Abstract
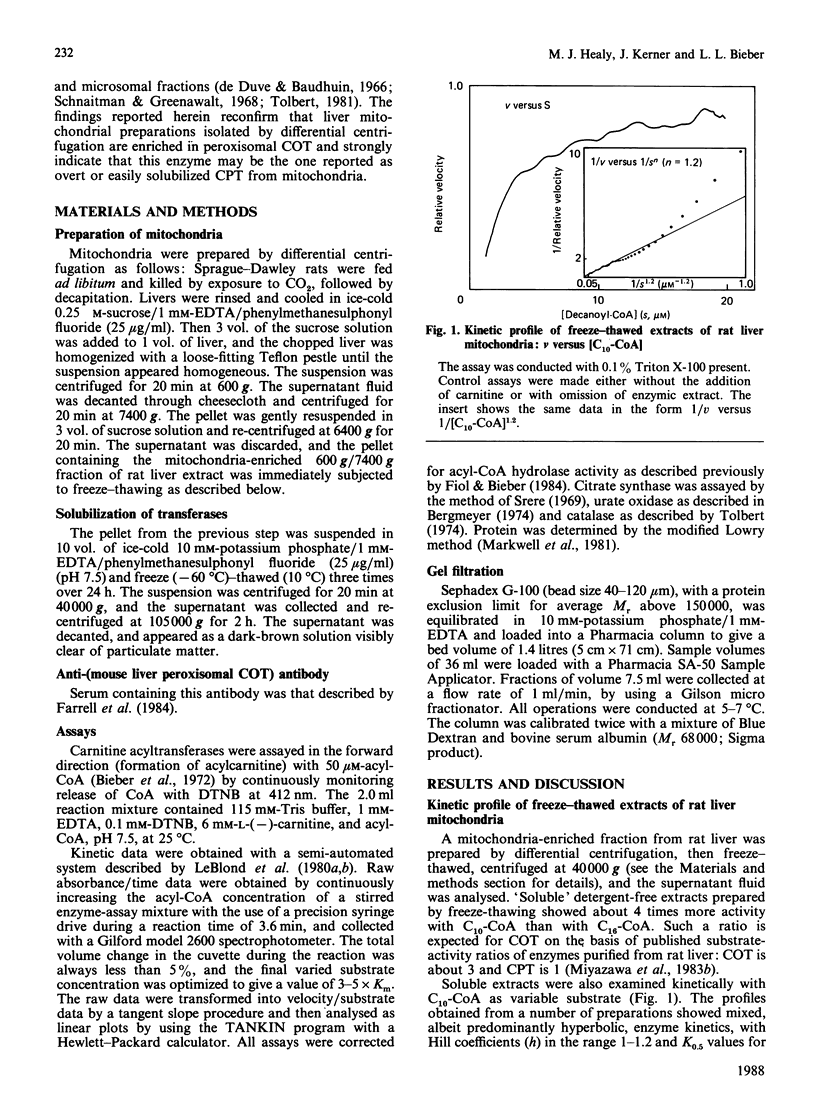
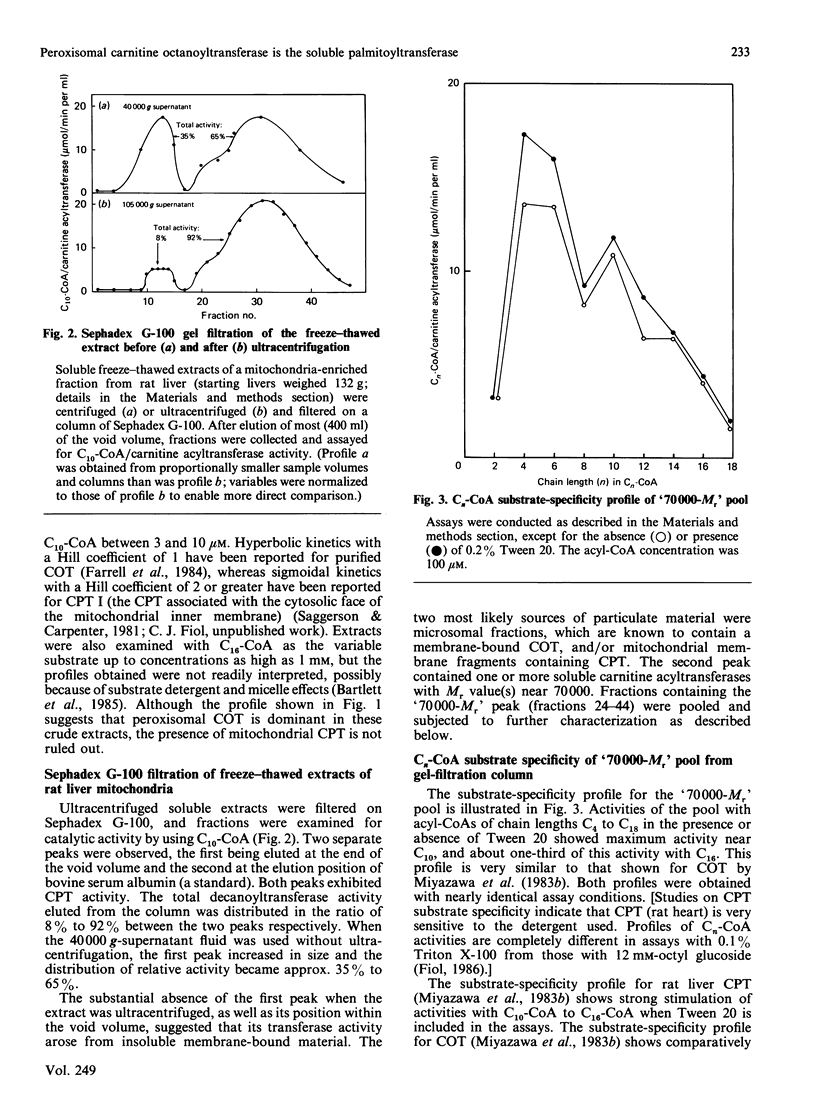
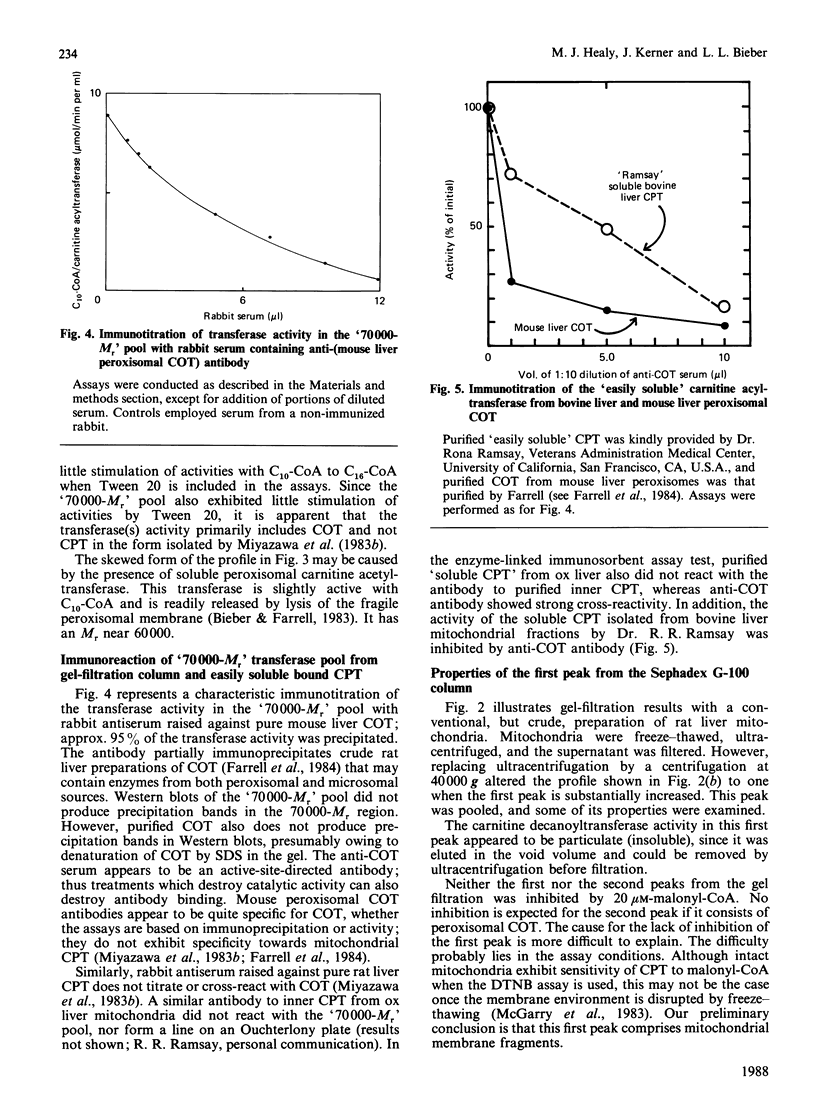
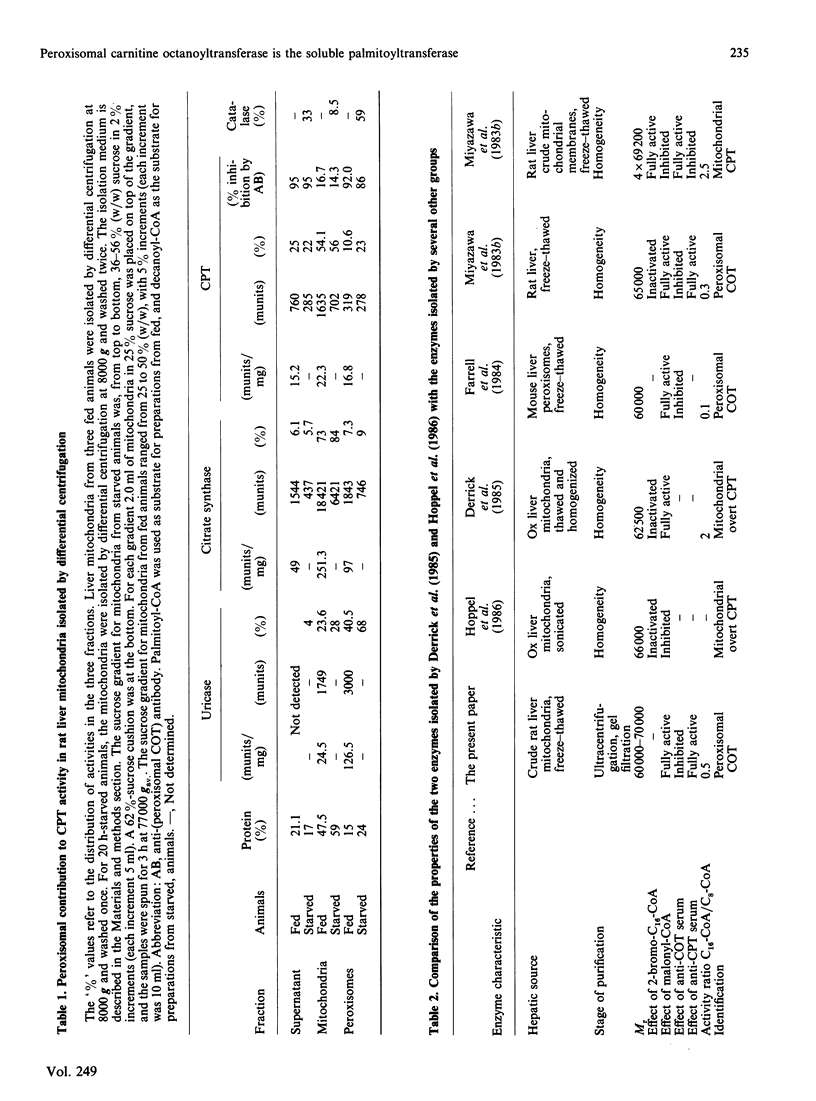
Liver mitochondria prepared by differential centrifugation are contaminated by significant quantities of peroxisomes and microsomal fractions. 'Easily solubilized carnitine palmitoyltransferase' prepared from liver mitochondria is thought to originate from the outer surface of the mitochondrial inner membrane. We have characterized the carnitine palmitoyltransferase activities of freeze-thaw extracts of rat liver mitochondrial preparations. Chromatography on Sephadex G-100 yields two broad peaks of carnitine decanoyltransferase activity: one eluted at the end of the void volume, which can be removed (precipitated) by ultracentrifugation; the second peak represents the soluble activity and is eluted at an Mr near 70,000. The activity in the soluble peak is precipitated by an antibody raised against carnitine octanoyltransferase purified from mouse liver peroxisomes. In contrast, antibody raised against carnitine palmitoyltransferase purified from liver mitochondrial membranes had no effect (P. Brady & L. Brady, personal communication). The carnitine acyltransferase activities of the Mr-70,000 peak in the presence or absence of Tween 20 showed maximum activity with decanoyl-CoA and about one-third of this activity with palmitoyl-CoA, similar to peroxisomal carnitine octanoyltransferase. These data show that 7500 g preparations of liver mitochondria isolated by differential centrifugation are enriched by peroxisomal carnitine octanoyltransferase (approx. 20% of the protein of the fraction is peroxisomal) and indicate that this enzyme may be the one reported as 'overt' or 'easily solubilized' mitochondrial carnitine palmitoyltransferase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartlett K., Bartlett P., Bartlett N., Sherratt H. S. Kinetics of enzymes requiring long-chain acyl-CoA esters as substrates: effects of substrate binding to albumin. Biochem J. 1985 Jul 15;229(2):559–560. doi: 10.1042/bj2290559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergstrom J. D., Reitz R. C. Studies on carnitine palmitoyl transferase: the similar nature of CPTi (inner form) and CPTo (outer form). Arch Biochem Biophys. 1980 Oct 1;204(1):71–79. doi: 10.1016/0003-9861(80)90008-9. [DOI] [PubMed] [Google Scholar]
- Bieber L. L., Abraham T., Helmrath T. A rapid spectrophotometric assay for carnitine palmitoyltransferase. Anal Biochem. 1972 Dec;50(2):509–518. doi: 10.1016/0003-2697(72)90061-9. [DOI] [PubMed] [Google Scholar]
- Clarke P. R., Bieber L. L. Isolation and purification of mitochondrial carnitine octanoyltransferase activities from beef heart. J Biol Chem. 1981 Oct 10;256(19):9861–9868. [PubMed] [Google Scholar]
- De Duve C., Baudhuin P. Peroxisomes (microbodies and related particles). Physiol Rev. 1966 Apr;46(2):323–357. doi: 10.1152/physrev.1966.46.2.323. [DOI] [PubMed] [Google Scholar]
- Farrell S. O., Fiol C. J., Reddy J. K., Bieber L. L. Properties of purified carnitine acyltransferases of mouse liver peroxisomes. J Biol Chem. 1984 Nov 10;259(21):13089–13095. [PubMed] [Google Scholar]
- Fiol C. J., Bieber L. L. Sigmoid kinetics of purified beef heart mitochondrial carnitine palmitoyltransferase. Effect of pH and malonyl-CoA. J Biol Chem. 1984 Nov 10;259(21):13084–13088. [PubMed] [Google Scholar]
- Ishii S., Ishii H., Suga T. The presence of peroxisomal carnitine palmitoyltransferase in chick embryo liver. J Biochem. 1985 Sep;98(3):747–755. doi: 10.1093/oxfordjournals.jbchem.a135332. [DOI] [PubMed] [Google Scholar]
- LeBlond D. J., Ashendel C. L., Wood W. A. Determination of enzyme kinetic parameters by continuous addition of substrate to a single reaction mixture and analysis by a tangent-slope procedure. I. Analysis of the method using computed progress curves. Anal Biochem. 1980 May 15;104(2):355–369. doi: 10.1016/0003-2697(80)90087-1. [DOI] [PubMed] [Google Scholar]
- LeBlond D. J., Ashendel C. L., Wood W. A. Determination of enzyme kinetic parameters by continuous addition of substrate to a single reaction mixture and analysis by a tangent-slope procedure. II. Application of the method to soluble and immobilized enzymes. Anal Biochem. 1980 May 15;104(2):370–385. doi: 10.1016/0003-2697(80)90088-3. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Tolbert N. E., Bieber L. L. Protein determination in membrane and lipoprotein samples: manual and automated procedures. Methods Enzymol. 1981;72:296–303. doi: 10.1016/s0076-6879(81)72018-4. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., McGroarty E. J., Bieber L. L., Tolbert N. E. The subcellular distribution of carnitine acyltransferases in mammalian liver and kidney. A new peroxisomal enzyme. J Biol Chem. 1973 May 25;248(10):3426–3432. [PubMed] [Google Scholar]
- McGarry J. D., Mills S. E., Long C. S., Foster D. W. Observations on the affinity for carnitine, and malonyl-CoA sensitivity, of carnitine palmitoyltransferase I in animal and human tissues. Demonstration of the presence of malonyl-CoA in non-hepatic tissues of the rat. Biochem J. 1983 Jul 15;214(1):21–28. doi: 10.1042/bj2140021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazawa S., Ozasa H., Furuta S., Osumi T., Hashimoto T. Purification and properties of carnitine acetyltransferase from rat liver. J Biochem. 1983 Feb;93(2):439–451. doi: 10.1093/oxfordjournals.jbchem.a134198. [DOI] [PubMed] [Google Scholar]
- Miyazawa S., Ozasa H., Osumi T., Hashimoto T. Purification and properties of carnitine octanoyltransferase and carnitine palmitoyltransferase from rat liver. J Biochem. 1983 Aug;94(2):529–542. doi: 10.1093/oxfordjournals.jbchem.a134384. [DOI] [PubMed] [Google Scholar]
- Murthy M. S., Pande S. V. Malonyl-CoA binding site and the overt carnitine palmitoyltransferase activity reside on the opposite sides of the outer mitochondrial membrane. Proc Natl Acad Sci U S A. 1987 Jan;84(2):378–382. doi: 10.1073/pnas.84.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay R. R. The soluble carnitine palmitoyltransferase from bovine liver. A comparison with the enzymes from peroxisomes and from the mitochondrial inner membrane. Biochem J. 1988 Jan 1;249(1):239–245. doi: 10.1042/bj2490239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggerson E. D., Carpenter C. A. Effects of fasting and malonyl CoA on the kinetics of carnitine palmitoyltransferase and carnitine octanoyltransferase in intact rat liver mitochondria. FEBS Lett. 1981 Sep 28;132(2):166–168. doi: 10.1016/0014-5793(81)81152-0. [DOI] [PubMed] [Google Scholar]
- Schnaitman C., Greenawalt J. W. Enzymatic properties of the inner and outer membranes of rat liver mitochondria. J Cell Biol. 1968 Jul;38(1):158–175. doi: 10.1083/jcb.38.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolbert N. E. Isolation of subcellular organelles of metabolism on isopycnic sucrose gradients. Methods Enzymol. 1974;31:734–746. doi: 10.1016/0076-6879(74)31077-4. [DOI] [PubMed] [Google Scholar]
- Tolbert N. E. Metabolic pathways in peroxisomes and glyoxysomes. Annu Rev Biochem. 1981;50:133–157. doi: 10.1146/annurev.bi.50.070181.001025. [DOI] [PubMed] [Google Scholar]
- Woldegiorgis G., Bremer J., Shrago E. Substrate inhibition of carnitine palmitoyltransferase by palmitoyl-CoA and activation by phospholipids and proteins. Biochim Biophys Acta. 1985 Nov 14;837(2):135–140. doi: 10.1016/0005-2760(85)90236-x. [DOI] [PubMed] [Google Scholar]


