Abstract
Purpose
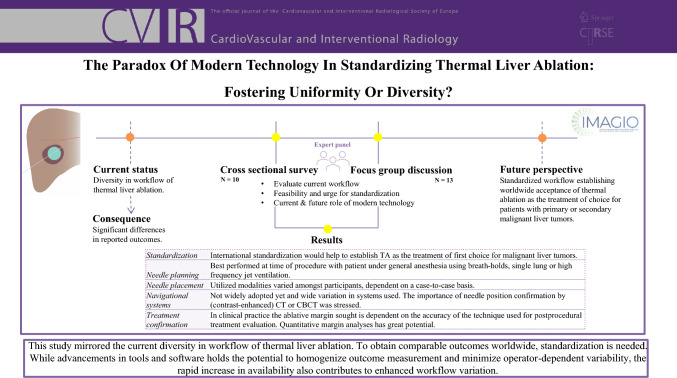
Currently, significant medical practice variation exists in thermal ablation (TA) of malignant liver tumors with associated differences in outcomes. The IMaging and Advanced Guidance for workflow optimization in Interventional Oncology (IMAGIO) consortium aims to integrate interventional oncology into the standard clinical pathway for cancer treatment in Europe by 2030, by development of a standardized low-complex-high-precision workflow for TA of malignant liver tumors. This study was conducted at the start of the IMAGIO project with the aim to explore the current state and future role of modern technology in TA of malignant liver tumors.
Materials and Methods
A cross-sectional questionnaire was conducted followed by an expert focus group discussion with core members and collaborating partners of the consortium.
Results
Of the 13 participants, 10 respondents filled in the questionnaire. During the focus group discussion, there was consensus on the need for international standardization in TA and several aspects of the procedure, such as planning based on cross-sectional images, the adoption of different techniques for needle placement and the importance of needle position- and post-ablative margin confirmation scans. Yet, also considerable heterogeneity was reported in the adoption of modern technology, particularly in navigational systems and computer-assisted margin assessment.
Conclusion
This study mirrored the current diversity in workflow of thermal liver ablation. To obtain comparable outcomes worldwide, standardization is needed. While advancements in tools and software hold the potential to homogenize outcome measurement and minimize operator-dependent variability, the rapid increase in availability also contributes to enhanced workflow variation.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s00270-024-03846-2.
Keywords: Thermal ablation, Liver tumor, Interventional oncology, Modern technology, Standardization
Introduction
Thermal ablation (TA) is an effective treatment for malignant liver tumors with clinical outcomes equivalent to surgical resection [1–5]. Yet, reported outcomes differ significantly between centers and countries, due to variability in patient selection, ablation technique and treatment evaluation [3, 6–8]. This hampers worldwide acceptance of ablation as treatment of first choice.
A multitude of developments in tools and software has become available [8–10]. The integration of these evolvements holds the potential to optimize technical success and clinical outcomes. However, the use of these techniques is still limited, mainly due to the imbalance of the additional costs and procedural benefits. Furthermore, various logistic and technical hurdles along with a certain conservatism among potential users hamper implementation in clinical practice.
An Innovative Health Initiative grant (No. 101112053) was rewarded to the ‘IMaging and Advanced Guidance for workflow optimization in Interventional Oncology’ (IMAGIO) consortium, consisting of 30 partners in academia, healthcare institutions and industry [11]. The purpose of the IMAGIO consortium is to make interventional oncology part of the standard clinical care pathway to cancer treatment in Europe by 2030. Among the main deliverables are to develop a standardized, accessible, and low-complexity-high-precision workflow for liver tumor TA and artificial intelligence algorithms to support patient selection, treatment planning, needle guidance and treatment evaluation.
At the start of the IMAGIO project, an international expert focus group discussion (FGD) was organized to evaluate the current and future role of modern technology in TA.
Materials and Methods
This was a mixed-method study comprising a semi-structured FGD preceded by a cross-sectional questionnaire.
Cross-sectional questionnaire:
Three weeks prior to the FGD, an online questionnaire was conducted comprising 15 questions, each accompanied by a rationale. The questions were designed by the FGD moderators (MB, CvL), also principal investigators in the IMAGIO project, and pertained to deliverables of the IMAGIO project (appendix A, Table 1).
Table 1.
Background of participants and respondents
| Participants, n (%) | Respondents, n (%) | |
|---|---|---|
| Total | 13 (100%) | 10 (100%) |
| Country | ||
| Austria | 1 (7.7%) | 1 (10%) |
| France | 1 (7.7%) | 1 (10%) |
| Italy | 4 (30.8%) | 2 (20%) |
| Netherlands | 6 (46.2%) | 5 (50%) |
| Switzerland | 1 (7.7%) | 1 (10%) |
| Medical specialty | ||
| Interventional radiologist | 12 (92.3%) | 10 (100%) |
| Hepatobiliary surgeon | 1 (7.7%) | 0 (0%) |
-
2.
Focus group discussion:
The FGD took place during the Cardiovascular and Interventional Radiological Society Europe (CIRSE) 2023 congress in Copenhagen. The results of the questionnaire were anonymously presented. The FGD was semi-structured with open-ended predefined questions, facilitated by two experienced moderators.
Two independent researchers (CV and PH) documented the raw data during the session, transcribed it and established inter-coder agreement and thematic analysis, followed by two peer debriefing sessions.
Participants
Since this study is part of the EU-funded IMAGIO study, the target audience consisted of European experts in the field. The selected body consisted of core members (n = 7) and collaborative partners (n = 6) of the IMAGIO consortium (Table 1). Twelve participants were employed by academic centers. One participant practiced in a large teaching hospital.
Questionnaire and Focus Group Discussion Findings
Appendix A, Table 1 illustrates the quantitative results of the questionnaire. Per topic, the results of the questionnaire and FGD are summarized.
Standardization
Nine respondents emphasized the importance of international standardization of liver tumor ablation, while one respondent did not due to the unclear specification of the proposed standardization. During the FGD, there was consensus that standardization would help to establish TA as treatment of first choice for malignant liver lesions. To compete with surgery, TA should universally be excellent with limited operator-dependency. Participants noted that some standardization already exists through guidelines from CIRSE, the Society of Interventional Radiology and the Society of Interventional Oncology.
Software-assisted Preprocedural Planning
The FGD revealed that all participants currently use preprocedural cross-sectional computed tomography (CT) and/or magnetic resonance imaging images for patient selection and needle trajectory planning. There was consensus that needle planning is best performed periprocedural, preferably with the patient under general anesthesia using respiratory control (i.e., breath-holds, single-lung or high-frequency jet ventilation). It was stressed that the position and morphology of the liver depend on the patient’s position and breathing and thus may vary considerably. Software-assisted planning prior to the intervention using pre-procedural images was therefore considered of limited use. Pre-procedural planning software, however, could be beneficial in hands-on simulation training for trainees and inexperienced interventional radiologists.
Needle Placement
Four of the respondents considered ultrasonography (US) as the modality of first choice as it allows real-time needle placement. In the event of poor visibility on US, additional modalities are required. During the FGD, there was consensus that image fusion could facilitate the localization and targeting of those lesions. Also, it was stated that the choice of the imaging modality, used for needle placement, depends on a case-by-case basis.
Navigational Tools
Four of the respondents integrated advanced navigational systems from seven different vendors into their clinical practice, while two respondents were exclusively using them within research projects. Considering the static nature of the CT-images that are currently being used for needle navigation, the importance of respiratory control and potential inaccuracies that arise from alternating liver morphology were once again highlighted during the FGD. There was a widespread agreement that confirming needle position and post-ablation margins with contrast-enhanced (cone bean) CT are crucial for optimization of outcomes.
Treatment Confirmation
To assess technical success, six of the respondents primarily used side-by-side visual (‘eye-balling’) qualitative assessment. Two respondents used visual three-dimensional assessment of ablative margins via pre- and post-ablation CT coregistration. The remaining two respondents employed quantitative assessment of the ablative margins with coregistration. The FGD considered quantitative margin analyses as a method with great potential, but further research is required before it can be widely implemented for clinical decision-making.
Discussion
Many developments and improvements in tools and software have become available in the field of TA of liver malignancies. Several studies have reported improved outcomes with their implementation [8, 9, 12].
Current clinical practice, however, demonstrates that the enhanced availability of these advancements seems to result in unwanted increased workflow variation. This is mirrored in the variability in treatment strategies observed among the participants in the FGD. A more standardized approach of TA is therefore essential to reduce operator-dependency and guarantee access to high-standard care for patients worldwide. Navigational systems, robotics and planning and confirmation software may help to standardize essential elements of the procedure such as needle placement and assessment of technical success. However, the adoption of such technology is currently heterogeneous and may paradoxically lead to more practice variation.
Different imaging modalities can be used for needle placement and the FGD brought forward that the modality of choice is best determined on a case-by-case basis, while none of the techniques is superior over the others in all cases. Irrespective of the modality used, confirmation of needle position as well as immediate post-ablation confirmation of adequate ablative margins were deemed critical. Three-dimensional planning can be beneficial when requiring the placement or repositioning of multiple probes [13].
The majority of participants used eye-balling comparison for treatment evaluation. This is remarkable as visual qualitative assessment has poor intra and interobserver variability and may lead to misjudgments in up to 44% of cases [9, 12]. Various quantification software packages are available to (semi-)automatically evaluate treatment margin [8]; yet, the results of the FGD indicate that the methodology is still not widely adopted in clinical practice and needs validation in clinical trials [14–16]. Besides, additional research on ablative tissue shrinkage is needed, as this may lead to underestimation of ablation margins.
This study has several limitations. Firstly, selection bias due to the purposive sampling with imbalanced nationality and expertise among the participants may limit the global applicability. Additionally, the incomplete questionnaire response rate and small number of participants may compromise the data robustness.
Conclusion
Current diversity in workflow and outcomes of thermal ablation of malignant liver tumors highlights the necessity for standardization. While advancements in tools and software hold the potential to homogenize outcome measurement and minimize operator-dependent variability, the enhanced availability also contributes to increased workflow variation.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The facilitation of the FGD was made possible through support of Philips Health Care, also a key contributor to the IMAGIO consortium.
Funding
The IMAGIO study is supported by the Innovative Health Initiative (IHI) Joint Undertaking (JU), under grant agreement No 101112053. The JU receives support from the European Union’s Horizon Europe research and innovation program and life science industries represented by COCIR, EFPIA / Vaccines Europe, EuropaBio and MedTech Europe. Further details can be found at https://www.ihi.europa.eu/projects-results/project-factsheets/imagio. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Declarations
Conflict of interest
Philips Health Care is a key contributor to the IMAGIO consortium. M.C. Burgmans has received an educational grant from the Dutch Cancer Society, MLDS, Health Holland and Medtronic and a consultancy fee from Philips Health Care. M.C. Burgmans, C. van der Leij and M. L. J. Smits serve as co-PIs within the IMAGIO consortium. R. Bale is a consultant for Interventional Systems (Kitzbühel, Austria). All other authors declare that they have no conflict of interest.
Ethical Approval
For this type of study, formal consent is not required.
Consent for Publication
Consent for publication was obtained for every individual person’s data included in the study.
Informed Consent
For this type of study, informed consent is not required.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Schullian P, Laimer G, Johnston E, Putzer D, Eberle G, Scharll Y, et al. Technical efficacy and local recurrence after stereotactic radiofrequency ablation of 2653 liver tumors: a 15-year single-center experience with evaluation of prognostic factors. Int J Hyperthermia. 2022;39(1):421–30. [DOI] [PubMed] [Google Scholar]
- 2.EASL Clinical Practice Guidelines. Management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. [DOI] [PubMed] [Google Scholar]
- 3.Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391(10127):1301–14. [DOI] [PubMed] [Google Scholar]
- 4.Puijk RS, Dijkstra M, van den Bemd BAT, Ruarus AH, Nieuwenhuizen S, Geboers B, et al. improved outcomes of thermal ablation for colorectal liver metastases: a 10-Year analysis from the prospective amsterdam CORE registry (AmCORE). Cardiovasc Intervent Radiol. 2022;45(8):1074–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kutlu OC, Chan JA, Aloia TA, Chun YS, Kaseb AO, Passot G, et al. Comparative effectiveness of first-line radiofrequency ablation versus surgical resection and transplantation for patients with early hepatocellular carcinoma. Cancer. 2017;123(10):1817–27. [DOI] [PubMed] [Google Scholar]
- 6.Goutté N, Sogni P, Bendersky N, Barbare JC, Falissard B, Farges O. Geographical variations in incidence, management and survival of hepatocellular carcinoma in a Western country. J Hepatol. 2017;66(3):537–44. [DOI] [PubMed] [Google Scholar]
- 7.Reinders MTM, van Meer S, Burgmans MC, de Jong KP, Klümpen HJ, de Man RA, et al. Trends in incidence, diagnosis, treatment and survival of hepatocellular carcinoma in a low-incidence country: data from the Netherlands in the period 2009–2016. Eur J Cancer. 2020;137:214–23. [DOI] [PubMed] [Google Scholar]
- 8.Rai P, Ansari MY, Warfa M, Al-Hamar H, Abinahed J, Barah A, et al. Efficacy of fusion imaging for immediate post-ablation assessment of malignant liver neoplasms: a systematic review. Cancer Med. 2023;12(13):14225–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laimer G, Schullian P, Jaschke N, Putzer D, Eberle G, Alzaga A, et al. Minimal ablative margin (MAM) assessment with image fusion: an independent predictor for local tumor progression in hepatocellular carcinoma after stereotactic radiofrequency ablation. Eur Radiol. 2020;30(5):2463–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Floridi C, Cellina M, Irmici G, Bruno A, Rossini N, Borgheresi A, et al. Precision imaging guidance in the era of precision oncology: an update of imaging tools for interventional procedures. J Clin Med. 2022;11(14):4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.IMaging and Advanced Guidance for workflow optimization in Interventional Oncology (IMAGIO)[internet]. 2023 [cited 2024 Aug 5]. Available from: https://imagioproject.eu/
- 12.Hendriks P, Boel F, Oosterveer TT, Broersen A, de Geus-Oei LF, Dijkstra J, et al. Ablation margin quantification after thermal ablation of malignant liver tumors: How to optimize the procedure? A systematic review of the available evidence. Eur J Radiol Open. 2023;11:100501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bale R, Widmann G, Haidu M. Stereotactic radiofrequency ablation. Cardiovasc Interv Radiol. 2011;34(4):852–6. [DOI] [PubMed] [Google Scholar]
- 14.Oosterveer TTM, van Erp GCM, Hendriks P, Broersen A, Overduin CG, van Rijswijk CSP, et al. Study protocol PROMETHEUS: prospective multicenter study to evaluate the correlation between safety margin and local recurrence after thermal ablation using image co-registration in patients with hepatocellular carcinoma. Cardiovasc Interv Radiol. 2022;45(5):606–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ablation With Confirmation of Colorectal Liver Metastases (ACCLAIM) [Clinicaltrials.gov]. 2022 [Prospective Trial for Microwave Ablation as a Local Cure]. Available from: https://clinicaltrials.gov/study/NCT05265169#contacts-and-locations.
- 16.Lin YM, Paolucci I, Anderson BM, O’Connor CS, Rigaud B, Briones-Dimayuga M, et al. Study protocol COVER-ALL: clinical impact of a volumetric image method for confirming tumour coverage with ablation on patients with malignant liver lesions. Cardiovasc Intervent Radiol. 2022;45(12):1860–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.