Abstract
Objective
Opioid overdose is a public health epidemic adversely impacting individuals and communities. To combat this, California passed a law mandating that prescribers offer a naloxone prescription in certain circumstances. Our objective was to evaluate associations with California's naloxone prescription mandate and emergency department (ED) overdose visits/hospitalizations, opioid and naloxone prescribing, and 30‐day mortality.
Methods
This retrospective cohort study included data from January 1, 2018, to December 31, 2019, and included all Kaiser Permanente Southern California (KPSC) members aged >10 years across 15 KPSC EDs. Exposure was defined as presentation to the ED within the study period. The primary outcome was ED visits for opioid overdose pre‐ and post‐implementation of California's naloxone prescription mandate.
Results
A total of 1.1 million ED visits (534K pre/576K post) were included in the study population. ED opioid overdose visits were 344 (6.4/10,000) pre‐policy and 351 (6.1/10,000) post‐policy implementation, while non‐opioid overdose visits were 309 (5.8/10,000) pre‐implementation and 411 (7.1/10,000) post‐implementation. The unadjusted rate of visits with opioid prescriptions decreased significantly (14.9% pre to 13.5% post) after implementation. ED naloxone prescriptions increased substantially (104 pre vs. 6031 post). Primary adjusted interrupted time series analysis found no statistical difference between monthly opioid overdose visits pre versus post (odds ratio 1.02, 95% confidence interval [CI] 0.98‒1.07). Difference‐in‐differences analysis revealed no significant changes in hospitalization (coefficient [CE] = ‒0.05, 95% CI = ‒0.11 to 0.02) or 30‐day mortality (CE = ‒0.01, 95% CI = ‒0.03 to 0.01).
Conclusion
This study revealed that the implementation of California's naloxone prescription mandate was associated with significantly increased naloxone prescribing and decreased opioid prescribing, but no significant change in ED opioid overdose visits, hospitalizations, or 30‐day mortality. This indicates that increasing naloxone prescribing alone may not be sufficient to lower opioid overdose rates.
1. INTRODUCTION
1.1. Background
The proliferation of opioid‐related harm was declared a public health emergency in the United States in October 2017. 1 From 1999 to 2008, there was a fourfold increase in opioid overdose‐related mortality, and the number has since continued to rise. 1 From October 2020 to September 2021, there were over 100,000 drug overdose deaths in the United States, most of which (>70%) involved opioids. 2 Additionally, opioid misuse cost over $1 trillion in 2017 alone, 3 , 4 while the minority of those with an opioid use disorder (OUD) receive treatment for their condition. 5 , 6 For these reasons, there have been recent efforts to mitigate the opioid epidemic in a variety of ways, and although these interventions are promising, the effectiveness of many efforts is not well known. 7 , 8
1.2. Importance
One key strategy to reduce opioid overdose mortality has been increasing access to naloxone. Naloxone is an opioid antagonist given intravenously, intramuscularly, or intranasally to quickly reverse the effects of opioid overdose. 9 Similar to many states, California has modified its laws to provide civil and criminal immunity to medical providers that prescribe and dispense naloxone, to permit the medication to be prescribed to “third parties” such as the friends and family members of a person at risk of overdose, and to permit it to be distributed under non‐patient‐specific orders. 10 , 11 , 12 , 13 To further bolster naloxone access, California passed a law, effective January 1, 2019, mandating prescribers offer naloxone (or another FDA‐approved opioid reversal agent) to any patient who is (1) taking ≥90 morphine milligram equivalents (MME) of an opioid medication per day; (2) prescribed an opioid while taking a concurrent benzodiazepine; and (3) at increased risk for overdose, including patients with a history of overdose. 14
1.3. Goal of this investigation
The primary objective of this study was to assess and quantify associations of this California co‐prescription mandate on emergency department (ED) opioid overdose visits. The secondary objectives were to evaluate any associations with ED naloxone prescribing, opioid prescribing, hospitalizations, and 30‐day mortality.
2. METHODS
2.1. Study design
An interrupted time series design was used to evaluate changes in the monthly rates of opioid overdose, non‐opioid overdose, opioid prescribing, and naloxone prescribing after implementation of the California co‐prescription law in January 2019, while controlling for pre‐policy trends. We used the interrupted time series approach because it is a recommended study design for these types of observational policy evaluations (REFS), and additionally included a control group for comparison (non‐opioid overdoses or poisonings) to assist with accurate interpretations of potential changes. 15 , 16 In additional analyses, a difference‐in‐differences design was used to assess changes in hospitalization and 30‐day mortality between opioid and non‐opioid overdose groups before and after the naloxone prescription law took effect. This was done as it is the recommended analysis for observational studies in order to mitigate background changes in outcomes. 17 , 18
2.2. Study setting
This study was based on a retrospective cohort of all ED visits from January 1, 2018, to December 31, 2019, at 15 hospitals in the Kaiser Permanente Southern California (KPSC) system. KPSC is an integrated health care delivery system currently providing care to more than 4.6 million members throughout the southern California region. KPSC members’ socioeconomic and racial/ethnic characteristics are generally reflective of the local and statewide population. 19 EDs vary in size, ranging from approximately 25,000 to 90,000 annual visits. All facilities use an electronic medical record system for patient encounters, including the ordering and dispensing of prescription medications.
The Bottom Line
This study evaluated California's naloxone co‐prescription law's impact on opioid‐related health outcomes. Looking at over 1 million emergency department visits from 2018 to 2019, this study found that while this law led to over a 50‐fold increase in naloxone prescribing, there was no change in emergency department visits, hospitalizations, or 30‐day mortality related to opioid overdose. While improved naloxone access is a great step in the right direction, this change, in isolation, may not be sufficient to reduce opioid overdose rates and mortality.
2.3. Selection of participants
The initial sample included 1,201,233 ED visits from 688,358 KPSC members in 2018‒2019 (Figure 1). Non‐KP members were excluded due to their incomplete information on past medical and medication records. For members with multiple visits, each visit was treated as a separate encounter. After excluding visits in which patients were under 10 years of age (n = 90,943) or were discharged to a hospice facility (n = 138), the primary sample consisted of 1,110,152 ED patient visits. These visits were further categorized into three mutually exclusive overdose‐type groups based on the International Classification of Diseases, tenth revision (ICD‐10) diagnosis codes recorded during their encounter, including opioid overdose and non‐opioid overdose (Table A1). The primary sample was used to analyze changes in opioid and non‐opioid overdose rates before and after policy implementation. For analyses of opioid and naloxone prescribed/filled at discharge, this study used a secondary sample (n = 1,089,234) that further excluded 20,918 visits from the primary sample in which patients were transferred to another facility, discharged against medical advice, or deceased (Figure 1).
FIGURE 1.
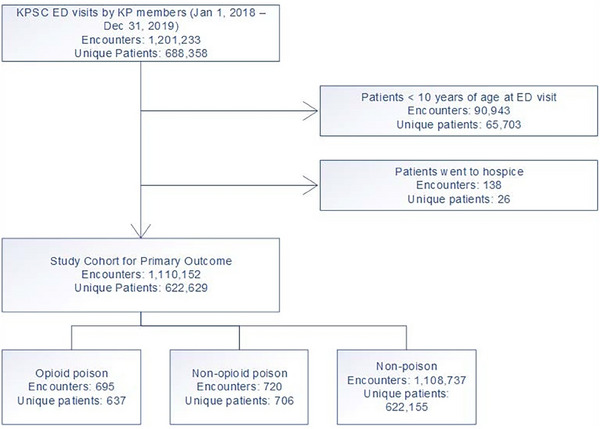
Inclusion criteria for study population.
The institutional review board of KPSC approved this study with a waiver of informed consent due to its retrospective nature and use of de‐identified data.
2.4. Measurements and outcomes
Opioid and non‐opioid overdose visits were identified using the ICD‐10 diagnosis codes (Table A1). Opioid (oral) and naloxone (nasal and intramuscular) discharge prescriptions were determined using KPSC pharmacy order records (regardless of whether the prescription was filled). Opioid and naloxone prescriptions dispensed to patients within 2 days of discharge were ascertained using outpatient pharmacy records. Hospitalizations and ED visits were obtained from inpatient records and out‐of‐network claims. Thirty‐day mortality status was assessed from multiple sources, including the KPSC inpatient records, KPSC membership system, California state death master files, Social Security Administration death master files, and out‐of‐network claims (Figure 1).
2.5. Covariates
Self‐reported demographic characteristics extracted from the electronic health records included age at the time of ED visit (categorized in this study as 10‒15 [reference group], 16‒22, 23‒34, 35‒50, and >50), sex (female [reference group] and male), and race/ethnicity (non‐Hispanic White [reference group], non‐Hispanic Black, Hispanic, non‐Hispanic Asian/Pacific Islander, and others). Charlson comorbidity index (none [reference group], 1, 2, and 3+) was calculated using ICD‐10 diagnosis codes from all encounters within the past 12 months prior to the ED visit. 20
2.6. Statistical analysis
Descriptive statistics were used to compare characteristics (e.g., age, sex, race/ethnicity, and Charlson comorbidity index) and outcomes of interests among opioid overdose visits, non‐opioid overdose visits, and other non‐overdose visits pre‐ and post‐implementation. The significance of differences pre‐ and post‐implementation was assessed using the chi‐squared test (for categorical variables) and the Kruskal‒Wallis test (for continuous variables).
Interrupted times series and segmented logistic regression were used to estimate changes in the monthly rates of opioid overdose, non‐opioid overdose, opioid prescribing, and naloxone prescribing after policy implementation, controlling for pre‐existing trends, and seasonal effects. 15 , 16 The model included terms for the pre‐implementation monthly trend, change in monthly trend, and change in level of outcome post‐implementation. Change in monthly trend indicated the difference in trends pre‐ and post‐implementation. Change in the level of outcome referred to the immediate difference in the outcome between the last month of the pre‐implementation period (December 2018) to the first month of the post‐implementation period (January 2019). Difference‐in‐differences and a linear regression model were used to examine the differences in hospitalization and 30‐day mortality between opioid and non‐opioid overdose groups pre‐ and post‐implementation. 17 , 18 The model included terms for time trend among the control group (eg, non‐opioid overdose), difference in the outcome between two groups pre‐implementation, and differences in the outcome between two groups between pre‐ and post‐implementation (eg, difference‐in‐differences estimate). All models controlled for age, sex, race/ethnicity, and Charlson comorbidity index. 21 Robust standard errors were clustered at the patient level to account for correlation across multiple ED visits made by the same patient. All analyses were conducted in July 2021 using SAS 9.4.
3. RESULTS
3.1. Characteristics of study subjects
There were 1,110,152 encounters (533,860 pre‐ and 576,292 post‐intervention) from a total of 622,829 patients included in the primary analysis. Study patients were 57.2% female (57.1% pre‐ and 57.2% post‐intervention) and had a mean age of 55.2 years (standard deviation [SD] ±22.56) with a range of 10‒108.1 (Table 1). Of these, there were 695 encounters for opioid overdose, with a mean age of 48.1 years (SD ±19.19), and 720 encounters for poisoning from other substances with a mean age of 35.7 years (SD ±22.13).
TABLE 1.
Demographics and clinical characteristics of 15 Southern California Kaiser Permanente emergency department (ED) encounters for patients before (January 1, 2018‒December 31, 2018) and after (January 1, 2019‒December 31, 2019) implementation of a California naloxone mandate.
| Total (N = 1,110,152) | 2018 (N = 533,860) | 2019 (N = 576,292) | |
|---|---|---|---|
| Age group | |||
| 10‒15 | 46,504 (4.2%) | 22,342 (4.2%) | 24,162 (4.2%) |
| 16‒22 | 61,589 (5.5%) | 30,121 (5.6%) | 31,468 (5.5%) |
| 22‒34 | 149,306 (13.4%) | 70,875 (13.3%) | 78,431 (13.6%) |
| 35‒50 | 185,300 (16.7%) | 88,904 (16.7%) | 96,396 (16.7%) |
| >50 | 667,453 (60.1%) | 321,618 (60.2%) | 345,835 (60%) |
| Sex | |||
| Female | 634,792 (57.2%) | 304,875 (57.1%) | 329,917 (57.2%) |
| Race/ethnicity | |||
| Hispanic | 456,078 (41.1%) | 217,908 (40.8%) | 238,170 (41.3%) |
| White | 385,835 (34.8%) | 187,322 (35.1%) | 198,513 (34.4%) |
| Black | 150,152 (13.5%) | 72,838 (13.6%) | 77,314 (13.4%) |
| Asian | 92,094 (8.3%) | 43,870 (8.2%) | 48,224 (8.4%) |
| Pacific Islander | 8029 (0.7%) | 3832 (0.7%) | 4197 (0.7%) |
| Other | 8036 (0.7%) | 3594 (0.7%) | 4442 (0.8%) |
| Unknown | 4200 (0.4%) | 1742 (0.3%) | 2458 (0.4%) |
| Multiple | 3408 (0.3%) | 1656 (0.3%) | 1752 (0.3%) |
| Native American/Alaskan | 2320 (0.2%) | 1098 (0.2%) | 1222 (0.2%) |
| Disposition | |||
| Admission | 233,304 (21%) | 114,038 (21.4%) | 119,266 (20.7%) |
| Charlson comorbidity categories | |||
| 0 | 417,145 (37.6%) | 195,941 (36.7%) | 221,204 (38.4%) |
| 1 | 230,103 (20.7%) | 110,275 (20.7%) | 119,828 (20.8%) |
| 2 | 141,499 (12.7%) | 67,838 (12.7%) | 73,661 (12.8%) |
| 3+ | 321,405 (29%) | 159,806 (29.9%) | 161,599 (28%) |
| Length of stay (among hospitalized patients) | |||
| N | 120,870 | 60,113 | 60,757 |
| Mean (SD) | 4.3 (6.13) | 4.3 (6.04) | 4.3 (6.21) |
| Died within 30 days after ED visit | |||
| Yes | 20,377 (1.8%) | 9971 (1.9%) | 10,406 (1.8%) |
| History of substance use disorder in the past 12 months before ED visit | |||
| Yes | 85,508 (7.7%) | 42,513 (8%) | 42,995 (7.5%) |
| Opioid and benzodiazepine prescriptions in the past 12 months before ED visit | |||
| Yes | 175,344 (15.8%) | 90,947 (17%) | 84,397 (14.6%) |
| Daily MME >90 in the past 12 months before ED visit | |||
| Yes | 7266 (0.7%) | 4327 (0.8%) | 2939 (0.5%) |
Abbreviations: MME, morphine milligram equivalents; SD, standard deviation.
3.2. Main results
For opioid overdose, there were 344 (6.4/10,000) encounters in 2018, and 351 (6.1/10,000) encounters in 2019 (Table 2). Among this group, 63.3% of patients had a history of substance use disorder within 12 months prior to ED visit (65.4% pre‐ and 61.3% post‐intervention), 41.3% had active opioid and concurrent benzodiazepine prescriptions within 12 months prior to ED visit (42.4% pre‐ and 40.2% post‐intervention), and 6.8% had an opioid prescription greater than 90 MME within 12 months (9% pre‐ and 4.6% post‐intervention; p = 0.0002). Hospital admission rate for those presenting to the ED for opioid overdose changed from 30.2% prior to the intervention to 21.9% afterward (p = 0.0127). The mean length of stay was 3.9 days (SD ±3.44) in 2018 and 5.7 days (SD ±6.98) in 2019 (p = 0.1793). Thirty‐day mortality changed from 2% prior to the intervention to 1.4% post‐implementation (p = 0.5368).
TABLE 2.
Descriptive statistics of patients presenting for an opioid overdose encounter within the 15 Southern California Kaiser Permanente emergency departments (EDs) before (2018) or after (2019) California naloxone law was implemented.
| Total (N = 695) | 2018 (N = 344) | 2019 (N = 351) | |
|---|---|---|---|
| Age group (years) | |||
| 10‒15 | 2 (0.3%) | 1 (0.3%) | 1 (0.3%) |
| 16‒22 | 57 (8.2%) | 19 (5.5%) | 38 (10.8%) |
| 22‒34 | 157 (22.6%) | 76 (22.1%) | 81 (23.1%) |
| 35‒50 | 139 (20%) | 71 (20.6%) | 68 (19.4%) |
| >50 | 340 (48.9%) | 177 (51.5%) | 163 (46.4%) |
| Sex | |||
| Female | 349 (50.2%) | 175 (50.9%) | 174 (49.6%) |
| Race/ethnicity | |||
| White | 358 (51.5%) | 166 (48.3%) | 192 (54.7%) |
| Hispanic | 214 (30.8%) | 113 (32.8%) | 101 (28.8%) |
| Black | 96 (13.8%) | 51 (14.8%) | 45 (12.8%) |
| Asian | 11 (1.6%) | 6 (1.7%) | 5 (1.4%) |
| Other | 7 (1%) | 1 (0.3%) | 6 (1.7%) |
| Multiple | 4 (0.6%) | 3 (0.9%) | 1 (0.3%) |
| Pacific Islander | 3 (0.4%) | 2 (0.6%) | 1 (0.3%) |
| Native American/Alaskan | 2 (0.3%) | 2 (0.6%) | 0 (0%) |
| Disposition | |||
| Admission | 181 (26%) | 104 (30.2%) | 77 (21.9%) |
| Charlson comorbidity categories | |||
| 0 | 251 (36.1%) | 110 (32%) | 141 (40.2%) |
| 1 | 151 (21.7%) | 78 (22.7%) | 73 (20.8%) |
| 2 | 91 (13.1%) | 45 (13.1%) | 46 (13.1%) |
| 3+ | 202 (29.1%) | 111 (32.3%) | 91 (25.9%) |
| Length of stay (among hospitalized patients) | |||
| N | 120 | 76 | 44 |
| Mean (SD) | 4.6 (5.08) | 3.9 (3.44) | 5.7 (6.98) |
| Died within 30 days after ED visit | |||
| Yes | 12 (1.7%) | 7 (2%) | 5 (1.4%) |
| History of substance use disorder in the past 12 months before ED visit | |||
| Yes | 440 (63.3%) | 225 (65.4%) | 215 (61.3%) |
| Opioid and benzo prescriptions in the past 12 months before ED visit | |||
| Yes | 287 (41.3%) | 146 (42.4%) | 141 (40.2%) |
| Daily MME >90 in the past 12 months before ED visit | |||
| Yes | 47 (6.8%) | 31 (9%) | 16 (4.6%) |
Abbreviations: MME, morphine milligram equivalents; SD, standard deviation.
The non‐opioid overdose group did not display any significant changes among all measured parameters pre‐ and post‐intervention. Non‐opioid overdose encounters went from 309 in 2018 to 411 in 2019. Of the total non‐opioid overdose encounters, 17.5% had a history of substance use disorder within the previous year (21.7% pre‐ and 14.4% post‐intervention), 21.3% had been prescribed both opioids and benzodiazepines within the previous year (20.7% pre‐ and 21.7% post‐intervention), and 0.3% had a prescription for opioids greater than 90 MME (0.3% pre‐ and 0.2% post‐intervention). Hospital admission went from 11.3% in 2018 to 12.4% in 2019 (p = 0.6577). Length of stay remained similar over the course of the study with a mean of 2.8 days (SD ±1.88) in 2018 and 2.5 days (SD ±2.88) in 2019 (p = 0.1881). Thirty‐day mortality changed slightly from 0% pre‐ to 0.5% post‐intervention (p = 0.2851; Table 3).
TABLE 3.
Descriptive statistics of patients presenting with non‐opioid overdose encountered within the 15 included Southern California Kaiser Permanente emergency departments (EDs) stratified by 1‐year interval pre‐intervention and post‐intervention.
| Total (N = 720) | 2018 (N = 309) | 2019 (N = 411) | |
|---|---|---|---|
| Age group (years) | |||
| 10‒15 | 110 (15.3%) | 49 (15.9%) | 61 (14.8%) |
| 16‒22 | 197 (27.4%) | 91 (29.4%) | 106 (25.8%) |
| 22‒34 | 124 (17.2%) | 66 (21.4%) | 58 (14.1%) |
| 35‒50 | 105 (14.6%) | 39 (12.6%) | 66 (16.1%) |
| >50 | 184 (25.6%) | 64 (20.7%) | 120 (29.2%) |
| Sex | |||
| Female | 461 (64%) | 201 (65%) | 260 (63.3%) |
| Race/ethnicity | |||
| Hispanic | 324 (45%) | 140 (45.3%) | 184 (44.8%) |
| White | 224 (31.1%) | 99 (32%) | 125 (30.4%) |
| Black | 80 (11.1%) | 37 (12%) | 43 (10.5%) |
| Asian | 56 (7.8%) | 19 (6.1%) | 37 (9%) |
| Other | 15 (2.1%) | 6 (1.9%) | 9 (2.2%) |
| Multiple | 8 (1.1%) | 5 (1.6%) | 3 (0.7%) |
| Pacific Islander | 7 (1%) | 2 (0.6%) | 5 (1.2%) |
| Unknown | 6 (0.8%) | 1 (0.3%) | 5 (1.2%) |
| Disposition | |||
| Admission | 86 (11.9%) | 35 (11.3%) | 51 (12.4%) |
| Charlson comorbidity categories | |||
| 0 | 455 (63.2%) | 201 (65%) | 254 (61.8%) |
| 1 | 129 (17.9%) | 62 (20.1%) | 67 (16.3%) |
| 2 | 54 (7.5%) | 18 (5.8%) | 36 (8.8%) |
| 3+ | 82 (11.4%) | 28 (9.1%) | 54 (13.1%) |
| Length of stay (among hospitalized patients) | |||
| N | 35 | 20 | 15 |
| Mean (SD) | 2.7 (2.33) | 2.8 (1.88) | 2.5 (2.88) |
| Died within 30 days after ED visit | |||
| Yes | 2 (0.3%) | 0 (0%) | 2 (0.5%) |
| History of substance use disorder in the past 12 months before ED visit | |||
| Yes | 126 (17.5%) | 67 (21.7%) | 59 (14.4%) |
| Opioid and benzo prescriptions in the past 12 months before ED visit | |||
| Yes | 153 (21.3%) | 64 (20.7%) | 89 (21.7%) |
| Daily MME >90 in the past 12 months before ED visit | |||
| Yes | 2 (0.3%) | 1 (0.3%) | 1 (0.2%) |
Abbreviations: MME, morphine milligram equivalents; SD, standard deviation.
Interrupted time series analysis showed no significant changes in the rate of ED visits for opioid overdose (odds ratio [OR] = 1.02, 95% confidence interval [CI] = 0.98‒1.07) and non‐opioid overdose (OR = 0.97, 95% CI = 0.93‒1.01) (Table 4). Difference‐in‐differences analysis revealed no significant change in hospitalizations (coefficient [CE] = −0.05, 95% CI = −0.11 to 0.02), 30‐day mortality (CE = −0.01, 95% CI = −0.03 to 0.01), or length of stay when hospitalized (CE = 1.77, 95% CI = −1.92 to 5.46) when comparing opioid and non‐opioid overdose in the pre‐ versus post‐intervention period (Table 5).
TABLE 4.
Adjusted odds ratio (OR) from interrupted time series logistic regression for emergency department (ED) encounters before (January 1, 2018‒December 31, 2018), immediately after (January 1, 2019‒31, 2019), and 1 year after (February 1, 2019‒December 31, 2019) implementation of California naloxone law.
| Any opioid poison | Any non‐opioid poison | Any naloxone prescribed at discharge | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | LCI | UCI | OR | LCI | UCI | OR | LCI | UCI | |
| Pre‐policy trend | 0.99 | 0.96 | 1.02 | 1.02 | 0.98 | 1.05 | 1.60 | 1.45 | 1.76 |
| Immediate change in level post‐policy | 0.93 | 0.69 | 1.27 | 1.21 | 0.91 | 1.62 | 13.23 | 10.26 | 17.06 |
| Change in trend between pre‐ and post‐policy | 1.02 | 0.98 | 1.07 | 0.97 | 0.93 | 1.01 | 0.62 | 0.56 | 0.68 |
| No. of observations | 1,110,127 | 1,110,127 | 1,089,211 | ||||||
Note: All analyses controlled for age group (10‒15 [reference group], 16‒22, 23‒34, 35‒50, and >50), sex (female [reference group] and male), race/ethnicity (NH White [reference group], NH Black, Hispanic, NH Asian/Pacific Islanders, and others), and Charlson score (none [reference group], 1, 2, and 3+). The first two analyses for opioid poison and non‐opioid poison excluded 25 visits, while last analysis for naloxone prescribed at discharge excluded 23 visits due to missing data on sex.
Abbreviations: LCI, lower confidence interval; UCI, upper confidence interval.
TABLE 5.
Adjusted difference‐in‐difference estimates of the impact of the implementation of California naloxone law on patient outcomes.
| Outcome: admission | Outcome: 30‐day mortality | Outcome: length of stay | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CE | LL | UL | p‐Value | CE | LL | UL | p‐Value | CE | LL | UL | p‐Value | |
| Year × poison type | ‒0.06 | ‒0.14 | 0.02 | 0.12 | ‒0.01 | ‒0.03 | 0.01 | 0.53 | 1.77 | ‒1.92 | 5.46 | 0.34 |
| No. of observations | 1415 | 1415 | 155 | |||||||||
Note: All analyses controlled for age group (10‒15 [reference group], 16‒22, 23‒34, 35‒50, and >50), sex (female [reference group] and male), race/ethnicity (NH White [reference group], NH Black, Hispanic, NH Asian/Pacific Islanders, and others), and Charlson score (none [reference group], 1, 2, and 3+).
Abbreviations: CE, coefficient; LL, lower limit; UL, upper limit.
Data from 2019 revealed a substantial increase in the frequency of naloxone prescription from the previous year. Naloxone orders increased from 104 in 2018 to 6031 in 2019 (p < 0.0001). Of those who were prescribed naloxone, 77.8% (74% pre‐ and 77.8% post‐implementation) filled the prescription within 2 days of discharge from the ED. There was also a significant decrease in opioids ordered within the study period. In 2019, 13.5% of the study population received an opioid prescription within 2 days of ED discharge, a decrease from 14.9% in 2018 (p < 0.0001). Furthermore, the daily MME dispensed per order also decreased from 34.7 (SD ±27.66) to 32.1 (SD ±26.24) post‐intervention (p < 0.0001). There was also a corresponding decrease in dispensed opioids greater than 90 MME (0.5% pre‐ and 0.3% post‐intervention, p < 0.0001). An immediate decreasing trend in the rate of monthly opioid prescription at discharge also took place (OR = 0.90, 95% CI = 0.88‒0.92).
3.3. Limitations
The main limitation of our study is its retrospective study design. To address this, we used an interrupted time series that included an extended preintervention period and emphasized changes that occurred in the period immediately following the intervention as well as the 12 months following the intervention. Segmented regression analysis of an interrupted time series is an accepted powerful study design that allows us to distinguish the effects of the intervention from secular change. 15 , 16 However, because of the retrospective study design, we cannot account for all other confounding factors that may have contributed to the rates of overdose during this time period. Our retrospective study design relied on data abstraction using the electronic medical record, and identifying cohorts for the subgroup analysis using ICD‐10 codes, which are potentially subject to inaccuracy.
Furthermore, while this has become a state‐wide mandate with an additional level of implementation into our electronic medical record, it is possible that there is still some variation in compliance on the individual provider level. Therefore, it is possible that this intervention may not necessarily produce the same results in all different settings, depending on the level of its adoption. Also, despite our trends indicating significant changes in prescription rates and subtle trends in improvement of overdose deaths, it is important to acknowledge the limited duration of our study. This included the post‐intervention period of only 12 months, which may limit interpretation of sustaining the outcome over a longer period of time.
4. DISCUSSION
Overall, we found that the implementation of California's law mandating naloxone be offered to patients at increased risk of opioid overdose was not associated with a significant decrease in ED opioid overdose visits. There was also no significant change in admission rates, length of stay, or 30‐day mortality of these patients. Although this was not significant when considering the difference‐in‐differences analysis, we did observe an 8.3% reduction in opioid overdose admission rates and a rise in the length of stay post‐admission by an average of 1.8 days, potentially indicating that those who were admitted after the intervention were a greater acuity subset of patients requiring a higher level of care.
The most significant results were the effect of the policy implementation on opioid and naloxone prescribing. There were statistically significant declines in number of opioid prescriptions (−1.4%) as well as total MME prescribed per order (−14.1 MME). Despite not being the intent of this policy, we found that the law was associated with a 7.6% decrease in opioid prescriptions within the first month, with a subsequent slow rise in opioid prescription, indicating a potential need for continuing education on the importance of appropriate opioid prescribing. This unintended consequence of the law on opioid prescribing indicates an area of need for future studies. In contrast to the decrease in total opioid prescriptions, we observed a 5800% increase in the prescription of naloxone after the law came into effect. Although naloxone prescribing greatly increased, opioid overdose‐related ED visits remained unchanged. While this demonstrates the policy's effectiveness in increasing naloxone prescribing, it also shows a need for further interventions to combat the opioid epidemic in the future.
Being within an integrated healthcare system has also allowed us to track the dispensing rates of both opioids and naloxone. We see that throughout the study period, 97.2% of patients filled their opioid prescriptions (97.5% pre‐ and 97% post‐implementation) and 77.8% of patients filled their naloxone prescriptions (74% pre‐ and 78% post‐intervention) within 2 days. This demonstrates that nearly four of five patients are actively filling their naloxone prescription, a far greater number than in previous studies. 22 This is potentially a result of increased medication adherence within an integrated healthcare system that is often not affiliated with other systems, although further research is necessary in this area.
It is important to acknowledge the impact that COVID‐19 may also have on opioid overdose as the pandemic progresses. Studies reveal concern of increased opioid overdose as social isolation and hesitation toward seeking care expand. 23 Similarly, studies have already shown negative impacts of the COVID‐19 pandemic on opioid overdose rates. 24 For this reason, further interventions are needed to decrease opioid overdose in a more urgent fashion.
In summary, implementation of a California law mandating that naloxone be offered to patients at increased risk of opioid‐related overdose was associated with a large increase in the prescription and dispensing of naloxone to high‐risk patients. This adoption was not associated with a significant impact on opioid overdose‐related ED visits, hospitalizations, or mortality. While this policy may be a step forward in combating the opioid epidemic, future studies are needed to understand how other supplemental interventions and policies may decrease opioid‐related mortality and overdose.
CONFLICT OF INTEREST STATEMENT
The authors declare they have no conflicts of interest.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
The authors thank the patients of Kaiser Permanente for helping us improve care through the use of information collected through our electronic health record systems. This research was supported by a grant from the Regional Research Committee of Kaiser Permanente Southern California (grant no. KP‐RRC‐20200501).
Biography
Ali Ghobadi, MD, is an Emergency Physician and Assistant Chief at Kaiser Permanente Orange County Medical Center in Anaheim, CA, USA.

Ghobadi A, Hanna M, Tovar S, et al. Impact of California's naloxone co‐prescription law on emergency department visits, 30‐day mortality, and prescription patterns. JACEP Open. 2024;5:e13236. 10.1002/emp2.13236
Supervising Editor: Brittany Punches, PhD, RN
REFERENCES
- 1. Jones MR, Viswanath O, Peck J, Kaye AD, Gill JS, Simopoulos TT. A brief history of the opioid epidemic and strategies for pain medicine. Pain Ther. 2018;7(1):13‐21. doi: 10.1007/s40122-018-0097-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ahmad FB, Rossen LM, Sutton P. In: Rossen LM, Lipphardt A, Ahmad FB, Keralis JM, Chong Y, Eds. Provisional Drug Overdose Death Counts. National Center for Health Statistics; 2022. [Google Scholar]
- 3. Luo F, Li M, Florence C. State‐level economic costs of opioid use disorder and fatal opioid overdose—United States, 2017. MMWR Morb Mortal Wkly Rep. 2021;70:541‐546. doi: 10.15585/mmwr.mm7015a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Florence C, Luo F, Rice K. The economic burden of opioid use disorder and fatal opioid overdose in the United States, 2017. Drug Alcohol Depend. 2021;218:108350. doi: 10.1016/j.drugalcdep.2020.108350external [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sharfstein JM, Olsen Y. Making amends for the opioid epidemic. JAMA. 2019;321(15):1446‐1447. doi: 10.1001/jama.2019.3505 [DOI] [PubMed] [Google Scholar]
- 6. Saini J, Johnson B, Qato DM. Self‐reported treatment need and barriers to care for adults with opioid use disorder: the US national survey on drug use and health, 2015 to 2019. Am J Public Health. 2022;112:284‐295. doi: 10.2105/AJPH.2021.306577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vadivelu N, Kai AM, Kodumudi V, Sramcik J, Kaye AD. The opioid crisis: a comprehensive overview. Curr Pain Headache Rep. 2018;22(3):16. doi: 10.1007/s11916-018-0670-z [DOI] [PubMed] [Google Scholar]
- 8. Rao IJ, Humphreys K, Brandeau ML. Effectiveness of policies for addressing the US opioid epidemic: a model‐based analysis from the Stanford‐Lancet Commission on the North American Opioid Crisis. Lancet Reg Health Am. 2021;3:100031. doi: 10.1016/j.lana.2021.100031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bell J, Strang J. Medication treatment of opioid use disorder. Biol Psychiatry. 2020;87(1):82‐88. doi: 10.1016/j.biopsych.2019.06.020 [DOI] [PubMed] [Google Scholar]
- 10. Puzantian T, Gasper JJ. Provision of naloxone without a prescription by California pharmacists 2 years after legislation implementation. JAMA. 2018;320(18):1933‐1934. doi: 10.1001/jama.2018.12291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Davis C, Carr D. State legal innovations to encourage naloxone dispensing. J Am Pharm Assoc (2003). 2017;57(2S):S180‐S184. doi: 10.1016/j.japh.2016.11.007 [DOI] [PubMed] [Google Scholar]
- 12.Cal. Civ. Code § 4052.01.
- 13.Cal. Civ. Code § 1714.22.
- 14.Ca. Bus. & Prof. §§ 740‐742.
- 15. Ansari F, Gray K, Nathwani D, et al. Outcomes of an intervention to improve hospital antibiotic prescribing: interrupted time series with segmented regression analysis. J Antimicrob Chemother. 2003;52(5):842‐848. doi: 10.1093/jac/dkg459 [DOI] [PubMed] [Google Scholar]
- 16. Taljaard M, McKenzie JE, Ramsay CR, Grimshaw JM. The use of segmented regression in analysing interrupted time series studies: an example in pre‐hospital ambulance care. Implement Sci. 2014;9:77. doi: 10.1186/1748-5908-9-77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dimick JB, Ryan AM. Methods for evaluating changes in health care policy: the difference‐in‐differences approach. JAMA. 2014;312(22):2401‐2402. doi: 10.1001/jama.2014.16153 [DOI] [PubMed] [Google Scholar]
- 18. Zhou H, Taber C, Arcona S, Li Y. Difference‐in‐differences method in comparative effectiveness research: utility with unbalanced groups. Appl Health Econ Health Policy. 2016;14(4):419‐429. doi: 10.1007/s40258-016-0249-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Koebnick C, Langer‐Gould AM, Gould MK, et al. Sociodemographic characteristics of members of a large, integrated health care system: comparison with US Census Bureau data. Perm J. 2012;16(3):37‐41. doi: 10.7812/tpp/12-031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD‐9‐CM and ICD‐10 administrative data. Med Care. 2005;43(11):1130‐1139. doi: 10.1097/01.mlr.0000182534.19832.83 [DOI] [PubMed] [Google Scholar]
- 21. Austin SR, Wong YN, Uzzo RG, Beck JR, Egleston BL. Why summary comorbidity measures such as the Charlson Comorbidity Index and Elixhauser Score Work. Med Care. 2015;53(9):e65‐e72. doi: 10.1097/MLR.0b013e318297429c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Guy GP Jr, Haegerich TM, Evans ME, Losby JL, Young R, Jones CM. Vital signs: pharmacy‐based naloxone dispensing—United States, 2012‒2018. MMWR Morb Mortal Wkly Rep. 2019;68:679‐686. doi: 10.15585/mmwr.mm6831e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Linas BP, Savinkina A, Barbosa C, et al. A clash of epidemics: impact of the COVID‐19 pandemic response on opioid overdose. J Substance Abuse Treat. 2021;120:108158. doi: 10.1016/j.jsat.2020.108158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Glober N, Mohler G, Huynh P, et al. Impact of COVID‐19 pandemic on drug overdoses in Indianapolis. J Urban Health. 2020;97:802‐807. doi: 10.1007/s11524-020-00484-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


