Abstract
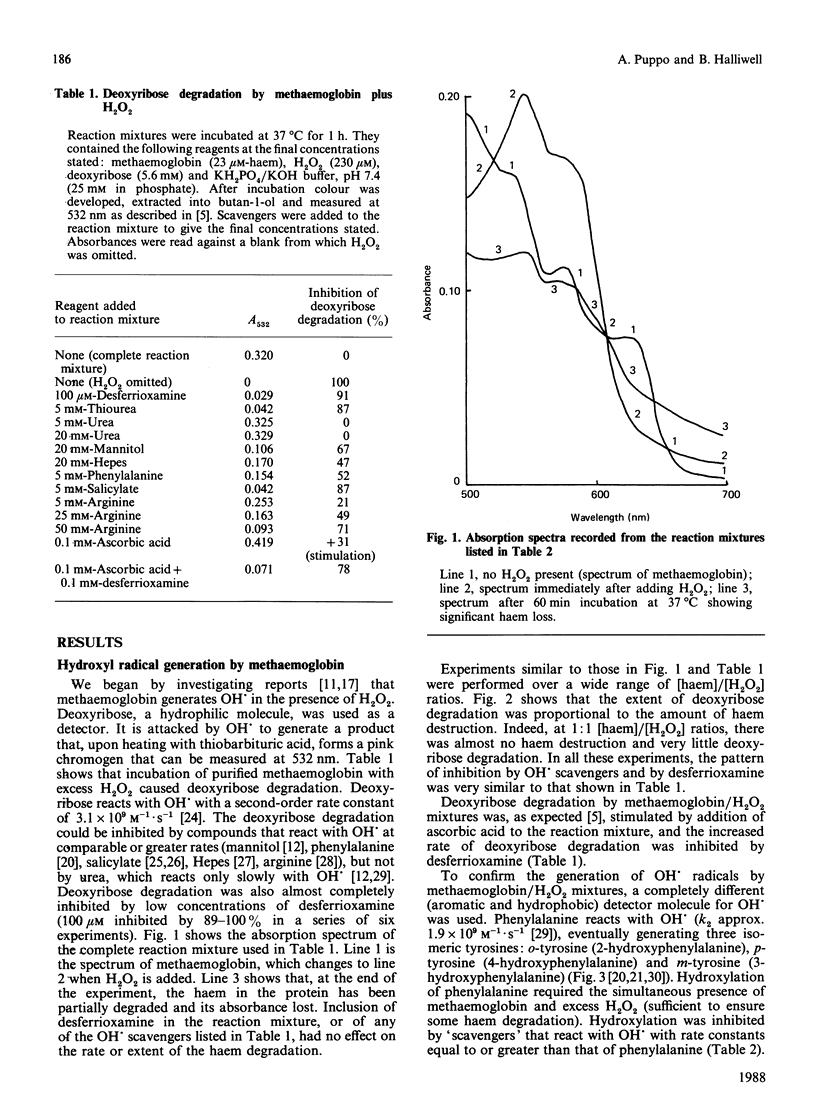
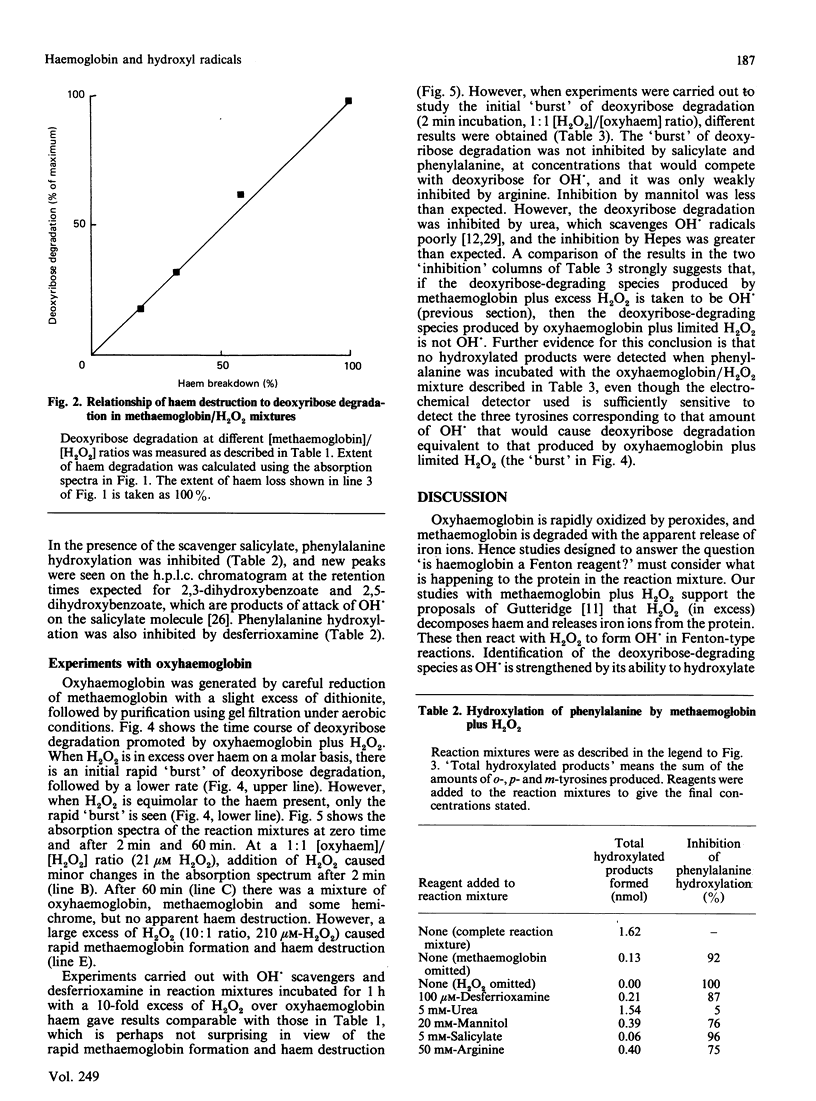
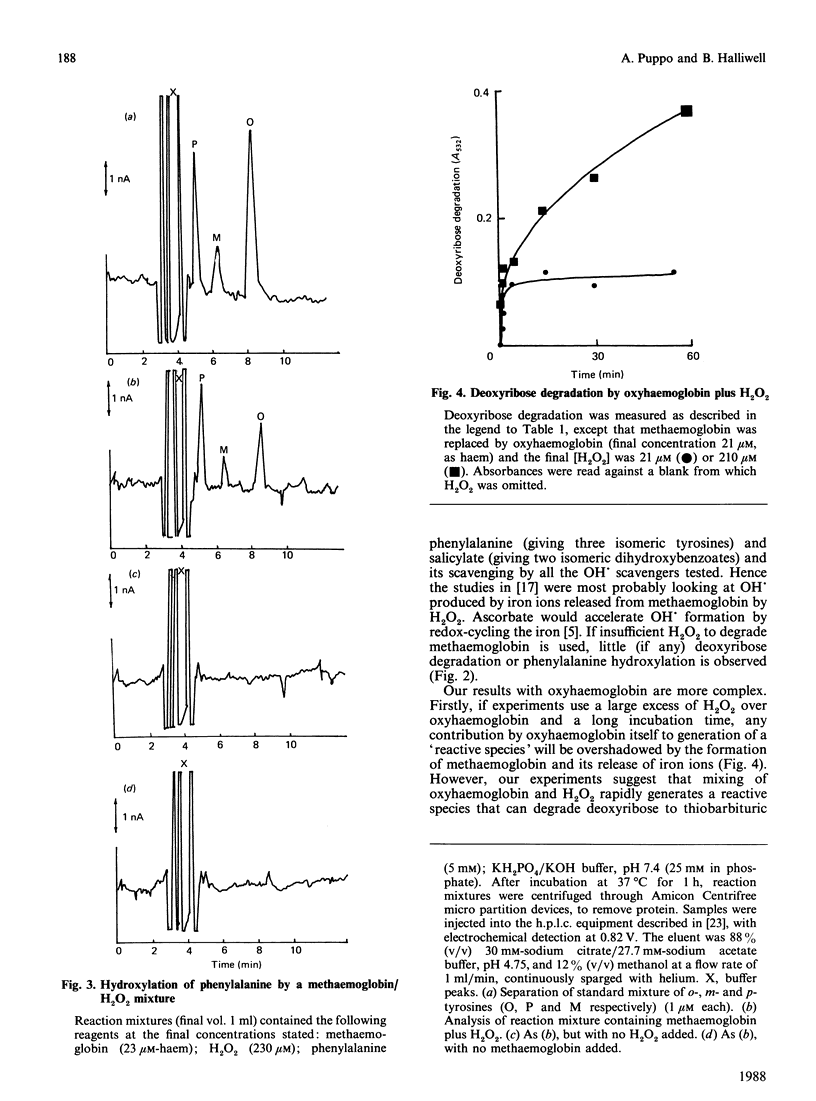
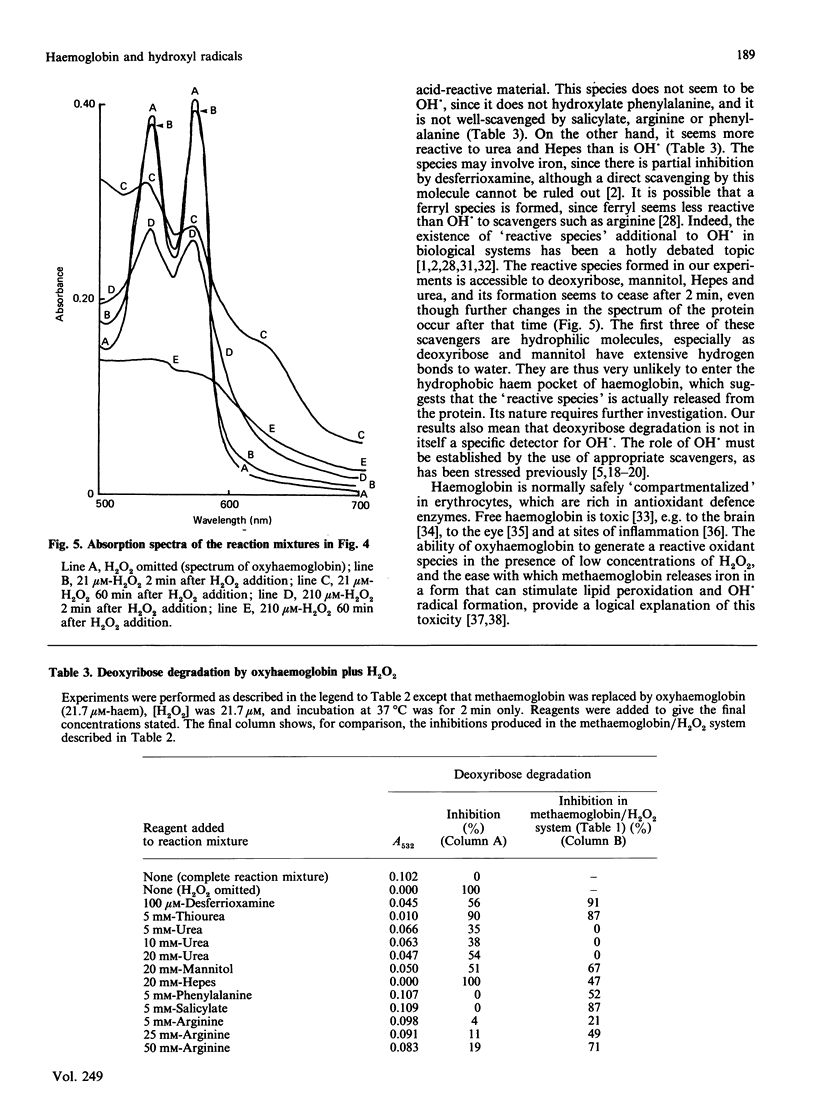
The ability of oxyhaemoglobin and methaemoglobin to generate hydroxyl radicals (OH.) from H2O2 has been investigated using deoxyribose and phenylalanine as 'detector molecules' for OH.. An excess of H2O2 degrades methaemoglobin, releasing iron ions that react with H2O2 to form a species that appears to be OH.. Oxyhaemoglobin reacts with low concentrations of H2O2 to form a 'reactive species' that degrades deoxyribose but does not hydroxylate phenylalanine. This 'reactive species' is less amenable to scavenging by certain scavengers (salicylate, phenylalanine, arginine) than is OH., but it appears more reactive than OH. is to others (Hepes, urea). The ability of haemoglobin to generate not only this 'reactive species', but also OH. in the presence of H2O2 may account for the damaging effects of free haemoglobin in the brain, the eye, and at sites of inflammation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aruoma O. I., Halliwell B. Superoxide-dependent and ascorbate-dependent formation of hydroxyl radicals from hydrogen peroxide in the presence of iron. Are lactoferrin and transferrin promoters of hydroxyl-radical generation? Biochem J. 1987 Jan 1;241(1):273–278. doi: 10.1042/bj2410273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benatti U., Morelli A., Guida L., De Flora A. The production of activated oxygen species by an interaction of methemoglobin with ascorbate. Biochem Biophys Res Commun. 1983 Mar 29;111(3):980–987. doi: 10.1016/0006-291x(83)91396-7. [DOI] [PubMed] [Google Scholar]
- Biemond P., van Eijk H. G., Swaak A. J., Koster J. F. Iron mobilization from ferritin by superoxide derived from stimulated polymorphonuclear leukocytes. Possible mechanism in inflammation diseases. J Clin Invest. 1984 Jun;73(6):1576–1579. doi: 10.1172/JCI111364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cederbaum A. I., Dicker E., Rubin E., Cohen G. Effect of thiourea on microsomal oxidation of alcohols and associated microsomal functions. Biochemistry. 1979 Apr 3;18(7):1187–1191. doi: 10.1021/bi00574a011. [DOI] [PubMed] [Google Scholar]
- Clemens M. R., Einsele H., Remmer H., Waller H. D. An essential requirement for ferrous-haemoglobin in the hydrogen peroxide stimulated oxidation of red blood cell membrane lipids. Biochem Pharmacol. 1985 Apr 15;34(8):1339–1341. doi: 10.1016/0006-2952(85)90516-7. [DOI] [PubMed] [Google Scholar]
- Czapski G., Goldstein S. When do metal complexes protect the biological system from superoxide toxicity and when do they enhance it? Free Radic Res Commun. 1986;1(3):157–161. doi: 10.3109/10715768609083147. [DOI] [PubMed] [Google Scholar]
- Doly M., Bonhomme B., Vennat J. C. Experimental study of the retinal toxicity of hemoglobinic iron. Ophthalmic Res. 1986;18(1):21–27. doi: 10.1159/000265409. [DOI] [PubMed] [Google Scholar]
- Grootveld M., Halliwell B. An aromatic hydroxylation assay for hydroxyl radicals utilizing high-performance liquid chromatography (HPLC). Use to investigate the effect of EDTA on the Fenton reaction. Free Radic Res Commun. 1986;1(4):243–250. doi: 10.3109/10715768609051634. [DOI] [PubMed] [Google Scholar]
- Grootveld M., Halliwell B. Aromatic hydroxylation as a potential measure of hydroxyl-radical formation in vivo. Identification of hydroxylated derivatives of salicylate in human body fluids. Biochem J. 1986 Jul 15;237(2):499–504. doi: 10.1042/bj2370499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutteridge J. M. Iron promoters of the Fenton reaction and lipid peroxidation can be released from haemoglobin by peroxides. FEBS Lett. 1986 Jun 9;201(2):291–295. doi: 10.1016/0014-5793(86)80626-3. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M. The antioxidant activity of haptoglobin towards haemoglobin-stimulated lipid peroxidation. Biochim Biophys Acta. 1987 Feb 14;917(2):219–223. doi: 10.1016/0005-2760(87)90125-1. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M. Thiobarbituric acid-reactivity following iron-dependent free-radical damage to amino acids and carbohydrates. FEBS Lett. 1981 Jun 15;128(2):343–346. doi: 10.1016/0014-5793(81)80113-5. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Grootveld M. The measurement of free radical reactions in humans. Some thoughts for future experimentation. FEBS Lett. 1987 Mar 9;213(1):9–14. doi: 10.1016/0014-5793(87)81455-2. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M., Aruoma O. I. The deoxyribose method: a simple "test-tube" assay for determination of rate constants for reactions of hydroxyl radicals. Anal Biochem. 1987 Aug 15;165(1):215–219. doi: 10.1016/0003-2697(87)90222-3. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Formation of thiobarbituric-acid-reactive substance from deoxyribose in the presence of iron salts: the role of superoxide and hydroxyl radicals. FEBS Lett. 1981 Jun 15;128(2):347–352. doi: 10.1016/0014-5793(81)80114-7. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Oxygen free radicals and iron in relation to biology and medicine: some problems and concepts. Arch Biochem Biophys. 1986 May 1;246(2):501–514. doi: 10.1016/0003-9861(86)90305-x. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. The importance of free radicals and catalytic metal ions in human diseases. Mol Aspects Med. 1985;8(2):89–193. doi: 10.1016/0098-2997(85)90001-9. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Superoxide-dependent formation of hydroxyl radicals in the presence of iron chelates: is it a mechanism for hydroxyl radical production in biochemical systems? FEBS Lett. 1978 Aug 15;92(2):321–326. doi: 10.1016/0014-5793(78)80779-0. [DOI] [PubMed] [Google Scholar]
- Hiller K. O., Hodd P. L., Willson R. L. Antiinflammatory drugs: protection of a bacterial virus as an in vitro biological measure of free radical activity. Chem Biol Interact. 1983 Dec;47(3):293–305. doi: 10.1016/0009-2797(83)90165-5. [DOI] [PubMed] [Google Scholar]
- Ishimitsu S., Fujimoto S., Ohara A. Hydroxylation of phenylalanine by the hypoxanthine-xanthine oxidase system. Chem Pharm Bull (Tokyo) 1984 Nov;32(11):4645–4649. doi: 10.1248/cpb.32.4645. [DOI] [PubMed] [Google Scholar]
- Kanner J., Harel S. Initiation of membranal lipid peroxidation by activated metmyoglobin and methemoglobin. Arch Biochem Biophys. 1985 Mar;237(2):314–321. doi: 10.1016/0003-9861(85)90282-6. [DOI] [PubMed] [Google Scholar]
- O'Connell M., Halliwell B., Moorhouse C. P., Aruoma O. I., Baum H., Peters T. J. Formation of hydroxyl radicals in the presence of ferritin and haemosiderin. Is haemosiderin formation a biological protective mechanism? Biochem J. 1986 Mar 15;234(3):727–731. doi: 10.1042/bj2340727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panter S. S., Sadrzadeh S. M., Hallaway P. E., Haines J. L., Anderson V. E., Eaton J. W. Hypohaptoglobinemia associated with familial epilepsy. J Exp Med. 1985 Apr 1;161(4):748–754. doi: 10.1084/jem.161.4.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond R., Halliwell B., Chauhan J., Darbre A. Superoxide-dependent formation of hydroxyl radicals: detection of hydroxyl radicals by the hydroxylation of aromatic compounds. Anal Biochem. 1981 Dec;118(2):328–335. doi: 10.1016/0003-2697(81)90590-x. [DOI] [PubMed] [Google Scholar]
- Rush J. D., Koppenol W. H. Oxidizing intermediates in the reaction of ferrous EDTA with hydrogen peroxide. Reactions with organic molecules and ferrocytochrome c. J Biol Chem. 1986 May 25;261(15):6730–6733. [PubMed] [Google Scholar]
- Sadrzadeh S. M., Graf E., Panter S. S., Hallaway P. E., Eaton J. W. Hemoglobin. A biologic fenton reagent. J Biol Chem. 1984 Dec 10;259(23):14354–14356. [PubMed] [Google Scholar]
- Trotta R. J., Sullivan S. G., Stern A. Lipid peroxidation and haemoglobin degradation in red blood cells exposed to t-butyl hydroperoxide. The relative roles of haem- and glutathione-dependent decomposition of t-butyl hydroperoxide and membrane lipid hydroperoxides in lipid peroxidation and haemolysis. Biochem J. 1983 Jun 15;212(3):759–772. doi: 10.1042/bj2120759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasil M., Halliwell B., Grootveld M., Moorhouse C. P., Hutchison D. C., Baum H. The specificity of thiourea, dimethylthiourea and dimethyl sulphoxide as scavengers of hydroxyl radicals. Their protection of alpha 1-antiproteinase against inactivation by hypochlorous acid. Biochem J. 1987 May 1;243(3):867–870. doi: 10.1042/bj2430867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White C. T., Murray A. J., Greene J. R., Smith D. J., Medina F., Makovec G. T., Martin E. J., Bolin R. B. Toxicity of human hemoglobin solution infused into rabbits. J Lab Clin Med. 1986 Aug;108(2):121–131. [PubMed] [Google Scholar]
- Yoshino S., Blake D. R., Hewitt S., Morris C., Bacon P. A. Effect of blood on the activity and persistence of antigen induced inflammation in the rat air pouch. Ann Rheum Dis. 1985 Jul;44(7):485–490. doi: 10.1136/ard.44.7.485. [DOI] [PMC free article] [PubMed] [Google Scholar]


