Abstract
Influenza A viruses possess two virion surface proteins, hemagglutinin (HA) and neuraminidase (NA). The HA binds to sialyloligosaccharide viral receptors, while the NA removes sialic acids from the host cell and viral sialyloligosaccarides. Alterations of the HA occur during adaptation of influenza viruses to new host species, as in the 1957 and 1968 influenza pandemics. To gain a better understanding of the contributions of the HA and possibly the NA to this process, we generated cell lines expressing reduced levels of the influenza virus receptor determinant, sialic acid, by selecting Madin-Darby canine kidney cells resistant to a lectin specific for sialic acid linked to galactose by α(2-3) or α(2-6) linkages. One of these cell lines had less than 1/10 as much N-acetylneuraminic acid as its parent cell line. When serially passaged in this cell line, human H3N2 viruses lost sialidase activity due to a large internal deletion in the NA gene, without alteration of the HA gene. These findings indicate that NA mutations can contribute to the adaptation of influenza A virus to new host environments and hence may play a role in the transmission of virus across species.
Influenza A viruses possess two surface spike proteins, hemagglutinin (HA) and neuraminidase (NA) (12). The HA protein, a trimeric type I membrane protein, is responsible for binding to sialyloligosaccharides (oligosaccharides containing terminal sialic acid linked to galactose) on host cell surface glycoproteins or glycolipids (reviewed in reference 28). This protein is also responsible for fusion between viral and host cell membranes, following virion internalization by endocytosis. Neuraminidase (NA), a tetrameric type II membrane protein, is a sialidase that cleaves terminal sialic acid residues from the glycoconjugates of host cells and the HA and NA and thus is recognized as a receptor-destroying enzyme (1). This sialidase activity is necessary for efficient release of progeny virions from the host cell surface, as well as prevention of progeny aggregation due to the binding activity of viral HAs with other viral glycoproteins (18, 23). Thus, the receptor-binding activity of the HA and the receptor-destroying activity of the NA likely act as counterbalances, allowing efficient replication of influenza A virus.
Influenza A viruses of all known subtypes have been isolated from a variety of animals, including humans, wild and domestic birds, pigs, horses, and sea mammals (27). Viruses responsible for the 1957 and 1968 influenza pandemics were reassortants between human and avian viruses with the PB1, HA, and/or NA genes derived from the latter (10, 13, 22). Such interspecies transmission of avian virus genes forces adaptation of the gene products to the new environment (i.e., human respiratory organs).
Comparative studies have demonstrated that HA receptor specificity differs among influenza A viruses, depending on the animal species from which they were isolated (20, 21). Thus, amino acid alterations are needed for efficient viral growth in new animal hosts. To gain a better understanding of how influenza A viruses adapt to new environments and thus acquire the ability to cause epidemics or epizootics, we produced cell lines with reduced expression of terminal sialic acid residues on the cell surface. We then passaged influenza A viruses in this altered cellular environment and determined the molecular basis of the subsequent growth adapation.
MATERIALS AND METHODS
Viruses and cells.
Human H3N2 viruses isolated from a single patient, either in embryonated chicken eggs (A/Tottori/872/AM2AL3/94; AM2AL3) or Madin-Darby canine kidney (MDCK) cells (A/Tottori/872/K4/94; K4), were obtained from T. Ito (Tottori University, Tottori, Japan). Virus stocks were grown either in 10-day-old embryonated chicken eggs (AM2AL3 virus) or on MDCK cells (K4 virus) in minimal essential medium (MEM) supplemented with 0.3% bovine serum albumin and 0.5 mg of trypsin/ml. MDCK cells were maintained in MEM supplemented with 5% newborn calf serum (Sigma, St. Louis, Mo.).
Generation of lectin-resistant cell lines.
MDCK cells grown to 75% confluency were washed three times with phosphate-buffered saline and incubated with Maakia amurensis (MAA) lectin (100 mg/ml; Boehringer Mannheim, Mannheim, Germany) or Sambucus nigra (SNA) lectin (100 mg/ml; Boehringer Mannheim) in MEM containing 0.3% bovine serum albumin. After a 48-h incubation, the medium was replaced with growth medium (MEM–5% fetal calf serum). Lectin selection was repeated as above two additional times. Surviving cell colonies were then cloned, and the SNA- and MAA-selected cell lines were designated MDCK-Sn10 and MDCK-Ma, respectively.
Fluorometric HPLC method for determination of sialic acid content.
The sialic acid (N-acetylneuraminic acid [NeuAc] and N-glycolylneuraminic acid [NeuGc]) content of both cell lines and the purified virus was determined fluorometrically by high-performance liquid chromatography as described previously (25). Each sample was placed in a 5-ml ground glass-topped vial and mixed with 100 μl (25 mM) of sulfuric acid. The vials were then heated at 60°C for 12 h to hydrolyze sialo-sugar chains. After cooling, 50 μl of 1,2-diamino-4,5-methylene dioxybenzene was added to 50 μl of the hydrolyte, and the mixture was heated to 60°C for 2.5 h in the dark to develop the fluorescence of the sialic acids. A 10-μl aliquot of the resulting solution was then injected into an 880-PU high-performance liquid chromatograph (JASCO, Tokyo, Japan) equipped with a sample injector valve (model 7125; Reodyne,) and a fluorescent spectrophotometer (650-105; Hitachi, Tokyo, Japan) with a 20-μl flow cell and a recorder (Chromatopac C-R5A; Shimadzu, Kyoto, Japan). The fluorescence spectrophotometer was positioned at an excitation wavelength of 373 nm and an emission wavelength of 448 nm. Standard mixtures (200 pmol/μl) of NeuAc (Sigma) and NeuGc (Sigma) were used to establish calibration curves.
Fluorometric sialidase activity assay.
Virus sialidase activity (5 × 102 PFU was measured with 2′-(4-methylumbelliferyl)-α-d-N-acetylneuraminic acid (Sigma) as a substrate as described previously (6). Briefly, the fluorogenic substrate, diluted 1:2 with 0.5 M sodium acetate (pH 4.6), was added to an equal volume of virus samples and incubated for 30 min at 37°C. Reactions were stopped with 200 ml of 0.5 M Na2CO3 (pH 10.7), and fluorescence was then measured at an excitation wavelength of 360 nm and an emission wavelength of 460 nm. All reactions were performed in duplicate.
Sequence analysis of the NA and HA genes.
Total viral RNA (vRNA) was obtained from virus sample with use of the Qiaspin vRNA purification kit as instructed by the manufacturer (Qiagen, Inc., Valencia, Calif.). For cDNA production, the oligonucleotide Uni-12, complementary to the conserved 12 vRNA 3′-terminal nucleotides of influenza A virus gene segments, was used as a primer for the Moloney murine leukemia virus reverse transcriptase (Promega, Madison, Wis.) reaction. The NA gene cDNA was amplified during 30 rounds of PCR with the NA gene-specific primers JN2-43s (5′ cRNA sense sequence: 5′-TGGCTCGTTTCTCTCACTATTGCC-3′) and JN2-1410r (3′ cRNA antisense sequence, 5′-TTATATAGGCATGAGATTGATGTCCG-3′) and 10 U of Pwo DNA polymerase (Boehringer Mannheim). The resulting PCR products were subcloned into the vector pCR2.1 (Invitrogen, Carlsbad, Calif.) and used for automated fluorescent sequencing. The HA genes were cloned in a similar fashion with the HA gene-specific primers JH3-Up (5′ cRNA sense primer sequence, 5′-AGCAAAAGCAGGGGATAATTCTATTAACCATGAAGAC-3′) and JH3-Down (3′ cRNA antisense primer sequence, 5′-AGTAGAAACAAGGGTGTTTTTAATTAATGCACTC-3′). For each isolate, three clones were examined to obtain the NA and HA consensus sequences.
RESULTS
Generation of lectin-resistant cell lines.
To produce cell lines with a decreased level of sialic acid expression on the cell surface, we used two lectins, SNA and MAA, that differ in sialic acid-binding specificity. The MAA lectin binds to sialic acid linked to galactose by α(2-3) linkages (26), while the SNA lectin is specific for sialic acids linked to galactose or N-acetylgalactosamine by α(2-6) linkages (24). The MDCK cell line, which supports the growth of influenza viruses, was used as a parent cell line for lectin selection. When incubated in the presence of either lectin, the majority of cells died within a week. Resistant cell clones were then grown out for stock cultures. The cell lines resulting from MAA and SNA lectin selection were designated MDCK-Ma and MDCK-Sn10, respectively.
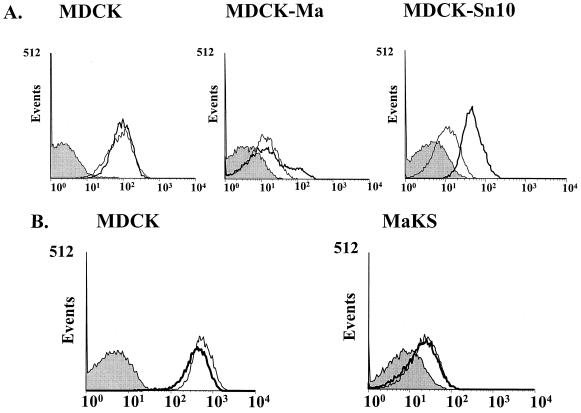
Fluorescent-activated cell sorting (FACS) with digoxigenin-labeled MAA and SNA lectins (Fig. 1A) demonstrated high levels of binding of MDCK cells to both lectins, as we previously reported (9). MDCK-Sn10 cells, selected with α(2-6) linkage-specific lectin, retained strong binding to the α(2-3)-specific MAA lectin but showed SNA lectin binding weaker than that of the MDCK parent. By contrast, MDCK-Ma cells, selected with the α(2-3) linkage-specific lectin, bound both lectins much more weakly than MDCK cells.
FIG. 1.
Binding of lectins to lectin-resistant cell lines. For each cell line, cells were incubated with digoxigenin-labeled MAA or SNA lectins, followed by fluorescein isothiocyanate-labeled antidigoxigenin antibody, and then analyzed by FACS. Bold lines, binding of the MAA lectin; narrow lines, binding of the SNA lectin; shaded profiles, negative control (no lectin added).
Viral growth in MDCK-Sn10 and MDCK-Ma cell lines.
To learn how influenza viruses adapt to cells with reduced receptor expression, we chose two influenza virus variants (AM2AL3 and K4) with known sialic acid receptor linkage specificity (9). The K4 virus specifically recognizes NeuAc linked to galactose by α(2-6) linkages [NeuAcα(2-6)Gal], while the AM2AL3 virus is specific for NeuAcα(2-3)Gal. Both viruses replicated almost as well in MDCK-Sn10 cells as in MDCK cells (Table 1). However, the titers of both viruses in MDCK-Ma cells were 1 log lower than in MDCK cells. We also noted that after infection with either virus, even at a multiplicity of infection of 10, a small percentage of MDCK-Ma cells continued to grow to confluency without any cytopathic effects. Virus production could not be detected in these surviving cells by hemagglutination assay upon replacement of the medium with that containing trypsin, which promotes virus growth. The cells were also negative by immunochemical staining for both influenza virus HA and NP proteins (data not shown), thus demonstrating that the cells were not persistently infected. We designated the surviving cells MaKS.
TABLE 1.
Replication of influenza viruses in lectin-resistant cell linesa
| Cell line | Titer (TCID50/ml)
|
|
|---|---|---|
| AM2AL3 | K4 | |
| MDCK | 1.8 × 109 | 5.6 × 104 |
| MDCK-Sn10 | 5.6 × 108 | 3.2 × 104 |
| MDCK-Ma | 1.8 × 108 | 5.6 × 103 |
The susceptibility of each cell line was determined by infecting cells with AM2AL3 or K4 stock virus and determining the dose required to infect 50% of tissue culture cells (TCID50).
FACS analysis with both SNA and MAA lectins demonstrated that the MaKS cells, like the MDCK-Ma cells from which they were derived, bound the α(2-6)-specific SNA lectin much more weakly than did MDCK cells (Fig. 1B). In addition, the MAA lectin-binding peak of MaKS cells was much narrower than that of the MDCK-Ma cell line, with loss of a small shoulder peak representing a higher MAA-binding population (Fig. 1).
To determine whether reduced amounts of sialic acid were responsible for the reduced lectin binding of MaKS cells, we quantified the sialic acid levels present in the MaKS cells by liquid chromatographic analysis. The MaKS cell line showed much lower levels of both NeuAc and NeuGc (8.2 and 0.4 pmol/μg of protein, respectively) than did MDCK cells (216.0 and 2.5 pmol/μg of protein, respectively), although the NeuGc content was much lower. These data demonstrate an extensive reduction of sialic acid receptor determinant in MaKS cells.
Adaptation of virus in MaKS cells.
To determine how AM2AL3 and K4 viruses propagate and adapt to growth in cells expressing very low levels of virus receptor, we serially passaged both viruses in MaKS cells in liquid culture. Since both viruses replicated more poorly in MaKS cells than in MDCK cells (Table 2), passages 1 through 3 were performed without dilution, and passages 4 through 13 were performed at 1:1,000 dilution. After passage 8, the diameter of plaques produced by either variant had changed from large (greater than 3 mm) to smaller (approximately 1 mm). By passage 10 and higher, only smaller plaques were present when the viruses were assayed with MDCK cells (data not shown). After 13 serial passages, both viruses were able to grow in MaKS cells as well as or better than in MDCK cells (Table 2). Virus stocks produced from either variant after passage 13 were amplified and designated AL3(MaKS)-13 and K4(MaKS)-13, respectively.
TABLE 2.
Replication of viruses adapted to growth in lectin-selected cellsa
| Cell line | Titer (TCID50)
|
|||
|---|---|---|---|---|
| AM2AL3 | AL3(MaKS)-13 | K4 | K4(MaKS)-13 | |
| MDCK | 1.8 × 109 | 5.6 × 104 | 5.6 × 104 | 5.6 × 104 |
| MaKS | 5.6 × 106 | 5.6 × 104 | 1.8 × 103 | 1.8 × 105 |
| Ratio, MDCK titer/MaKS titer | 321 | 1 | 31 | 0.3 |
The susceptibility of each cell line was determined by infecting cells with AM2AL3 (grown in eggs), K4 (grown in MDCK cells), AL3(MaKS)-13 (grown in MaKs cells), or K4(MaKS)-13 (grown in MaKs cells) stock virus and determining the dose required to infect 50% of tissue culture cells (TCID50). Note that both viruses adapted in MaKS cells grow in these cells as well as [AL3(MaKS)-13] or better than [K4(MaKS)-13] in MDCK cells, while the original viruses grow better in MDCK cells.
Mutational analysis of the HA and NA genes of AL3 (MaKS)-13 and K4(MaKS)-13 viruses.
To determine the molecular basis of virus adaptation to a cellular environment characterized by a reduced receptor concentration, we first reverse transcribed the HA genes of the AL3(MaKS)-13 and K4(MaKS)-13 viruses, amplified the cDNAs by PCR, and sequenced the resulting products. Neither of the genes contained mutations by comparison with the corresponding HA genes from the two parental viruses.
Since changes in NA sialidase activity likely influence HA receptor-binding activity, we next determined the NA sequence of the AL3(MaKS)-13 and K4(MaKS)-13 viruses. Sequence analysis of the NA genes of both variants revealed large internal deletions (Fig. 2). In AL3(MaKS)-13, the deletion extended from nucleotides 220 to 1253, shifting a reading frame and thus generating a stop codon immediately after the deletion. The coding capacity of this NA is 66 amino acids, corresponding to the cytoplasmic tail, the transmembrane domain, stalk region, and a short portion of the head region. Similarly, the K4(MaKS)-13 isolate contained a deletion in the NA gene from bases 130 to 1193, bringing a stop codon into frame at codon 39. Like the AL3(MaKS)-13 virus, the gene no longer encoded a full catalytic head region. Thus, viruses passaged in a cell line with very low receptor expression lost their NA catalytic activity.
FIG. 2.
Structures of the NA genes of the AL3(MaKS)-13 and K4(MaKS)-13 mutants. (A) The AL3(MaKS)-13 NA contains a 936-nucleotide deletion (from bases 220 to 1253) that removes a large portion of the NA gene coding sequence. This mutation also brings a TAG stop codon into frame two bases beyond the deletion, so that the gene potentially encodes only a 66-amino-acid peptide, corresponding to the cytoplasmic tail, transmembrane region, stalk, and a portion of the NA head. (B) The K4(MaKS)-13 NA gene contains a 1,066-nucleotide deletion (from bases 130 to 1193) that removes a large portion of the NA gene coding sequence. This mutation also brings a TAG stop codon into frame four bases beyond the deletion, so that the gene potentially encodes only a 38-amino-acid peptide, corresponding to the cytoplasmic tail and transmembrane region of the NA gene.
To confirm this result, we tested the AL3(MaKS)-13 and K4 (MaKS)-13 variants for sialidase activity, using a fluorescent sialidase substrate [2′-(4-methylumbelliferyl)-α-d-N-acetylneuraminic acid]. Unlike the parental viruses, neither of the NA deletion mutants had detectable sialidase activity (Fig. 3).
FIG. 3.
Sialidase activity of the parental AM2AL3 and K4 viruses and the AL3(MaKS)-13 and K4(MaKS)-13 mutants. For each sample, virus (5 × 102 PFU) was incubated in duplicate for 1 h at 37°C in the presence of a fluorogenic sialidase substrate (4-methylumbelliferyl-a-d-N-acetylneuraminic acid). The fluorescence of released 4-methylumbelliferone was determined with a fluorometer (Labsystems Fluoroskan II) with excitation at 360 nm and emission at 460 nm.
Extent of sialylation of viral glycoproteins.
During normal infection, viruses with reduced sialidase activity fail to grow efficiently and aggregate at the cell surface (18, 23). Why, then, do AL3(MaKS)-13 and K4 (MaKS)-13 viruses, which lack sialidase activity, grow in MaKS cells? One possible explanation would be that since the sialic acid content of these cells is low, the extent of sialylation of the HA and NA oligosaccharides may also be low, preventing the aggregation of viruses at the infected cell surface, even when viral sialidase activity is absent. To test this hypothesis, we compared the sialic acid content in purified virus preparations between AM2AL3 and K4 viruses grown in MDCK cells and AL3(MaKS)-13 virus grown in MaKS cells. The NeuAc content was similar among the virus samples, although the AM2AL3 virus had lower sialic acid content (0.9 pmol of NeuAc/g of protein) than the other samples (A/Tottori/872/K4/94, 3.8 pmol of NeuAc/g of protein; AL3[MaKS]-13, 2.6 pmol of NeuAc/g of protein). Thus, viruses lacking sialidase activity can grow efficiently in cells expressing a reduced level of sialic acid because the viral glycoproteins are not sialylated extensively compared with those in normal cell lines and are not bound by the HA, thus preventing viral aggregation.
DISCUSSION
In previous studies, the passage of influenza A viruses in the presence of an exogenous bacterial sialidase activity and antibodies to the viral NA led to deletion of the viral NA gene (14, 15, 29). Moreover, NA mutants obtained by such passaging were able to grow in cell cultures lacking exogenous sialidase activity, as well as in eggs and mice, as a result of compensatory mutations in the HA protein that reduce the molecule's affinity for sialic acid residues (8). We demonstrate here that influenza A viruses can adapt to growth in cells with greatly reduced receptor expression by large NA gene deletion mutations that abolish sialidase activity. Even though the reduction of viral receptors could theoretically affect the receptor-binding HA protein, we found that only the NA gene was altered.
What is the molecular basis of this finding? In normal cellular environments where sialic acid receptors are abundant, the loss of NA activity can be compensated for by reduction of the viral HA affinity for sialic acid, allowing efficient release of progeny from the host cell surface and preventing virion aggregation (8). In the absence of high levels of viral receptors, as in our MaKS cells, a reduction of HA affinity is not necessary to release viral progeny and allow the growth of NA deletion mutants. In fact, high-affinity binding of the HA protein must be maintained for viral replication in cells expressing low levels of viral receptor. Sialidase activity, however, is not required for virion release and prevention of virion aggregation in such an environment, since the amounts of sialic acid on cell surface molecules are quite low and the sialic acid contents of NA deletion virions are similar to that of wild-type virions (see Results). In fact, sialidase activity is likely deleterious for viral growth because it further removes receptor determinant sialic acid from the cell surface. We have recently shown that influenza A virus lacking an NA stalk, and thus unable to grow in eggs, acquired a stalk insertion of up to 22 amino acids through nonhomologous RNA-RNA recombination (16). Taken together, these finding indicate that influenza viruses can adapt to new host environments by undergoing radical genetic changes, including large insertions and deletions.
We stress that in both this and previous studies (8, 14), viruses lost sialidase activity by internal deletions in the NA gene segment that spared segment ends encoding the cytoplasmic tail and transmembrane region. Thus, the preserved regions of the NA gene in these mutants may be necessary for functions such as virion morphogenesis and stability, a possibility awaiting confirmation in future studies.
MaKS cells have a lower sialic acid content than their parental (MDCK) cells. Although similar cell lines have been produced from CHO cells (19), they have not proven useful for influenza virus studies because of their inability to support efficient influenza virus replication. By contrast, MaKS cells were derived from MDCK cells, a standard cell line in studies of influenza viruses, and should be useful in viral receptor-based analyses. For example, since exogenously added gangliosides are known to be incorporated into host cell membranes (4), one could therefore incubate known gangliosides with MaKS cells and test their ability to serve as viral receptors.
During the past century, three influenza A virus pandemics arose when the HA or both the HA and NA genes of emerging viruses were introduced into a human population. Comparative studies of viruses from different host animals suggest that in these pandemic strains, mutations were introduced in the HA gene (3). Whether similar mutations are required in the NA to enable the virus to cross host species barriers remains unknown; however, the substrate specificity of the human virus N2 NA, which was derived from an avian virus, gradually changed during its replication in humans (2). Results of the current study indicate that NA mutations can indeed contribute to the ability of influenza A viruses to adapt to new environments. Support for this hypothesis comes from a study in which a reassortant virus with a human virus NA and the remaining genes from a duck virus failed to replicate in ducks (7), even though the NA of the human virus originated from an avian virus (22). This suggests that mutations likely occurred in the NA gene during adaptation in humans. Comparative studies of viral NAs from different animal hosts, in conjunction with recently developed plasmid-based reverse genetics (5, 17), may yield useful insights into how these surface glycoproteins contribute to adaptive changes among influenza A viruses in nature.
ACKNOWLEDGMENTS
We thank Krisna Wells for excellent technical assistance and John Gilbert for editing the manuscript.
Support for this work came from Public Health Service research grants from the National Institute of Allergy and Infectious Diseases.
REFERENCES
- 1.Air G M, Laver W G. The neuraminidase of influenza virus. Proteins Struct Func Genet. 1989;6:341–356. doi: 10.1002/prot.340060402. [DOI] [PubMed] [Google Scholar]
- 2.Baum L G, Paulson J C. The N2 neuraminidase of human influenza virus has acquired a substrate specificity complementary to the hemagglutinin receptor specificity. Virology. 1991;180:10–15. doi: 10.1016/0042-6822(91)90003-t. [DOI] [PubMed] [Google Scholar]
- 3.Bean W J, Schell M, Katz J, Kawaoka Y, Naeve C, Gorman O, Webster R G. Evolution of the H3 influenza virus hemagglutinin from human and nonhuman hosts. J Virol. 1992;66:1129–1138. doi: 10.1128/jvi.66.2.1129-1138.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carroll S M, Paulson J C. Differential infection of receptor-modified host cells by receptor-specific influenza viruses. Virus Res. 1985;3:165–179. doi: 10.1016/0168-1702(85)90006-1. [DOI] [PubMed] [Google Scholar]
- 5.Fodor E, Devenish L, Engelhardt O G, Palese P, Brownlee G G, Garcia-Sastre A. Rescue of influenza A virus from recombinant DNA. J Virol. 1999;73:9679–9682. doi: 10.1128/jvi.73.11.9679-9682.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hara S, Takemori Y, Yamaguchi M, Nakamura M, Ohkura Y. Fluorometric high-performance liquid chromatography of N-acetyl- and N-glycolylneuraminic acids and its application to their microdetermination in human and animal sera, glycoproteins, and glycolipids. Anal Biochem. 1987;164:138–145. doi: 10.1016/0003-2697(87)90377-0. [DOI] [PubMed] [Google Scholar]
- 7.Hinshaw V S, Webster R G, Naeve C W, Murphy B R. Altered tissue tropism of human-avian reassortant influenza viruses. Virology. 1983;128:260–263. doi: 10.1016/0042-6822(83)90337-9. [DOI] [PubMed] [Google Scholar]
- 8.Hughes M T, Matrosovich M, Rodgers E M, McGregor M, Kawaoka Y. Influenza A viruses lacking sialidase activity can undergo multiple cycles of replication in cell culture, eggs, or mice. J Virol. 2000;74:5206–5212. doi: 10.1128/jvi.74.11.5206-5212.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ito T, Suzuki Y, Takada A, Kawamoto A, Otsuki K, Masuda H, Yamada M, Suzuki T, Kida H, Kawaoka Y. Differences in sialic acid-galactose linkages in the chicken egg amnion and allantois influence human influenza virus receptor specificity and variant selection. J Virol. 1997;71:3357–3362. doi: 10.1128/jvi.71.4.3357-3362.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawaoka Y, Krauss S, Webster R G. Avian-to-human transmission of the PB1 gene of influenza A viruses in the 1957 and 1968 pandemics. J Virol. 1989;63:4603–4608. doi: 10.1128/jvi.63.11.4603-4608.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kobasa D, Rodgers M E, Wells K, Kawaoka Y. Neuraminidase hemadsorption activity, conserved in avian influenza A viruses, does not influence viral replication in ducks. J Virol. 1997;71:6706–6713. doi: 10.1128/jvi.71.9.6706-6713.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lamb R A, Krug R M. Orthomyxoviridae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 1353–1395. [Google Scholar]
- 13.Laver W G, Webster R G. Studies on the origin of pandemic influenza. III. Evidence implicating duck and equine influenza viruses as possible progenitors of the Hong Kong strain of human influenza. Virology. 1973;51:383–391. doi: 10.1016/0042-6822(73)90437-6. [DOI] [PubMed] [Google Scholar]
- 14.Liu C, Air G M. Selection and characterization of a neuraminidase-minus mutant of influenza virus and its rescue by cloned neuraminidase genes. Virology. 1993;194:403–407. doi: 10.1006/viro.1993.1276. [DOI] [PubMed] [Google Scholar]
- 15.Liu C, Eichelberger M C, Compans R W, Air G M. Influenza type A virus neuraminidase does not play a role in viral entry, replication, assembly, or budding. J Virol. 1995;69:1099–1106. doi: 10.1128/jvi.69.2.1099-1106.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitnaul L J, Matrosovich M, Castrucci M R, Tuzikov A B, Bovin N V, Kobasa D, Kawaoka Y. Balanced hemagglutinin and neuraminidase activities are critical for efficient replication of influenza A virus. J Virol. 2000;74:6015–6020. doi: 10.1128/jvi.74.13.6015-6020.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neumann G, Watanabe T, Ito H, Watanabe S, Goto H, Gao P, Hughes M, Perez D R, Donis R, Hoffmann E, Hobom G, Kawaoka Y. Generation of influenza A viruses entirely from cloned cDNAs. Proc Natl Acad Sci USA. 1999;96:9345–9350. doi: 10.1073/pnas.96.16.9345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palese P, Tobita K, Ueda M, Compans R W. Characterization of temperature-sensitive influenza virus mutants defective in neuraminidase. Virology. 1974;61:397–410. doi: 10.1016/0042-6822(74)90276-1. [DOI] [PubMed] [Google Scholar]
- 19.Ray M K, Yang J, Sundaram S, Stanely P. A novel glycosylation phenotype expressed by lec23, a Chinese hamster ovary mutant deficient in alpha-glucosidase I. J Biol Chem. 1991;266:18–25. [PubMed] [Google Scholar]
- 20.Rogers G N, Paulson J C. Receptor determinants of human and animal influenza virus isolates: differences in receptor specificity of the H3 hemagglutinin based on species of origin. Virology. 1983;127:361–373. doi: 10.1016/0042-6822(83)90150-2. [DOI] [PubMed] [Google Scholar]
- 21.Rogers G N, Prichett T J, Lane J L, Paulson J C. Differential sensitivity of human, avian, and equine influenza A viruses to a glycoprotein inhibitor of infection: selection of receptor specific variants. Virology. 1983;131:394–408. doi: 10.1016/0042-6822(83)90507-x. [DOI] [PubMed] [Google Scholar]
- 22.Scholtissek C, Rohde W, von Hoyningen V, Rott R. On the origin of the human influenza virus subtypes H2N2 and H3N2. Virology. 1978;87:13–20. doi: 10.1016/0042-6822(78)90153-8. [DOI] [PubMed] [Google Scholar]
- 23.Shibata S, Yamamoto-Goshima F, Maeno K, Hanaichi T, Fujita Y, Nakajima K, Imai M, Komatsu T, Sugiura S. Characterization of a temperature-sensitive influenza B virus mutant defective in neuraminidase. J Virol. 1993;67:3264–3273. doi: 10.1128/jvi.67.6.3264-3273.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shibuya N, Goldstein I J, Broekaert W F, Nsimba-Lubaki M, Peeters B, Peumans W J. The elderberry (Subbucus nigra L.) bark lectin recognizes the Neu5(alpha 2–6)Gal/GalNAc sequence. J Biol Chem. 1987;262:1596–1601. [PubMed] [Google Scholar]
- 25.Suzuki T, Horiike G, Yamazaki Y, Kawabe K, Masuda H, Miyamoto D, Masuta M, Nishimura S I, Yamagata T, Ito T, Kiha H, Kawaoka Y, Suzuki Y. Swine influenza virus strains recognize sialylsugar chains containing the molecular species of sialic acid predominantly present in the swine tracheal epithelium. FEBS Lett. 1997;404:192–196. doi: 10.1016/s0014-5793(97)00127-0. [DOI] [PubMed] [Google Scholar]
- 26.Wang W C, Cummings R D. The immobilized leukoagglutinin from the seeds of Maackia amurensis binds with high affinity to complex-type Asn-linked oligosaccharides containing terminal sialic acid-linked α-2,3 to penultimate galactose residues. J Biol Chem. 1988;263:4576–4585. [PubMed] [Google Scholar]
- 27.Webster R G, Bean W J, Gorman O T, Chambers T M, Kawaoka Y. Evolution and ecology of influenza A viruses. Microbiol Rev. 1992;56:152–179. doi: 10.1128/mr.56.1.152-179.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wiley D C, Skehel J J. The structure and function of the hemagglutinin membrane glycoprotein of influenza virus. Annu Rev Biochem. 1987;56:3665–394. doi: 10.1146/annurev.bi.56.070187.002053. [DOI] [PubMed] [Google Scholar]
- 29.Yang P, Bansal A, Liu C G, Air G M. Hemagglutinin specificity and neuraminidase coding capacity of neuraminidase-deficient influenza viruses. Virology. 1997;229:155–165. doi: 10.1006/viro.1996.8421. [DOI] [PubMed] [Google Scholar]