Abstract
Purpose
Transarterial radioembolization (TARE) has emerged as a promising therapeutic approach for unresectable intrahepatic cholangiocarcinoma (ICCA). We updated our previous meta-analysis with meta-regression to explore the efficacy of TARE in the context of ICCA.
Methods
We searched PubMed and Scopus for studies published up to September 1, 2023. The primary outcome was overall survival. Secondary outcomes were tumor overall response rate, severe adverse events, and downstaging to surgery. Meta-analysis employed a random-effects model, and meta-regression was utilized to explore sources of heterogeneity.
Results
We included 27 studies, involving 1365 patients. Pooled survival estimates at 1, 2, and 3 years were 52.6%, 27%, and 16.8%, respectively. Meta-regression revealed that the proportion of patients naïve to treatment was the only pre-TARE predictor of survival (1-, 2-, and 3-year survival of 70%, 45%, and 36% for treatment-naïve patients, mean survival 19.7 months vs. 44%, 18%, and 7% for non-naïve patients, mean survival 12.2 months). Overall response according to RECIST 1.1 and mRECIST was 19.6% and 67%, respectively. Effective downstaging to surgery was possible in varying rates (3–54%); the mean survival in these patients was 34.8 months (1-, 2-, and 3-year survival of 100%, 87%, and 64%). About 45.7% of patients experienced adverse events, but only 5.9% were severe.
Conclusions
Our study benchmarked the survival rates of patients undergoing TARE for unresectable ICCA and showed that this is a valid option in these patients, especially if naïve to previous treatments. Downstaging to surgery is feasible in selected patients with promising results.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s00270-024-03825-7.
Keywords: Radioembolization, Cholangiocarcinoma, Selective internal radiation therapy, Meta-analysis, Meta-regression
Introduction
Intrahepatic cholangiocarcinoma (ICCA) is a rare and aggressive type of liver cancer that arises from the bile ducts within the liver. ICCA ranks as the second most prevalent primary liver cancer, following hepatocellular carcinoma, accounting for less than 10% of cholangiocarcinomas [1, 2], but its incidence is rising. At present, hepatic resection represents the only potentially curative option, presenting a 10% chance of survival and disease-free status a decade post-treatment [3]. However, only 30–40% of ICCAs are diagnosed early enough to qualify for a curative resection. In unresectable ICCAs, the prognosis is poor, but several treatment options are available [4].
Chemotherapy is often the first line of treatment for inoperable ICCA. The combination of gemcitabine and cisplatin has been shown to offer some benefit in terms of tumor shrinkage and symptom relief, but the survival benefit and response rates are limited. The median progression-free survival with this regimen is merely 8 months, with an overall median survival of less than a year in more advanced cases [5, 6].
“TOPAZ-1” is the first phase 3 trial to demonstrate the benefit of immunotherapy, in particular durvalumab, in patients with biliary tract cancer reporting 24-month overall survival rate of 24.9% [7]. The introduction of immunotherapy represents a revolutionary treatment modality which might change the landscape of ICCA management, but more data from clinical trials are eagerly awaited [8–11].
Such dire survival statistics have driven specialists toward exploring multimodal and combined therapeutic approaches. Among these, intra-arterial therapies (IATs), such as transarterial chemoembolization (TACE) and transarterial radioembolization (TARE), have emerged as promising strategies.
Our previous meta-analysis [12], which included only nine studies, revealed encouraging results for patients with unresectable ICCA undergoing TARE, showing 1-, 2-, and 3-year pooled survival rates of 55.7%, 33.1%, and 20.2%, respectively. While these results were promising, recent studies have emerged over the past few years that further support and strengthen these findings. Notably, the phase 2 clinical trial conducted by Edeline et al. [13] emphasized the benefit of a combined approach involving first-line chemotherapy alongside TARE. This combination enabled downstaging to surgery in a significant proportion (22%) of patients, and the median overall survival was 22 months. These results support the inclusion of TARE in the treatment flowchart for patients with ICCA. However, the role of locoregional therapies in the guidelines remains unclear, as evidenced by the 2023 guidelines from the European Association for the Study of the Liver (EASL) [14], and the recommendation to support their use is weak.
We aimed to update our previous meta-analysis with meta-regression [12] and provide new benchmarks for the survival rates after TARE in patients with unresectable ICCA.
Secondary aims were to (i) evaluate the impact of patients’ and treatment’s characteristics on survival through meta-regression analysis and (ii) assess rates of tumor response and successful downstaging leading to surgical intervention and their impact on the primary outcome.
Methods
Literature Search Strategy
A systematic exploration of articles on radioembolization for ICCA, published until July 31, 2023, has been conducted using PubMed and Scopus databases. There were no restrictions on the starting date of the articles included in the search. The meta-analysis adhered to both the guidelines outlined in the Meta-analysis of Observational Studies in Epidemiology and the PRISMA guidelines. For further information and specifics, refer to the Supplementary Material 1.
Literature Screening and Inclusion Criteria
One author (MA.C) initially conducted a screening process to eliminate articles deemed irrelevant based on title, abstract, and publication keywords. The selection of studies proceeded through three levels of screening, as outlined in the Supporting Information. The final inclusion criteria of studies were: (i) a study population comprising patients treated for ICCA with TARE; (ii) a detailed description of the study population included in the studies; and (iii) availability of patient survival rate descriptions for at least 1-year post-TARE. In cases where a subsequent study provided a more comprehensive dataset or included the original dataset, the most recent and comprehensive report was chosen. These linked studies were identified based on authorship, institutions, design, length of follow-up, and study populations. If additional data or results were required, the corresponding author of each report was contacted via email. Any discrepancies in inclusion were resolved through discussions between the reviewers and a third investigator (C.M.).
Data Extraction and Quality Assessment
We extracted the following data according to a pre-specified sheet: study period and location, study design, study size, patients’ characteristics (age, gender, and performance status), tumor characteristics (burden, extension, multifocality, extrahepatic dissemination, and infiltrative pattern), treatment characteristics (previous treatments, concomitant chemotherapy, and type of microspheres), and clinical outcomes (adverse events, tumor response, downstaging to surgery, and overall survival). Tumor response rate was evaluated according to the response evaluation criteria in solid tumors (RECIST 1.1) criteria [15] and modified mRECIST criteria [16]; overall response rate was defined as complete + partial response rate; downstaging to surgery, refers to tumor shrinkage to satisfy the surgical criteria for resectability. Chemotherapy data were collected when retrieved studies clearly described that it was administered in addition to/after TARE. The quality of each selected study was assessed by two investigators (MA.C. and E.D.) through the Cochrane tool (RoB-2) [17] for randomized controlled trial (RCTs) and the Newcastle–Ottawa scale (NOS) for observational studies [18]. Any divergences were resolved by discussion between reviewers and a third investigator (C.M.).
Statistical Analysis
The primary outcome measure for the meta-analysis was overall survival after the first TARE procedure. Secondary outcomes measures were considered: (i) tumor overall response rate according to RECIST 1.1 and mRECIST criteria and (ii) the rate of patient undergoing surgery after successful downstaging of the disease.
Demographical characteristics and available clinical and tumor features were pooled together to obtain a description of the joint study population. Dichotomous variables, including survival rates, were estimated as pooled binomial proportions with 95% of confidence interval (C.I.) applying the Freeman-Tukey double arcsine transformation to retain studies with proportions at 0 or 1 margins and ensuring admissible confidence intervals for the pooled proportions. Continuous variables were pooled in weighted means with 95% C.I.; when studies reported this variable as median and range, the mean and variance were estimated as proposed by Wan et al. [19]. Studies were not weighted for their quality. The primary survival endpoints were fixed at 1, 2, and 3 years from TARE. Since most reports did not provide the number of patients at risk or tick-marks on Kaplan–Meier curves for censoring events, we were forced to assume it as a binomial proportion from survival rates as proposed by Tierney et al. [20]. Moreover, we pooled in summary mean survival from TARE both in the overall population and predicted its value through the meta-regression analysis. Statistical heterogeneity was explored by inconsistency (I2) statistics; the heterogeneity was considered substantial if I2 > 50% [21, 22]. Since the present meta-analysis was based on studies not identical in their methods and/or the characteristics of the included patients, a meta-regression analysis that included available covariates was performed. Covariates to be tested were selected on the basis of their clinical likelihood to modify the primary outcome measures and their presence in the selected literature. All comparisons were made by the random-effects model of DerSimonian and Laird [23], if not specified otherwise. Two-sided p < 0.050 were considered statistically significant. Meta-analysis and meta-regression were performed using the packages “meta” and “metafor” for R-Project 4.1.1.
Results
Results of the Literature Search
A total of 832 articles were initially identified based on our search criteria for screening (Fig. 1). After applying the exclusion criteria, 32 studies were selected following a thorough assessment of the full manuscripts. Five studies were excluded for overlapping cohorts. Consequently, the final list of included studies comprised 27 reports and 1365 patients (Table 1) [13, 24–49]. The quality of the included studied was deemed to be sufficient.
Fig. 1.
Literature search used in the present analysis outlining the included and excluded studies
Table 1.
Summary of studies included in the meta-analysis
| Author (year) | Study design | Nr | Type of microspheres | Enrollment period | Survival (months) | NOS/RoB-2 | |
|---|---|---|---|---|---|---|---|
| Saxena et al. (2010) [24] | Single center, prospective | 25 | Resin | Jan 2004 | May 2009 | 20 | 7 |
| Hoffmann et al. (2012) [25] | Single center, retrospective | 33 | Resin | Apr 2007 | Jan 2010 | 20 | 6 |
| Rafi et al. (2013) [26] | Single center, prospective | 19 | Resin | Dec 2002 | Oct 2010 | 12 | 8 |
| Camacho et al. (2014) [27] | Single center, prospective | 21 | Resin | Jan 2009 | Dec 2012 | 16 | 7 |
| Filippi et al. (2015) [28] | Single center, prospective | 17 | Resin | N/A | N/A | 16 | 7 |
| Soydal et al. (2016) [29] | Single center, retrospective | 16 | Resin | Jan 2008 | Dec 2014 | 10 | 7 |
| Shaker et al. (2018) [30] | Single center, retrospective | 17 | Resin, glass | Jan 2006 | Dec 2016 | 34 | 6 |
| Bourien et al. (2018) [32] | Single center, retrospective | 64 | Glass | Aug 2010 | Oct 2016 | 16 | 6 |
| Reimer et al. (2018) [31] | Single center, retrospective | 21 | Resin | Jan 2005 | Nov 2016 | N/A | 8 |
| Gangi et al. (2018) [33] | Single center, retrospective | 85 | Glass | May 2009 | May 2016 | 12 | 8 |
| Levillain et al. (2019) [34] | Multicenter, retrospective | 58 | Resin | Jan 2004 | Sep 2018 | 10 | 7 |
| White et al. (2019) [35] | Multicenter, prospective | 61 | Resin, glass | Dec 2013 | Feb 2017 | 9 | 8 |
| Edeline et al. (2020) [13] | Multicenter, prospective | 41 | Glass | Nov 2013 | Jun 2016 | 22 | Low |
| Bargellini et al. (2020) [37] | Multicenter, retrospective | 81 | Resin | Jul 2008 | Oct 2017 | 14,5 | 7 |
| Buettner et at. (2020) [38] | Multicenter, retrospective | 115 | Resin, glass | Jun 2006 | Feb 2017 | 11 | 6 |
| Koehler et al. (2020) | Multicenter, retrospective | 46 | Resin | N/A | N/A | 9,5 | 7 |
| Sarwar et al. (2021) [40] | Single center, retrospective | 31 | Resin | Oct 2015 | Sep 2020 | 22 | 8 |
| Paprottka et al. (2021) [42] | Single center, retrospective | 73 | Resin | N/A | N/A | 14 | 7 |
| Cheng et al. (2021) [41] | Single center, retrospective | 38 | Resin, glass | Jan 2013 | Dec 2018 | 11 | 6 |
| Paz-Fumagalli et al. (2021) [39] | Single center, retrospective | 28 | Glass | May 2016 | Feb 2020 | N/A | 7 |
| Robinson et al. (2022) [45] | Multicenter, prospective | 94 | Resin | Jul 2015 | Aug 2020 | 14 | 8 |
| Gupta et al. (2022) [46] | Single center, retrospective | 136 | Glass | Jun 2004 | Jan 2020 | 14 | 8 |
| Schatka et al. (2022) [44] | Single center, retrospective | 39 | Resin | Jan 2009 | Dec 2016 | 8 | 7 |
| Kumar et al. (2022) [43] | Single center, retrospective | 16 | Glass | May 2009 | Oct 2019 | 7 | 8 |
| Ahmed et al. (2023) [47] | Single center, retrospective | 13 | Glass | Dec 2018 | May 2021 | 29 | 6 |
| Schaarschmidt et al. (2023) [49] | Multicenter, retrospective | 128 | Resin, glass | May 2007 | May 2021 | 12 | 8 |
| Mosconi et al. (2023) [48] | Single center, retrospective | 49 | Resin | Jan 2016 | Jun 2021 | 16 | 8 |
NOS; Newcastle–Ottawa scale, RoB; risk of bias
Characteristics of the Included Studies
Seven studies had a prospective design, while the remaining 20 were retrospective. Eight studies were multicenter, of which three were both multicenter and prospective. Fifteen studies used resin microspheres [24–29, 31, 34, 36, 37, 40, 42, 44, 45, 48], seven used glass microspheres [13, 32, 33, 39, 43, 46, 47], and the remaining five used both [30, 35, 38, 41, 49]. The number of included patients ranged from 13 [47] to 136 [46]. The inclusion criteria varied among studies: four studies [13, 31, 39, 47] included only patients naïve to treatment, six studies included only patients previously treated with (and mostly refractory to) chemotherapy [25–27, 34, 41, 42], and the others included both with a rate of treatment-naïve patients varying from 8% [35] to 77% [40].
Characteristics of Included Patients
The pooled study cohort comprised 1365 individual patients with unresectable ICCA who underwent TARE. We summarized the results of the meta-analysis regarding demographic, clinical, and tumor characteristics in Table 2. Briefly, summary mean age was 64.2 (95% CI 62.6–65.9) years, and summary proportion of men was 49.2% (95% CI 45.8–52.6%). The tumor was bilobar (47.4%, 95% CI 39.4–55.4%) and multifocal (53.5%, 95% CI 45.5–61.4%). Extrahepatic disease, consisting mostly of lymph node metastases, was presented in a summary proportion of 36.2% (95%-29.6–43.4%). In the subgroup of studies (n = 13) distinguishing between mass-forming and infiltrative pattern ICCA, the summary proportion of the latter type was 38.4% (95% CI 16.8–65.8%). Noteworthy, the rate of patients naïve to treatment, intended as surgery or any IAT, was 25% (95% CI 8.2–55.7%) and 48.8% (95% CI 24.9–73.3%) received concomitant chemotherapy.
Table 2.
Pooled analysis of clinical features over study population submitted to radioembolization
| Variable | Number of studies | Weighted analysis (95% CI) | I2 (%) |
|---|---|---|---|
| Patient characteristics | |||
| Age (years) | 25 | 64.2 (62.6–65.9) | 84.2 |
| Male (%) | 27 | 49.2 (45.8–52.6) | 32.4 |
| Performance status < 2 (%) | 22 | 92.3 (83–96.7) | 75.7 |
| Naïve to treatment (%) | 27 | 25 (8.2–55.7) | 71.9 |
| Tumor characteristics | |||
| Burden > 25% | 11 | 46 (38–54.3) | 69.3 |
| Bilobar (%) | 23 | 47.4 (39.4–55.4) | 74.8 |
| Multifocal (%) | 19 | 53.5 (45.5–61.4) | 79.1 |
| Infiltrative pattern (%) | 13 | 38.4 (16.8–65.8) | 75.6 |
| Extrahepatic disease (%) | 26 | 36.2 (29.6–43.4) | 71.8 |
| Lymph node metastases (%) | 22 | 23.5 (17.1–31.3) | 82.4 |
| Distant metastases (%) | 22 | 3.7 (1.2–10.5) | 18.1 |
| Treatment and follow-up data | |||
| Use of glass microspheres | 27 | 40.2 (37.7–42.9)* | 67.7 |
| Any adverse events | 21 | 45.7 (26.2–66.6) | 91.4 |
| Severe (grade ≥ 3) adverse events | 21 | 5.9 (3–11.2) | 76.5 |
| Concomitant chemotherapy | 22 | 48.8 (24.9–73.3) | 89 |
| Objective response (RECIST 1.1) | 20 | 19.6 (13.6–27.3) | 80.3 |
| Objective response (mRECIST) | 4 | 67 (57.2–75.5)* | 79 |
| Downstage to surgery | 27 | 4.9 (3.9–6.2)* | 48.7 |
*Fixed-effect analysis
CI; confidence intervals, RECIST 1.1; response evaluation criteria in solid tumors, mRECIST; modified RECIST
Primary Outcome: Overall Survival
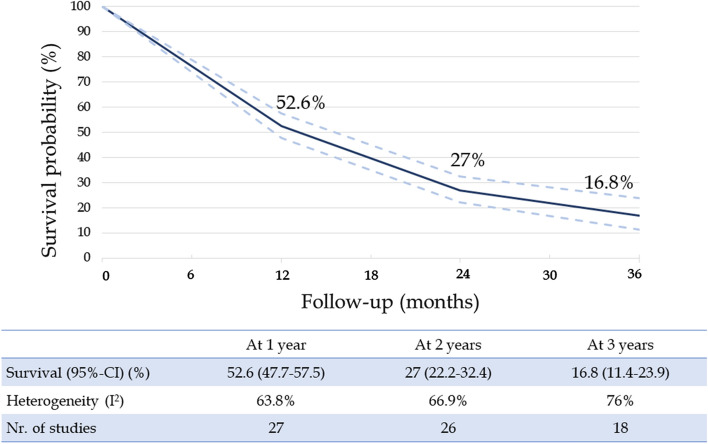
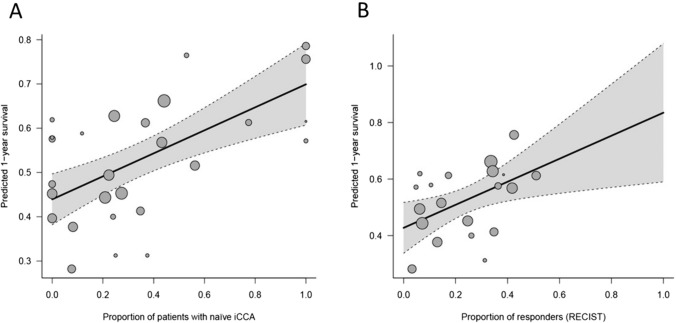
The summary survival estimates for 1-, 2-, and 3-year intervals were determined to be 52.6%, 27%, and 16.8%, respectively (Fig. 2), with a mean survival of 15.8 (95% CI 10.9–18.5) months after TARE and of 29 (95% CI 22–33.4) months after diagnosis. However, the heterogeneity between the studies was substantial (> 50%) for all these outcomes. Therefore, we conducted a meta-regression analysis to assess the influence of studies’ and patients’ characteristics on survival. (Table 3). These analyses identified the proportion of patients naïve to treatment as the sole pre-treatment determinant of survival (p < 0.001 for all three fixed timepoints) (Fig. 3A); the predicted 1-, 2-, and 3-year survival rates were 70%, 45%, and 36% in treatment-naïve patients and 44%, 18%, and 7% in patients receiving previous treatments. As extreme values at meta-regression, the precited mean survival of treatment-naïve ICCA patients was 19.7 (95% CI 11.5–27.9) months and that of non-naïve patients was 12.2 (95% CI 4.7–19.7) months.
Fig. 2.
Meta-analysis results for patient survival after TARE
Table 3.
Results from univariable meta-regression over main outcome considered
| Variable | Nr. studies | 1-year survival OR (95% CI) | Residual I2 | Nr. studies | 2-year survival OR (95%C.I.) | Residual I2 | Nr. studies | 3-year survival OR (95% CI) | Residual I2 |
|---|---|---|---|---|---|---|---|---|---|
| Studies’ characteristics | |||||||||
| Publication year | 27 | 1.01 (0.95–1.07) | 66.4% | 26 | 0.99 (0.91–1.07) | 73.0% | 18 | 0.98 (0.86–1.12) | 81.0% |
| Prospective | 27 | 1.12 (0.67–1.86) | 66.7% | 26 | 0.77 (0.38–1.54) | 73.5% | 18 | 1.24 (0.37–4.16) | 81.0% |
| Patient characteristics | |||||||||
| Age (years) | 25 | 1.05 (0.99–1.1) | 64.4% | 24 | 1.06 (0.99–1.14) | 71.2% | 16 | 1.05 (0.94–1.18) | 77.1% |
| Male (%) | 27 | 3.83 (0.54–26.96) | 62.8% | 26 | 6 (0.93–72.39) | 70.5% | 18 | 3.57 (0.06–203.57) | 81.2% |
| Performance status < 2 (%) | 22 | 0.55 (0.16–1.55) | 60.3% | 21 | 0.57 (0.17–1.96) | 58.8% | 14 | 0.98 (0.03–31.12) | 66.0% |
| Naïve to treatment (%) | 27 | 2.79 (1.64–4.77) | 43.7% | 27 | 3.27 (1.76–6.07) | 53.5% | 18 | 5.85 (2.25–15.23) | 62.6% |
| Tumor characteristics | |||||||||
| Burden > 25% | 11 | 0.31 (0.04–2.6) | 63.6% | 10 | 0.75 (0.04–14.03) | 66.2% | 7 | 0.26 (0.01–10.75) | 36.8% |
| Bilobar (%) | 23 | 0.58 (0.16–2.03) | 66.2% | 22 | 0.54 (0.12–2.52) | 71.3% | 15 | 0.33 (0.03–3.85) | 81.4% |
| Infiltrative pattern (%) | 13 | 0.44 (0.16–1.2) | 58.9% | 12 | 0.45 (0.13–1.5) | 64.2% | 8 | 0.25 (0.03–2.18) | 71.4% |
| Extrahepatic disease (%) | 26 | 0.5 (0.14–1.76) | 65.0% | 25 | 0.62 (0.12–3.33) | 74.3% | 17 | 1.14 (0.06–23.48) | 82.7% |
| Lymph node metastases (%) | 22 | 0.47 (0.12–1.94) | 68.5 | 21 | 1.21 (0.18–8.08) | 78.4% | 15 | 0.59 (0.02–19.81) | 84.7% |
| Distant metastases (%) | 22 | 1.27 (0.16–10.21) | 70.8% | 21 | 0.54 (0.04–7.6) | 78.0% | 15 | 2.06 (0.03–152.78) | 84.6% |
| Treatment data | |||||||||
| Use of glass microspheres | 27 | 1.4 (0.89–2.19) | 63.3% | 26 | 1.48 (0.84–2.61) | 71.5% | 18 | 2.36 (0.99–5.59) | 76.0% |
| Concomitant chemotherapy | 22 | 1.63 (0.77–3.46) | 70.3% | 21 | 2.19 (0.88–5.42) | 73.0% | 14 | 2.38 (0.53–10.72) | 79.0% |
*Indicates p < 0.05
CI; confidence interval; OR; odds ratio
Fig. 3.
Meta-regression results for patient survival at 1 year after TARE. This figure shows how 1-year survival rates reported in the retrieved literature were influenced by the proportion of patients naïve to treatment (Panel A) and patients achieving overall response according to RECIST 1.1 criteria (Panel B)
Secondary Outcomes: Tumor Response and Downstaging to Surgery
Tumor response was evaluated with imaging (CT and MR) according to RECIST 1.1 and mRECIST in 20 and four studies, respectively. The summary proportion of patients achieving objective response according to RECIST 1.1 criteria rate was 19.6% (95% CI 13.6–27.3%), and it was associated with an improved 1-year survival at meta-regression analysis (OR 5.01, 95% CI 1.43–17.6, p-value = 0.01) (Fig. 3B). In responders, the predicted 1-, 2- and 3-year survival rates were 83%, 51%, and 37% and the predicted mean survival was 23.8 (95% CI 10.6–36.9) months. In non-responders, these rates dropped to 43%, 18%, and 8% and the predicted mean survival was 11.6 (95% CI 2.6–20.6) months. At meta-regression analysis, no pre-treatment variable (type of microspheres and concomitant chemotherapy) was associated with the objective rate (data not shown).
The summary proportion of patients achieving objective response according to mRECIST criteria was 67% (95% CI 57.2–75.5%), and it was also associated with increased 1-year survival (p = 0.03). The precited 1-, 2-, and 3-year survival rates in responders were 81%, 63%, and 57% in responders (mean survival 20.1 months) and 16%, 0%, and 0% in non-responders (mean survival 4.8 months).
Finally, we evaluated the rate of successful downstage to surgery, which was 0% in 16 studies, and it ranged from 3% [41] to 54% [47] in the other studies (summary proportion 4.9%, 95% CI 3.9%-6.2%). At meta-regression analysis, the increasing proportion of patients successfully downstaged to surgery (i.e., hepatic resection) was associated with increased 1-year survival (OR 8.25, 95% CI 1.58–42.91, p = 0.01). At extreme values, the predicted 1-, 2-, and 3-year survival in patients undergoing surgery was estimated 100%, 87%, and 64%, with a predicted mean survival of 34.8 (95% CI 20–49.6) months.
Discussion
This updated meta-analysis benchmarked the prognosis of patients with ICCA undergoing TARE; the summary overall survival estimates at 1-, 2-, and 3-years after TARE were, respectively, 53%, 27%, and 17%, and the mean survival was 15.8 (95% CI 10.9–18.5) months. These estimates confirm our preliminary findings in a larger sample (27 vs. 9 studies) and more importantly are relatively higher than the survival rates of patients undergoing chemotherapy (10.9 months, 95% CI 9.9–11.6) or immunotherapy (12.7 months, 95% CI 11.5–13.6) according to a recent phase 3 trial on immunotherapy in patients with unresectable biliary tract cancer (60% of enrolled patients had ICCA) [7]. Conversely, the TOPAZ-1 trial [50] included patients with different types of cholangiocarcinoma and at a more advanced stage, and this clearly influences the differences in survival found between systemic therapy and TARE.
We observed that the assessed clinical outcomes exhibited substantial between-studies heterogeneity (> 50%). To elucidate the sources of this heterogeneity, we conducted a comprehensive meta-regression analysis, examining the potential influence of both study and patient characteristics on overall survival. We found that the proportion of patients naïve to treatment emerged as the sole pre-treatment determinant significantly impacting survival (p < 0.001 for all three fixed timepoints). Predicted survival rates at 1-, 2-, and 3-year intervals underscored this distinction, with rates of 70%, 45%, and 36% in treatment-naïve patients and with an estimated mean survival as high as 19.7 months (95% CI 11.5–27.9) compared to 44%, 18%, and 7% in those who had received previous treatments (summary mean survival 12.2 (95% CI 4.7–19-7) months). These findings provide critical insights for clinical decision making and rationale of treatment combinations. Of note, in the only phase 2 RCT trial evaluating TARE in patients with ICCA naïve to treatment and receiving concomitant chemotherapy [13], the median overall survival was 22 months (95% CI 14–52), with overall survival rates of 75% at 1 year and 45% at 2 years. These results are strikingly similar to our estimates in treatment-naïve patients, so our study provides real-life evidence supporting the survival benefit of this strategy and its implementation in clinical practice. For this reason, candidate selection of TARE is a crucial aspect. This point involves tumor burden, hepatic function, extrahepatic disease, and overall health. Literature indicates that chemotherapy and the latest systemic treatments alone have demonstrated lower survival rates compared to TARE in treatment-naïve patients [45, 51]. Despite the heterogeneity of patient populations in these studies, which may partially impact outcomes, the higher efficacy of radioembolization in terms of survival in treatment-naïve patients could be attributed to better local tumor control, stimulation of tumor-specific immune responses by releasing tumor antigen, and a higher rate of unresectable lesions being downstaged to surgery [51, 52]. In fact, recent observational studies have focused on the combination of TARE with systemic therapy. Reimer et al. [53] reported that patients who received TARE and concomitant systemic therapy showed better results in overall survival, progression-free survival (PFS), and hepatic PFS compared to treatment-naïve patients or those who received one or more cycles of chemotherapy. These results were similar in the RESiN study [45] for concomitant chemotherapy, while studies on immunotherapy plus TARE are lacking in the literature to our knowledge. Moreover, in our previous meta-analysis [12], we identified treatment-naïve patients with mass-forming ICC as the best candidates for TARE, rather than patients with infiltrative ICC or those who had undergone cycles of chemotherapy.
Regarding the choice of optimal TARE technique, in our meta-analysis, both types of microspheres (resin and glass microsphere) were used in included trials, and no difference was found in terms of prognosis. It is proved that they had different cutoffs of delivered dose for tumor target and liver, but this does not influence survival rates or toxicity [38, 41, 54].
Within the realm of IAT, both TACE and TARE are viable options for treating ICCA within IAT. Conventional TACE (c-TACE) and drug-eluting beads TACE (DEB-TACE) are two modalities, with DEB-TACE possibly offering better tumor response and disease control, though its impact on overall survival is unclear [55]. The choice between TACE and TARE for unresectable ICCA is debated, as the median survival rates are similar. But, TARE was associated with a lower rate of adverse events than TACE. [56, 57]. Regarding the safety profile, TARE is confirmed as a well-tolerated treatment for cholangiocarcinoma, with frequent mild side effects, such as temporary nausea, vomiting, and abdominal pain. Our analysis indicates that adverse events occur in 45.7% of cases, but only 5.9% of these are severe. While severe side effects are rare, the potential for complications like radiation-induced liver and lung disease and non-target gastrointestinal embolization underscores the importance of patient selection, comprehensive pre-procedural planning, and rigorous post-procedural follow-up.
In examining secondary outcomes, our analysis delved into tumor response and downstaging to surgery. These analyses were not feasible in the previous meta-analysis due to the limited number of studies reporting the data and therefore represent a novel finding of our study.
According to RECIST 1.1 criteria, one out five patients had an objective response. We could not identify pre-treatment factors associated with this outcome, but we were able to confirm the prognostic value of such definition and the survival benefit it confers. Responders within this category exhibited a predicted mean survival of 23.8 (95% CI 10.6–36.9) months (vs. 11.6 months, 95% CI 2.6–20.6, in non-responders), and the predicted 1-, 2-, and 3-year survival rates were 83%, 51%, and 37%. On the other hand, two-thirds of the patients achieved an objective response according to mRECIST criteria; responders displayed predicted 1-, 2-, and 3-year survival rates of 81%, 63%, and 57%, with a mean survival of 20.1 months. However, the number of studies reporting this information was limited (n = 4), so these data should be interpreted with caution. In light of these compelling results, there arises a pertinent question regarding the prognostic validation of the mRECIST criteria in comparison with RECIST 1.1. The data suggest that both criteria are valuable, but mRECIST might indicate an enhanced prognostic value for treatment outcomes and associated survival benefits. Future validation could potentially establish it as a more reliable tool for predicting patient outcomes.
Radioembolization shows promise as a transformative treatment for ICCA, potentially downstaging tumors to make them resectable and improve survival rates. However, the success of downstaging varies (3–54%), with a large heterogeneity across centers. This estimate might be understated since, in some studies, TARE was offered as a palliative therapy after multiple chemotherapy failures, making downstaging neither an aim nor a possibility. Nevertheless, survival data are very promising: The predicted mean survival was 34.8 months, with 1-, 2-, and 3-year survival rates of 100%, 87%, and 64%, respectively. Despite data heterogeneity and preliminary findings, downstaging to surgery remains a significant predictor of improved survival.
Our study has many limitations: First, there was a high heterogeneity among the baseline clinical and tumor features of the patients included in the retrieved studies. The heterogeneity remained substantial even after the meta-regression analysis. This likely mirrors the inherent diversity within the group of patients subjected to various previous treatments, encompassing surgical interventions, locoregional therapies, and the number of failed chemotherapy lines, among other factors. The profound differences in patient profiles, such as those undergoing radioembolization for post-surgical recurrence versus those opting for TARE due to progression after exhausting all available chemotherapy lines, contribute significantly to this heterogeneity. The heterogeneity complicates the interpretation of long-term outcomes across published experiences, posing challenges for comparing TARE outcomes with standard care and selecting the most appropriate treatment beyond established guidelines. Future research efforts may benefit from further refinement of patient categorization and increased granularity in data collection to address these inherent limitations.
In conclusion, out meta-analysis benchmarked the survival outcomes post-TARE across various clinical contexts. The results suggest that treatment-naïve ICCA patients, especially when assessed with mRECIST criteria, exhibit the most favorable outcomes, indicating promising downstaging effects and providing new possibilities for managing inoperable ICCA. Since the introduction of immunotherapy will revolutionize the management of patients with advanced biliary tract cancer, future studies should investigate the benefit of combining immunotherapy with TARE:
Supplementary Information
Below is the link to the electronic supplementary material.
Funding
Open access funding provided by Alma Mater Studiorum - Università di Bologna within the CRUI-CARE Agreement. This study was not supported by any funding.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
For this type of study, formal consent is not required.
Informed Consent
For this type of study, informed consent is not required.
Consent for Publication
For this type of study, consent for publication is not required.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Maria Adriana Cocozza and Elton Dajti have contributed equally to preparing this manuscript and shared co-first authorship.
References
- 1.Qurashi M, Vithayathil M, Khan SA. Epidemiology of cholangiocarcinoma. Eur J Surg Oncol. 2023. 10.1016/j.ejso.2023.107064. [DOI] [PubMed] [Google Scholar]
- 2.Vithayathil M, Khan SA. Current epidemiology of cholangiocarcinoma in Western countries. J Hepatol. 2022;77:1690–8. [DOI] [PubMed] [Google Scholar]
- 3.Spolverato G, Vitale A, Cucchetti A, Popescu I, Marques HP, Aldrighetti L, et al. Can hepatic resection provide a long-term cure for patients with intrahepatic cholangiocarcinoma? Cancer. 2015;121:3998–4006. [DOI] [PubMed] [Google Scholar]
- 4.Bartolini I, Risaliti M, Fortuna L, Agostini C, Ringressi MN, Taddei A, et al. Current management of intrahepatic cholangiocarcinoma: from resection to palliative treatments. Radiol Oncol. 2020;54:263–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–81. [DOI] [PubMed] [Google Scholar]
- 6.Krenzien F, Nevermann N, Krombholz A, Benzing C, Haber P, Fehrenbach U, et al. Treatment of intrahepatic cholangiocarcinoma—a multidisciplinary approach. Cancers. 2022;14:362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oh D-Y, Ruth He A, Qin S, Chen L-T, Okusaka T, Vogel A, et al. Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer. NEJM Evid. 2022. 10.1056/EVIDoa2200015. [DOI] [PubMed] [Google Scholar]
- 8.Greten TF, Schwabe R, Bardeesy N, Ma L, Goyal L, elley RK, et al. Immunology and immunotherapy of cholangiocarcinoma. Nat Rev Gastroenterol Hepatol. 2023;20:349–65. [DOI] [PubMed] [Google Scholar]
- 9.Kelley RK, Bridgewater J, Gores GJ, Zhu AX. Systemic therapies for intrahepatic cholangiocarcinoma. J Hepatol. 2020;72:353–63. [DOI] [PubMed] [Google Scholar]
- 10.Kawamura E, Matsubara T, Kawada N. New era of immune-based therapy in intrahepatic cholangiocarcinoma. Cancers. 2023;15:3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu X, Green BL, Xie C, Liu C, Chen X. Preclinical and clinical studies of immunotherapy for the treatment of cholangiocarcinoma. JHEP Rep. 2023;5: 100723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cucchetti A, Cappelli A, Mosconi C, Zhong J, Cescon M, Pinna AD, et al. Improving patient selection for selective internal radiation therapy of intra-hepatic cholangiocarcinoma: a meta-regression study. Liver Int. 2017;37:1056–64. [DOI] [PubMed] [Google Scholar]
- 13.Edeline J, Touchefeu Y, Guiu B, Farge O, Tougeron D, Baumgaertner I, et al. Radioembolization plus chemotherapy for first-line treatment of locally advanced intrahepatic cholangiocarcinoma: a phase 2 clinical trial. JAMA Oncol. 2020;6:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alvaro D, Gores GJ, Walicki J, Hassan C, Sapisochin G, Komuta M, et al. EASL-ILCA clinical practice guidelines on the management of intrahepatic cholangiocarcinoma. J Hepatol. 2023;79:181–208. [DOI] [PubMed] [Google Scholar]
- 15.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47. [DOI] [PubMed] [Google Scholar]
- 16.Lencioni R, Llovet J. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:052–60. [DOI] [PubMed] [Google Scholar]
- 17.Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019. 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 18.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle–Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2013. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 19.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- 22.Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods. 2006;11:193–206. [DOI] [PubMed] [Google Scholar]
- 23.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- 24.Saxena A, Bester L, Chua TC, Chu FC, Morris DL. Yttrium-90 radiotherapy for unresectable intrahepatic cholangiocarcinoma: a preliminary assessment of this novel treatment option. Ann Surg Oncol. 2010;17:484–91. [DOI] [PubMed] [Google Scholar]
- 25.Hoffmann R-T, Paprottka PM, Schön A, Bamberg F, Haug A, Dürr E-M, et al. Transarterial hepatic yttrium-90 radioembolization in patients with unresectable intrahepatic cholangiocarcinoma: factors associated with prolonged survival. Cardiovasc Intervent Radiol. 2012;35:105–16. [DOI] [PubMed] [Google Scholar]
- 26.Rafi S, Piduru SM, El-Rayes B, Kauh JS, Kooby DA, Sarmiento JM, et al. Yttrium-90 radioembolization for unresectable standard-chemorefractory intrahepatic cholangiocarcinoma: survival, efficacy, and safety study. Cardiovasc Intervent Radiol. 2013;36:440–8. [DOI] [PubMed] [Google Scholar]
- 27.Camacho JC, Kokabi N, Xing M, Prajapati HJ, El-Rayes B, Kim HS. Modified response evaluation criteria in solid tumors and european association for the study of the liver criteria using delayed-phase imaging at an early time point predict survival in patients with unresectable intrahepatic cholangiocarcinoma following yttrium-90 radioembolization. J Vasc Interv Radiol. 2014;25:256–65. [DOI] [PubMed] [Google Scholar]
- 28.Filippi L, Pelle G, Cianni R, Scopinaro F, Bagni O. Change in total lesion glycolysis and clinical outcome after 90Y radioembolization in intrahepatic cholangiocarcinoma. Nucl Med Biol. 2015;42:59–64. [DOI] [PubMed] [Google Scholar]
- 29.Soydal C, Kucuk ON, Bilgic S, Ibis E. Radioembolization with 90Y resin microspheres for intrahepatic cholangiocellular carcinoma: prognostic factors. Ann Nucl Med. 2016;30:29–34. [DOI] [PubMed] [Google Scholar]
- 30.Shaker TM, Chung C, Varma MK, Doherty MG, Wolf AM, Chung MH, et al. Is there a role for Ytrrium-90 in the treatment of unresectable and metastatic intrahepatic cholangiocarcinoma? Am J Surg. 2018;215:467–70. [DOI] [PubMed] [Google Scholar]
- 31.Reimer P, Virarkar MK, Binnenhei M, Justinger M, Schön MR, Tatsch K. Prognostic factors in overall survival of patients with unresectable intrahepatic cholangiocarcinoma treated by means of yttrium-90 radioembolization: results in therapy-naïve patients. Cardiovasc Intervent Radiol. 2018;41:744–52. [DOI] [PubMed] [Google Scholar]
- 32.Bourien H, Palard X, Rolland Y, Le Du F, Beuzit L, Uguen T, et al. Yttrium-90 glass microspheres radioembolization (RE) for biliary tract cancer: a large single-center experience. Eur J Nucl Med Mol Imaging. 2019;46:669–76. [DOI] [PubMed] [Google Scholar]
- 33.Gangi A, Shah J, Hatfield N, Smith J, Sweeney J, Choi J, et al. Intrahepatic cholangiocarcinoma treated with transarterial yttrium-90 glass microsphere radioembolization: results of a single institution retrospective study. J Vasc Interv Radiol. 2018;29:1101–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levillain H, Duran Derijckere I, Ameye L, Guiot T, Braat A, Meyer C, et al. Personalised radioembolization improves outcomes in refractory intra-hepatic cholangiocarcinoma: a multicenter study. Eur J Nucl Med Mol Imaging. 2019;46:2270–9. [DOI] [PubMed] [Google Scholar]
- 35.White J, Carolan-Rees G, Dale M, Patrick HE, See TC, Bell JK, et al. Yttrium-90 transarterial radioembolization for chemotherapy-refractory intrahepatic cholangiocarcinoma: a prospective. Obs Study J Vasc Interv Radiol. 2019;30:1185–92. [DOI] [PubMed] [Google Scholar]
- 36.Köhler M, Harders F, Lohöfer F, Paprottka PM, Schaarschmidt BM, Theysohn J, et al. Prognostic factors for overall survival in advanced intrahepatic cholangiocarcinoma treated with yttrium-90 radioembolization. J Clin Med. 2019;9:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bargellini I, Mosconi C, Pizzi G, Lorenzoni G, Vivaldi C, Cappelli A, et al. Yttrium-90 radioembolization in unresectable intrahepatic cholangiocarcinoma: results of a multicenter retrospective study. Cardiovasc Intervent Radiol. 2020;43:1305–14. [DOI] [PubMed] [Google Scholar]
- 38.Buettner S, Braat AJAT, Margonis GA, Brown DB, Taylor KB, Borgmann AJ, et al. Yttrium-90 radioembolization in intrahepatic cholangiocarcinoma: a multicenter retrospective analysis. J Vasc Interv Radiol. 2020;31:1035-1043.e2. [DOI] [PubMed] [Google Scholar]
- 39.Paz-Fumagalli R, Core J, Padula C, Montazeri S, McKinney J, Frey G, et al. Safety and initial efficacy of ablative radioembolization for the treatment of unresectable intrahepatic cholangiocarcinoma. Oncotarget. 2021;12:2075–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sarwar A, Ali A, Ljuboja D, Weinstein JL, Shenoy-Bhangle AS, Nasser IA, et al. Neoadjuvant yttrium-90 transarterial radioembolization with resin microspheres prescribed using the medical internal radiation dose model for intrahepatic cholangiocarcinoma. J Vasc Interv Radiol. 2021;32:1560–8. [DOI] [PubMed] [Google Scholar]
- 41.Cheng B, Villalobos A, Sethi I, Wagstaff W, Galt J, Brandon D, et al. Determination of tumor dose response thresholds in patients with chemorefractory intrahepatic cholangiocarcinoma treated with resin and glass-based y90 radioembolization. Cardiovasc Intervent Radiol. 2021;44:1194–203. [DOI] [PubMed] [Google Scholar]
- 42.Paprottka KJ, Galiè F, Ingrisch M, Geith T, Ilhan H, Todica A, et al. Outcome and safety after 103 radioembolizations with yttrium-90 resin microspheres in 73 patients with unresectable intrahepatic cholangiocarcinoma—an evaluation of predictors. Cancers. 2021;13:5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kumar P, Mhaskar R, Kim R, Anaya D, Frakes J, Hoffe S, et al. Unresectable intrahepatic cholangiocarcinoma treated with radiation segmentectomy/lobectomy using yttrium 90-labeled glass microspheres. J Clin Exp Hepatol. 2022;12:1259–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schatka I, Jochens HV, Rogasch JMM, Walter-Rittel TC, Pelzer U, Benckert J, et al. Transarterial yttrium-90 radioembolization in intrahepatic cholangiocarcinoma patients: outcome assessment applying a prognostic score. Cancers. 2022;14:5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Robinson TJ, Du L, Matsuoka L, Sze DY, Kennedy AS, Gandhi RT, et al. Survival and toxicities after yttrium-90 transarterial radioembolization of cholangiocarcinoma in the RESiN registry. J Vasc Interv Radiol. 2023;34:694-701.e3. [DOI] [PubMed] [Google Scholar]
- 46.Gupta AN, Gordon AC, Gabr A, Kalyan A, Kircher SM, Mahalingam D, et al. Yttrium-90 radioembolization of unresectable intrahepatic cholangiocarcinoma: long-term follow-up for a 136-patient cohort. Cardiovasc Intervent Radiol. 2022;45:1117–28. [DOI] [PubMed] [Google Scholar]
- 47.Ahmed O, Yu Q, Patel M, Hwang G, Pillai A, Liao C, et al. Yttrium-90 radioembolization and concomitant systemic gemcitabine, cisplatin, and capecitabine as the first-line therapy for locally advanced intrahepatic cholangiocarcinoma. J Vasc Interv Radiol. 2023;34:702–9. [DOI] [PubMed] [Google Scholar]
- 48.Mosconi C, Cacioppa LM, Cappelli A, Gramenzi AG, Vara G, Modestino F, et al. Update of the Bologna experience in radioembolization of Intrahepatic cholangiocarcinoma. Technol Cancer Res Treat. 2023;22:153303382311556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schaarschmidt BM, Kloeckner R, Dertnig T, Demircioglu A, Müller L, Auer TA, et al. Real-life experience in the treatment of intrahepatic cholangiocarcinoma by 90 Y radioembolization: a multicenter retrospective study. J Nucl Med. 2023;64:529–35. [DOI] [PubMed] [Google Scholar]
- 50.Rimini M, Fornaro L, Lonardi S, Niger M, Lavacchi D, Pressiani T, et al. Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer: an early exploratory analysis of real-world data. Liver Int. 2023;43:1803–12. [DOI] [PubMed] [Google Scholar]
- 51.Edeline J, Du FL, Rayar M, Rolland Y, Beuzit L, Boudjema K, et al. Glass microspheres 90Y selective internal radiation therapy and chemotherapy as first-line treatment of intrahepatic cholangiocarcinoma. Clin Nucl Med. 2015;40:851–5. [DOI] [PubMed] [Google Scholar]
- 52.Oh D-Y, Lee K-H, Lee D-W, Yoon J, Kim T-Y, Bang J-H, et al. Gemcitabine and cisplatin plus durvalumab with or without tremelimumab in chemotherapy-naive patients with advanced biliary tract cancer: an open-label, single-centre, phase 2 study. Lancet Gastroenterol Hepatol. 2022;7:522–32. [DOI] [PubMed] [Google Scholar]
- 53.Reimer P, Vilgrain V, Arnold D, Balli T, Golfieri R, Loffroy R, et al. Factors impacting survival after transarterial radioembolization in patients with unresectable intrahepatic cholangiocarcinoma: a combined analysis of the prospective cirt studies. Cardiovasc Intervent Radiol. 2024;47:310–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bozkurt M, Eldem G, Bozbulut UB, Bozkurt MF, Kılıçkap S, Peynircioğlu B, et al. Factors affecting the response to Y-90 microsphere therapy in the cholangiocarcinoma patients. Radiol Med. 2021;126:323–33. [DOI] [PubMed] [Google Scholar]
- 55.He M, Jiang N, Yin X, Xu A, Mu K. Conventional and drug-eluting beads transarterial chemoembolization in patients with unresectable intrahepatic cholangiocarcinoma: a systematic review and pooled analysis. J Cancer Res Clin Oncol. 2023;149:531–40. [DOI] [PubMed] [Google Scholar]
- 56.Cocozza MA, Braccischi L, De Cinque A, Bruno A, Cappelli A, Renzulli M, et al. Unresectable intrahepatic cholangiocarcinoma: TARE or TACE, which one to choose? Front Gastroenterol. 2023;2:1270264. [Google Scholar]
- 57.Mosconi C, Solaini L, Vara G, Brandi N, Cappelli A, Modestino F, et al. Transarterial chemoembolization and radioembolization for unresectable intrahepatic cholangiocarcinoma—a systemic review and meta-analysis. Cardiovasc Intervent Radiol. 2021;44:728–38. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.