Abstract
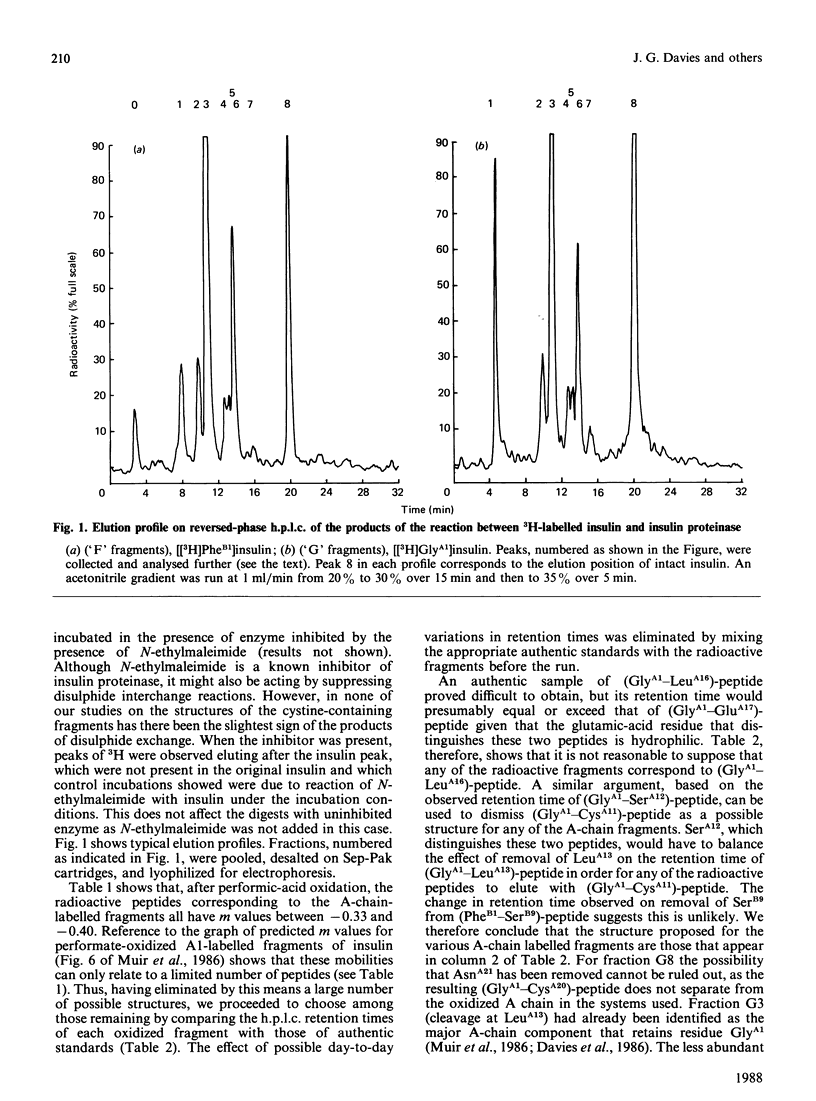
(1) We [Muir, Offord & Davies (1986) Biochem. J. 237, 631-637 and Davies, Muir & Offord (1986) Biochem. J. 240, 609-612] have previously identified a major product in the degradation of insulin by insulin proteinase (the N-terminal fragment produced by cleavage between residues LeuA13 and TyrA14, SerB9 and HisB10) together with evidence for a minor cleavage site between HisB10 and LeuB11 or between LeuB11 and ValB12. (2) We now present evidence for minor sites of cleavage between TyrA14 and GlnA15, GluB13 and AlaB14 as well as HisB10 and LeuB11.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assoian R. K., Tager H. S. Peptide intermediates in the cellular metabolism of insulin. J Biol Chem. 1982 Aug 10;257(15):9078–9085. [PubMed] [Google Scholar]
- Burghen G. A., Kitabchi A. E., Brush J. S. Characterization of a rat liver protease with specificity for insulin. Endocrinology. 1972 Sep;91(3):633–642. doi: 10.1210/endo-91-3-633. [DOI] [PubMed] [Google Scholar]
- Davies J. G., Muir A. V., Offord R. E. Identification of some cleavage sites of insulin by insulin proteinase. Biochem J. 1986 Dec 1;240(2):609–612. doi: 10.1042/bj2400609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J. G., Offord R. E. The preparation of tritiated insulin specifically labelled by semisynthesis at glycine-A1. Biochem J. 1985 Oct 15;231(2):389–392. doi: 10.1042/bj2310389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth W. C., Heinemann M. A., Kitabchi A. E. Purification of insulin-specific protease by affinity chromatography. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3698–3702. doi: 10.1073/pnas.69.12.3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halban P. A., Karakash C., Davies J. G., Offord R. E. The degradation of semisynthetic tritiated insulin by perfused mouse livers. Biochem J. 1976 Nov 15;160(2):409–412. doi: 10.1042/bj1600409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halban P. A., Offord R. E. The preparation of a semisynthetic tritiated insulin with a specific radioactivity of up to 20 Curies per millimole. Biochem J. 1975 Nov;151(2):219–225. doi: 10.1042/bj1510219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel F. G., Peavy D. E., Ryan M. P., Duckworth W. C. High performance liquid chromatographic analysis of insulin degradation by rat skeletal muscle insulin protease. Endocrinology. 1986 Jan;118(1):328–333. doi: 10.1210/endo-118-1-328. [DOI] [PubMed] [Google Scholar]
- Juul S. M., Jones R. H., Evans J. L., Neffe J., Sönksen P. H., Brandenburg D. Evidence for an early degradative event to the insulin molecule following binding to hepatocyte receptors. Biochim Biophys Acta. 1986 Apr 14;856(2):310–319. doi: 10.1016/0005-2736(86)90041-6. [DOI] [PubMed] [Google Scholar]
- Karakash C., Jeanrenaud B. Insulin binding and removal by livers of genetically obese rats. Diabetes. 1983 Jul;32(7):605–609. doi: 10.2337/diab.32.7.605. [DOI] [PubMed] [Google Scholar]
- Misbin R. I., Almira E. C. The fate of insulin in rat hepatocytes. Evidence for the release of an immunologically active fragment. Diabetes. 1984 Apr;33(4):355–361. doi: 10.2337/diab.33.4.355. [DOI] [PubMed] [Google Scholar]
- Muir A., Offord R. E., Davies J. G. The identification of a major product of the degradation of insulin by 'insulin proteinase' (EC 3.4.22.11). Biochem J. 1986 Aug 1;237(3):631–637. doi: 10.1042/bj2370631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullen R. A., Lindsay D. G., Wood S. P., Tickle I. J., Blundell T. L., Wollmer A., Krail G., Brandenburg D., Zahn H., Gliemann J. Receptor-binding region of insulin. Nature. 1976 Feb 5;259(5542):369–373. doi: 10.1038/259369a0. [DOI] [PubMed] [Google Scholar]
- Savoy L. A., Jones R. M., Pochon S., Davies J. G., Muir A. V., Offord R. E., Rose K. Identification by fast atom bombardment mass spectrometry of insulin fragments produced by insulin proteinase. Biochem J. 1988 Jan 1;249(1):215–222. doi: 10.1042/bj2490215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechter I., Berger A. On the size of the active site in proteases. I. Papain. Biochem Biophys Res Commun. 1967 Apr 20;27(2):157–162. doi: 10.1016/s0006-291x(67)80055-x. [DOI] [PubMed] [Google Scholar]
- Varandani P. T., Shroyer L. A. Identification of an insulin fragment produced by an insulin degrading enzyme, neutral thiopeptidase. Mol Cell Endocrinol. 1987 Apr;50(3):171–175. doi: 10.1016/0303-7207(87)90014-1. [DOI] [PubMed] [Google Scholar]



