Abstract
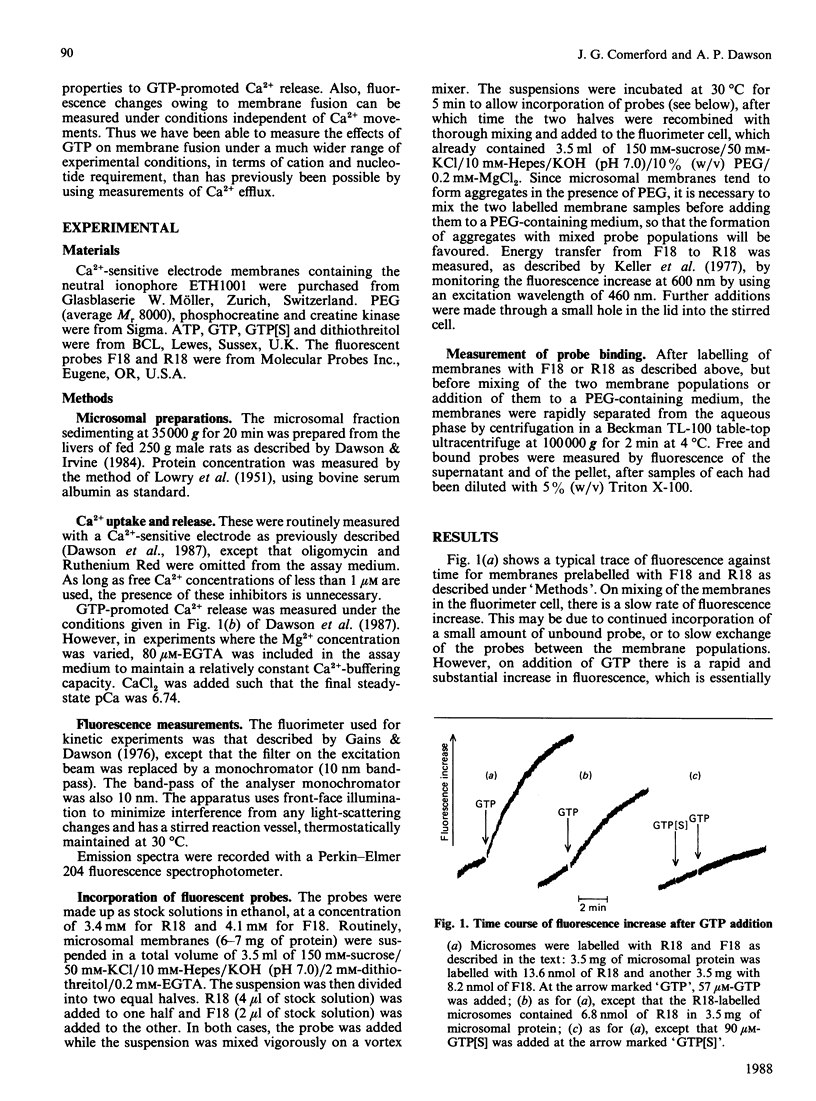
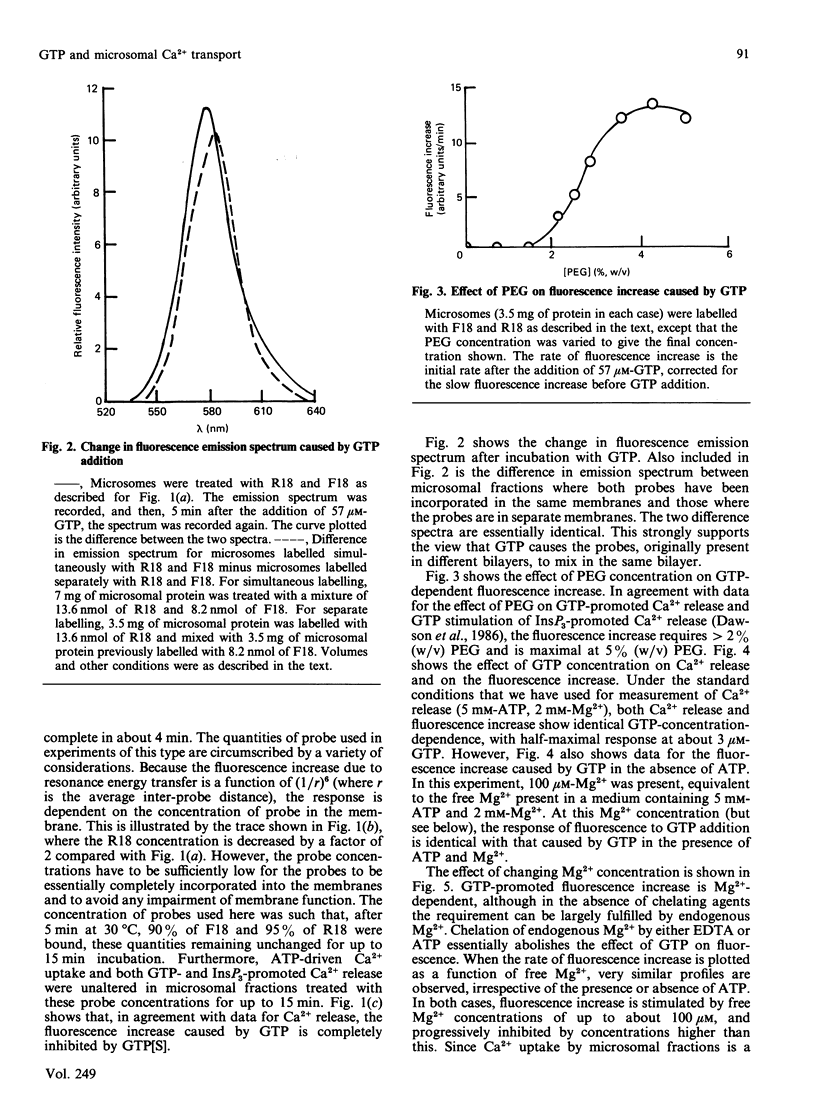
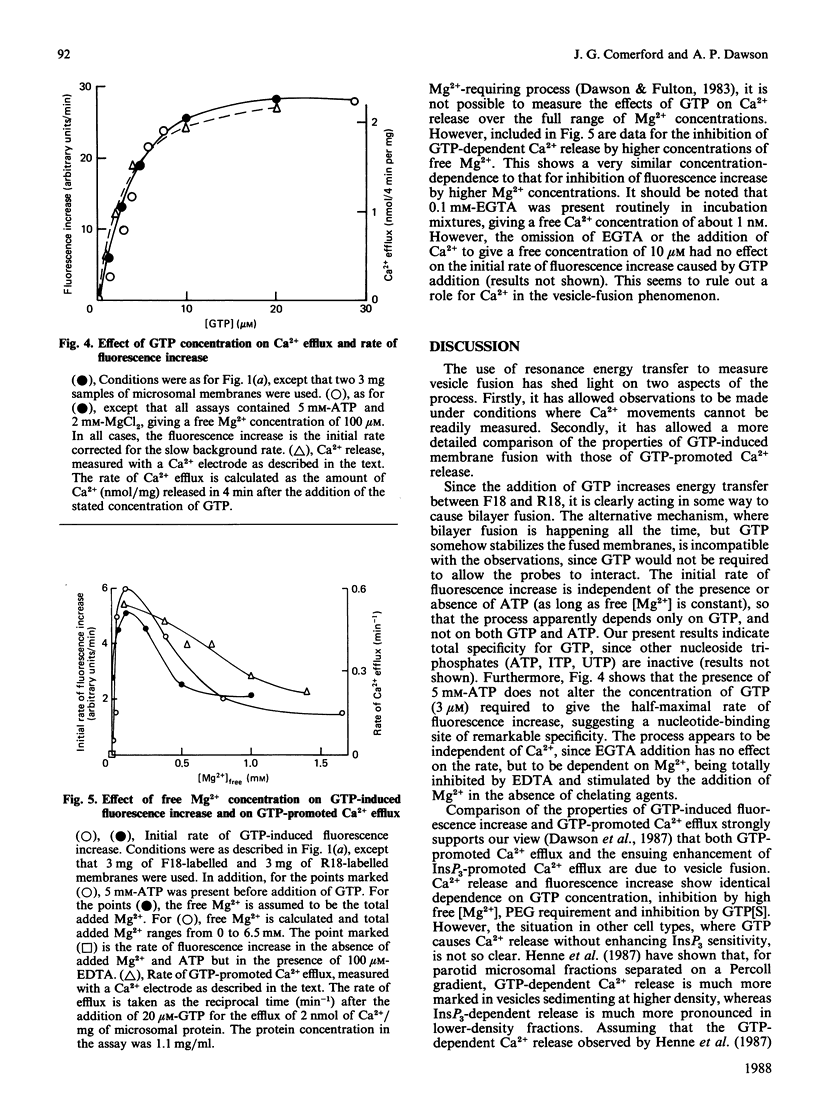
1. GTP-promoted fusion between microsomal vesicles was studied by using fluorescence-resonance-energy transfer between the fluorescent membrane probes octadecanoyl-aminofluorescein and octadecyl-rhodamine. 2. The fluorescence increase after GTP addition does not require the presence of ATP, is unaffected by changes in free [Ca2+] in the range 10 microM-1 nM, but requires Mg2+, although higher Mg2+ concentrations are inhibitory. 3. In terms of requirements for poly(ethylene glycol), dependence on GTP concentration and inhibition by high Mg2+ concentrations, there is excellent correlation between rate of increase in fluorescence and rate of GTP-promoted Ca2+ efflux measured under Ca2+ transport conditions. 4. The observations support our previous conclusions that GTP-induced membrane fusion plays a major role in causing GTP-promoted Ca2+ efflux from microsomal vesicles.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dawson A. P., Comerford J. G., Fulton D. V. The effect of GTP on inositol 1,4,5-trisphosphate-stimulated Ca2+ efflux from a rat liver microsomal fraction. Is a GTP-dependent protein phosphorylation involved? Biochem J. 1986 Mar 1;234(2):311–315. doi: 10.1042/bj2340311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson A. P., Fulton D. V. Some properties of the Ca2+-stimulated ATPase of a rat liver microsomal fraction. Biochem J. 1983 Feb 15;210(2):405–410. doi: 10.1042/bj2100405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson A. P. GTP enhances inositol trisphosphate-stimulated Ca2+ release from rat liver microsomes. FEBS Lett. 1985 Jun 3;185(1):147–150. doi: 10.1016/0014-5793(85)80759-6. [DOI] [PubMed] [Google Scholar]
- Dawson A. P., Hills G., Comerford J. G. The mechanism of action of GTP on Ca2+ efflux from rat liver microsomal vesicles. Biochem J. 1987 May 15;244(1):87–92. doi: 10.1042/bj2440087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson A. P., Irvine R. F. Inositol (1,4,5)trisphosphate-promoted Ca2+ release from microsomal fractions of rat liver. Biochem Biophys Res Commun. 1984 May 16;120(3):858–864. doi: 10.1016/s0006-291x(84)80186-2. [DOI] [PubMed] [Google Scholar]
- Gains N., Dawson A. P. A kinetic analysis of the changes in fluorescence on the interaction of 8-anilinonaphthalene-1-sulphonate with submitochondrial particles. Biochem J. 1976 Aug 15;158(2):295–305. doi: 10.1042/bj1580295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson G. A., Loew L. M. Phospholipid vesicle fusion monitored by fluorescence energy transfer. Biochem Biophys Res Commun. 1979 May 14;88(1):135–140. doi: 10.1016/0006-291x(79)91707-8. [DOI] [PubMed] [Google Scholar]
- Gill D. L., Ueda T., Chueh S. H., Noel M. W. Ca2+ release from endoplasmic reticulum is mediated by a guanine nucleotide regulatory mechanism. Nature. 1986 Apr 3;320(6061):461–464. doi: 10.1038/320461a0. [DOI] [PubMed] [Google Scholar]
- Henne V., Piiper A., Söling H. D. Inositol 1,4,5-trisphosphate and 5'-GTP induce calcium release from different intracellular pools. FEBS Lett. 1987 Jun 22;218(1):153–158. doi: 10.1016/0014-5793(87)81037-2. [DOI] [PubMed] [Google Scholar]
- Henne V., Söling H. D. Guanosine 5'-triphosphate releases calcium from rat liver and guinea pig parotid gland endoplasmic reticulum independently of inositol 1,4,5-trisphosphate. FEBS Lett. 1986 Jul 7;202(2):267–273. doi: 10.1016/0014-5793(86)80699-8. [DOI] [PubMed] [Google Scholar]
- Keller P. M., Person S., Snipes W. A fluorescence enhancement assay of cell fusion. J Cell Sci. 1977 Dec;28:167–177. doi: 10.1242/jcs.28.1.167. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nicchitta C. V., Joseph S. K., Williamson J. R. Polyethylene glycol-stimulated microsomal GTP hydrolysis. Relationship to GTP-mediated Ca2+ release. FEBS Lett. 1986 Dec 15;209(2):243–248. doi: 10.1016/0014-5793(86)81120-6. [DOI] [PubMed] [Google Scholar]
- Paiement J. Physiological concentrations of GTP stimulate fusion of the endoplasmic reticulum and the nuclear envelope. Exp Cell Res. 1984 Apr;151(2):354–366. doi: 10.1016/0014-4827(84)90386-0. [DOI] [PubMed] [Google Scholar]
- Paiement J., Rindress D., Smith C. E., Poliquin L., Bergeron J. J. Properties of a GTP sensitive microdomain in rough microsomes. Biochim Biophys Acta. 1987 Mar 26;898(1):6–22. doi: 10.1016/0005-2736(87)90105-2. [DOI] [PubMed] [Google Scholar]
- Ueda T., Chueh S. H., Noel M. W., Gill D. L. Influence of inositol 1,4,5-trisphosphate and guanine nucleotides on intracellular calcium release within the N1E-115 neuronal cell line. J Biol Chem. 1986 Mar 5;261(7):3184–3192. [PubMed] [Google Scholar]
- Vanderwerf P., Ullman E. F. Monitoring of phospholipid vesicle fusion by fluorescence energy transfer between membrane-bound dye labels. Biochim Biophys Acta. 1980 Feb 28;596(2):302–314. doi: 10.1016/0005-2736(80)90363-6. [DOI] [PubMed] [Google Scholar]
- Wolf B. A., Florholmen J., Colca J. R., McDaniel M. L. GTP mobilization of Ca2+ from the endoplasmic reticulum of islets. Comparison with myo-inositol 1,4,5-trisphosphate. Biochem J. 1987 Feb 15;242(1):137–141. doi: 10.1042/bj2420137. [DOI] [PMC free article] [PubMed] [Google Scholar]


