholar]
- Lefkowitch J. H., Muschel R., Price J. B., Marboe C., Braunhut S. Copper and copper-binding protein in fibrolamellar liver cell carcinoma. Cancer. 1983 Jan 1;51(1):97–100. doi: 10.1002/1097-0142(19830101)51:1<97::aid-cncr2820510120>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Margalioth E. J., Schenker J. G., Chevion M. Copper and zinc levels in normal and malignant tissues. Cancer. 1983 Sep 1;52(5):868–872. doi: 10.1002/1097-0142(19830901)52:5<868::aid-cncr2820520521>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Margalioth E. J., Udassin R., Maor J., Schenker J. G. Serum copper level in ovarian carcinoma. Cancer. 1985 Aug 15;56(4):856–859. doi: 10.1002/1097-0142(19850815)56:4<856::aid-cncr2820560425>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Miatto O., Casaril M., Gabrielli G. B., Nicoli N., Bellisola G., Corrocher R. Diagnostic and prognostic value of serum copper and plasma fibrinogen in hepatic carcinoma. Cancer. 1985 Feb 15;55(4):774–778. doi: 10.1002/1097-0142(19850215)55:4<774::aid-cncr2820550415>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Ohtake H., Hasegawa K., Koga M. Zinc-binding protein in the livers of neonatal, normal and partially hepatectomized rats. Biochem J. 1978 Sep 15;174(3):999–1005. doi: 10.1042/bj1740999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah-Reddy I., Khilanani P., Bishop C. R. Serum copper levels in non-Hodgkin's lymphoma. Cancer. 1980 Apr 15;45(8):2156–2159. doi: 10.1002/1097-0142(19800415)45:8<2156::aid-cncr2820450824>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Suzuki K. T., Sunaga H., Yajima T. Separation of metallothionein into isoforms by column switching on gel permeation and ion-exchange columns with high-performance liquid chromatography-atomic-absorption spectrophotometry. J Chromatogr. 1984 Oct 26;303(1):131–136. doi: 10.1016/s0021-9673(01)96052-2. [DOI] [PubMed] [Google Scholar]
- Suzuki K. T., Uehara H., Sunaga H., Shimojo N. Induction and detection of a third isometallothionein (metallothionein-II') in rat liver. Toxicol Lett. 1985 Jan;24(1):15–20. doi: 10.1016/0378-4274(85)90133-x. [DOI] [PubMed] [Google Scholar]
- Tessmer C. F., Hrgovcic M., Thomas F. B., Wilbur J., Mumford D. M. Long-term serum copper studies in acute leukemia in children. Cancer. 1972 Aug;30(2):358–365. doi: 10.1002/1097-0142(197208)30:2<358::aid-cncr2820300209>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Tessmer C. F., Hrgovcic M., Wilbur J. Serum copper in Hodgkin's disease in children. Cancer. 1973 Feb;31(2):303–315. doi: 10.1002/1097-0142(197302)31:2<303::aid-cncr2820310206>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Thorling E. B., Thorling K. The clinical usefulness of serum copper determinations in Hodgkin's disease. A retrospective study of 241 patients from 1963-1973. Cancer. 1976 Jul;38(1):225–231. doi: 10.1002/1097-0142(197607)38:1<225::aid-cncr2820380134>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Ujjani B., Krakower G., Bachowski G., Krezoski S., Shaw C. F., 3rd, Petering D. H. Host zinc metabolism and the Ehrlich ascites tumour. Zinc redistribution during tumour-related stress. Biochem J. 1986 Jan 1;233(1):99–105. doi: 10.1042/bj2330099. [DOI] [PMC free article] [PubMed] [Google Scholar]


