Abstract
Studies have shown that the cachexia index (CXI) is a useful predictor of cachexia in patients with colorectal cancer. However, the application of the CXI is limited stemming from the intricacy and additional cost of radiographic examinations. This study aimed to develop an easy-to-use and practical CXI based on fat-free mass index (FFMI-CXI) to evaluate the prognostic value of FFMI-CXI in CRC. A total of 656 patients with CRC were enrolled in the Investigation on Nutrition Status and Clinical Outcome of Common Cancers (INSCOC) study. The FFMI-CXI was calculated as [FFMI (kg)/height (m)2 × serum albumin (g/L)]/neutrophil-lymphocyte ratio. The cutoff value for FFMI-CXI was determined through the analysis of ROC curves and Youden’s index for both male and female cohorts. Kaplan–Meier survival curves with log-rank tests were conducted to compare time–event relationships between different groups. Cox proportional hazards regression models incorporating both univariate and multivariate variables were employed to explore the independent prognostic factors associated with OS. Logistic regression analysis was performed to assess the association of the FFMI-CXI with secondary outcomes. The major outcome was 5-year overall survival (OS). Based on the cutoff values, 331 patients had low FFMI-CXI, and 325 patients had high FFMI-CXI. Patients in the low FFMI-CXI subgroup were significantly older and had advanced TNM stage, malnutrition, high systemic inflammation, long hospitalizations, high hospitalization costs, adverse short-term outcomes, and all-cause mortality. Multivariate Cox regression analyses revealed that FFMI-CXI (HR 0.47, 95% CI 0.33–0.66; p < 0.001) and TNM stage (HR 3.38, 95% CI 2.63–4.35; p < 0.001) were independently associated with OS in CRC patients. K-M survival curves revealed that the CRC patients with a high FFMI-CXI had significantly more favorable OS than those with low FFMI-CXI (62.84% vs. 84.31%; log-rank p < 0.001). Furthermore, the FFMI-CXI was valuable for predicting 90-day outcomes, malnutrition, cancer cachexia, length of hospitalization, and hospitalization expenses. This study revealed that the FFMI-CXI can be used as a prognostic indicator in patients with CRC. Patients with low FFMI-CXI should receive more attention.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-75485-z.
Keywords: Colorectal cancer, FFMI-based cachexia index, Cancer cachexia, Prognosis
Subject terms: Cancer, Medical research, Oncology
Introduction
Among malignant cancers, colorectal cancer (CRC) ranks as one of the most prevalent. Despite the advancements in minimally invasive surgery and adjuvant therapy for patients with CRC, there is still a need to accurately identify those with a poor prognosis.
Cachexia is a multifaceted syndrome distinguished by persistent skeletal muscle mass depletion (with or without concurrent loss of adipose tissue) and progressive functional decline that cannot be treated with traditional nutritional interventions1. Up to 80% of patients with malignancies develop cachexia, with the highest incidence occurring in gastrointestinal cancer2. There is a nearly 50% prevalence of cachexia in patients with colorectal cancer3. Cancer-associated cachexia, recognized as a cancer-related adverse event, correlates with decreased physical function, diminished tolerance to cancer treatment, and inferior survival outcomes. Additionally, cachexia contributes to 20% of cancer-related deaths4 and is associated with increasing weight loss grades5, often progressing to heart and respiratory failure.
The cachexia index (CXI) was created by Jafri et al. to assess the extent of cachexia in non-small cell lung cancer patients3. As previously reported, the CXI was calculated as follows: skeletal muscle index (SMI) × serum albumin (ALB)/neutrophil-to-lymphocyte ratio (NLR). The skeletal muscle area (SMA) was measured as the skeletal muscle area at the lumbar spine (L3) level using computed tomography (CT) images as described in the literature. The SMI is calculated from the SMA/height (m2). Recent studies have shown that the cachexia index (CXI) serves as a valuable predictor of cachexia in patients with colorectal cancer, gastric cancer, hepatocellular carcinoma, diffuse large B-cell lymphoma, and small cell lung cancer6–9.
However, the application of the CXI is limited stemming from the intricacy and additional cost of radiographic examinations. Several methods have been used to evaluate the SMI, including CT, magnetic resonance imaging, dual energy X-ray absorptiometry, and bioelectrical impedance analysis (BIA). Compared with the DXA method, BIA is a reliable method for measuring body composition in patients with different types of cancer10–12. Fat-free mass (FFM) refers to body composition, including muscle and bone mass, and organs such as the liver in humans. FFM detected by BIA is a simple technique that can be used to identify patients with signs of sarcopenia who are at increased risk of treatment-related complications and mortality. The FFM index (FFMI) was calculated as FFMI/height (m2), which is one of the parameters used to evaluate muscle mass13. Therefore, the present study aimed to develop an easy-to-use and practical FFMI-based CXI (FFMI-CXI) to assess the predictive power of FFMI-CXI in CRC and predict the risk of cachexia.
Methods
Patient
Our study participants were drawn from the Investigation of Nutrition Status and its Clinical Outcome of Common Cancers (INSCOC), which was a multicentre prospective study initiated by our research team in 2013. Registration for this cohort can be found at http://www.chictr.org.cn (registration number: ChiCTR1800020329). Patients meeting the predefined inclusion criteria were enrolled in this study from the INSCOC cohort between 2012 and 2019. Participants had to meet the following criteria: (a) be over the age of 18; (b) patients with pathologically confirmed colon cancer; (c) be able to provide detailed anthropometric and serological information and (d) obtain informed consent in written form. Criteria for exclusion included: (a) patients with incomplete clinical records; (b) patients with history of other malignancies; (c) patients with severe comorbidities, acute infections, or pregnancy and (d) patients with a hospitalization duration of less than 48 h. Patients who were hospitalized more than twice during the study period had only data from the initial survey included. Patients underwent annual follow-up assessments via either in-person interviews or telephone inquiries until death, final recorded contact, or the conclusion of the last follow-up. 20 October 2020 marked the end of the follow-up. This study followed the principles outlined in the Declaration of Helsinki and received approval from the ethics committees of all the institutions involved in the research. Participants’ Informed consent was obtained in writing before participation in the study. The data in this study were analyzed while ensuring the confidentiality of personal information.
Calculation of the FFMI-CXI and outcomes
BIA was conducted under fasting conditions or 2 hours postprandially via the body composition analyzer InBody S10 (Biospace, Seoul, Korea). And the timepoint of the BIA assessment was the same time as that of other clinical or biological assessments. The instrument automatically processed and computed pertinent metrics related to human body composition, encompassing weight, FFM, and muscle mass. In order to calculate the FFMI-CXI, we used the following formula: [FFMI (kg)/height (m)2 × serum ALB (g/L)]/NLR. Neutrophil to lymphocyte ratio was represented by the NLR. The primary endpoint was overall survival (OS) at 5 years, and the secondary endpoints were short-term outcomes, cancer cachexia, malnutrition, duration of hospitalization and associated healthcare expenditures. OS was determined as the interval from the date of cancer diagnosis until either death from any cause or the last follow-up visit. Following anticancer therapy administration, short-term outcomes were specified as mortality from any cause occurring within a 90-day period. The following criteria were used to diagnose cancer cachexia according to the International Expert Consensus on Cancer Cachexia in 2011: (a) the loss of weight over the past six months was greater than 5% (without simple starvation); (b) having a BMI of less than 20 and a weight loss of more than 2%; and (c) weight loss exceeding 2% coupled with an appendicular skeletal muscle index indicative of sarcopenia.
Clinical and laboratory assessment
Experienced physicians assessed the following baseline characteristics of patients: sex, age, height, weight, body mass index, comorbid conditions (hypertension and diabetes), lifestyle (drinking and smoking), family history, tumor-node-metastasis (TNM) stage, treatment method (radiotherapy, chemotherapy or surgery), Karnofsky Performance score, hospitalization length, cost of hospitalization, score on Patient-Generated Subjective Global Assessment (PG-SGA), European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 items (EORTC QLQ-C30) version 3.0 and cachexia status.
Statistical analysis
To determine the sex-specific optimal cutoff value for FFMI-CXI, receiver operating characteristic (ROC) curve analysis, together with the application of Youden’s index, was employed. Normally distributed data was described using the mean ± standard deviation (SD). Data that was not normally distributed was represented by the median (interquartile range) or frequency (percentage). Continuous variables with normal distributions were analyzed using Student’s t tests, while continuous variables with nonnormal distributions were tested using the Mann‒Whitney test. OS was determined via the Kaplan–Meier methodology and subsequently analyzed through the log-rank test for comparison. Cox proportional hazards regression models, incorporating both univariate and multivariate variables, were employed to calculate hazard ratio (HR) and corresponding 95% confidence intervals. Sensitivity analysis was performed excluding patients who experienced mortality following the 90-day interval, using repeated survival analyses. The associations of the FFMI-CXI with short-term outcomes, cancer cachexia, malnutrition, duration of hospitalization and associated healthcare expenditures were assessed using logistic regression analysis. The risk was calculated using odds ratios (OR) and 95% confidence intervals (CI). Statistical significance was established if the p-value was less than 0.05. Analyses were performed using the following statistical software: R software (version 4.3.2; http://www.r-Project.org) and SPSS (version 26.0; IBM Corporation, Armonk, NY).
Results
Patient characteristics
Initially, a total of 699 patients with CRC were enrolled from the INSCOC study. After excluding patients without data on critical information, including stage (12 patients) and FFMI (17 patients) and loss to follow-up (14 patients), 656 patients with CRC were included in this study (Supplemental Fig. S1). The cutoff values for FFMI-CXI were 245.74 for men and 395.14 for women according to the ROC curves and Youden’s index (Supplemental Fig. S2). Based on the cutoff values, 331 patients had low FFMI-CXI, and 325 patients had high FFMI-CXI. The characteristics of the patients are shown in Table 1. The baseline characteristics of the total cohort are summarized in Supplemental Table 1. Compared to the high FFMI-CXI group, the low FFMI-CXI group had an older patient population. There was a significant association between a low FFMI-CXI and hypertension, smoking status, drinking status, advanced TNM stage, malnutrition status, high systemic inflammation, longer hospitalization duration, higher hospitalization costs, worse 90-day outcomes, and mortality from all causes (Table 1).
Table 1.
Characteristics by level of FFMI-based cachexia index in patients with colorectal cancer.
| Characteristic | FFMI-based cachexia | ||
|---|---|---|---|
| Low n = 331 | High n = 325 | p-value | |
| Sex, male, n (%) | 169 (51.1) | 226 (57.20) | < 0.001 |
| Age, years, (median (IQR)) | 61.03 ± 11.27 | 57.82 ± 9.97 | < 0.001 |
| BMI (median (IQR)) | 20.31 (22.51, 25.06) | 23.05 (20.83, 25.32) | 0.064 |
| Hypertension, yes, n (%) | 78 (23.6) | 52 (40.0) | 0.015 |
| Diabetes, yes, n (%) | 44 (13.3) | 28 (8.6) | 0.055 |
| Smoking yes, n (%) | 109 (32.9) | 140 (43.1) | 0.007 |
| Drinking, yes, n (%) | 58 (17.5) | 79 (24.30) | 0.033 |
| Family history, yes, n (%) | 57 (17.2) | 69 (21.20) | 0.192 |
| TNM stage, n (%) | |||
| Stage I | 21 (6.3) | 14 (4.3) | 0.003 |
| Stage II | 77 (23.3) | 64 (19.7) | |
| Stage III | 113 (34.1) | 157 (48.3) | |
| Stage IV | 120 (36.3) | 90 (27.7) | |
| Surgery, yes, n (%) | 141 (42.6) | 39 (12.0) | < 0.001 |
| Radiotherapy, yes, n (%) | 4 (1.2) | 2 (0.6) | 0.686 |
| Chemotherapy, yes, n (%) | 148 (44.7) | 238 (73.20) | < 0.001 |
| White blood cells, median (IQR) | 6.89 (5.25, 9.16) | 4.79 (3.92, 5.96) | < 0.001 |
| Neutrophil, median (IQR) | 4.61 (3.29, 6.91) | 2.44 (1.76, 3.33) | < 0.001 |
| Lymphocyte, median (IQR) | 1.23 (0.86, 1.59) | 1.69 (1.39, 2.08) | < 0.001 |
| Platelets, median (IQR) | 221.00 (180.00, 288.00) | 203.00 (160.50, 246.00) | 0.334 |
| Red blood cells, median (IQR) | 4.09 (3.71, 4.46) | 4.46 (4.14, 4.84) | < 0.001 |
| Hemoglobin, median (IQR) | 120.0 (104.00, 134.00) | 133 (120.00, 144.50) | < 0.001 |
| Albumin, median (IQR) | 36.7 (32.3, 40.6) | 40.60 (38.00, 42.85) | < 0.001 |
| CRP, median (IQR) | 8.15 (3.08, 40.85) | 3.14 (3.02, 6.79) | < 0.001 |
| KPS score, median (IQR) | 90.00 (80.00, 90.00) | 90.00 (90.00, 100.00) | < 0.001 |
| PG-SGA score, median (IQR) | 6.00 (4.00, 9.00) | 4 (2.00, 7.00) | < 0.001 |
| Cachexia, yes, n (%) | 127 (38.40) | 100 (30.8) | 0.064 |
| NRS2002, median (IQR) | 1 (0,1) | 1 (0, 2.5) | < 0.001 |
| Global quality of life score, median (IQR) | 47.00 (43.00, 54.00) | 45.00 (42.00, 49.00) | < 0.001 |
| Short-term outcome, yes, n (%) | 21 (6.3) | 3 (0.9) | < 0.001 |
| Status, death, n (%) | 123 (32.7) | 51 (15.7) | < 0.001 |
| Length of hospitalization, median (IQR) | 12.00 (9.00, 16.00) | 9.00 (5.00, 11.50) | < 0.001 |
| Hospitalization expenses, median (IQR) | 26319.5 (12440.8, 56243.0) | 13245.9 (7341.75, 21398.25) | < 0.001 |
Kaplan–Meier survival analysis
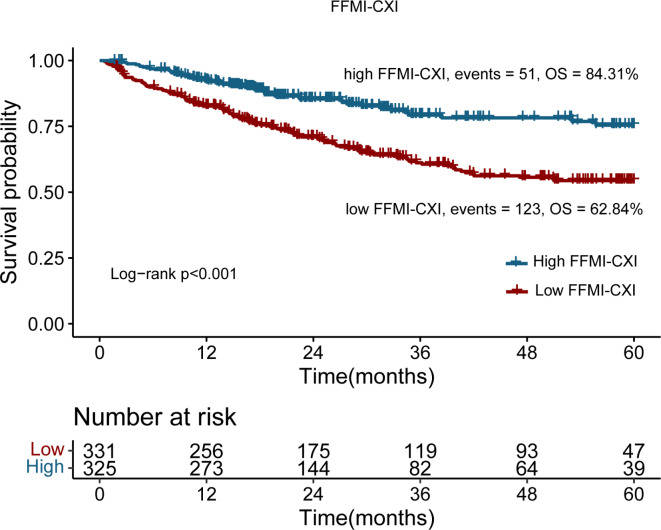
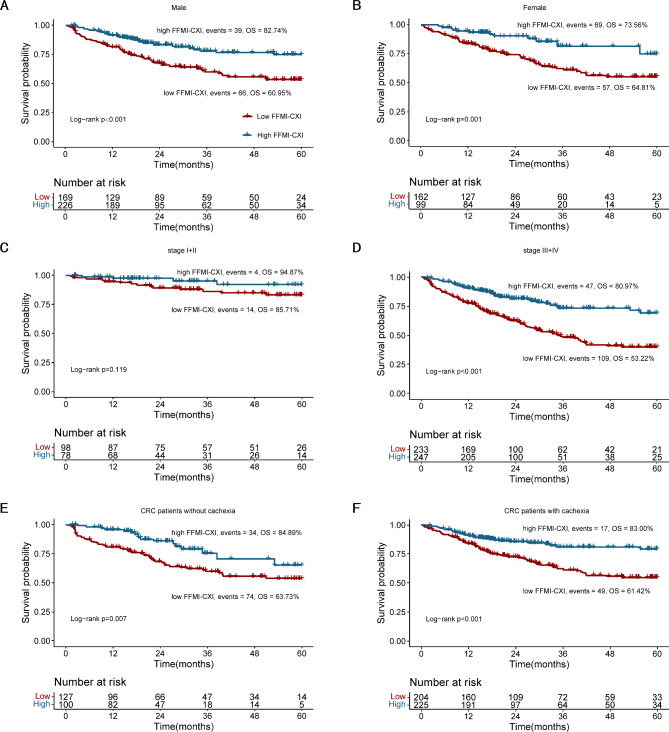
Survival was compared between the FFMI-CXI groups based on the cutoff point. The results indicated that the 5-year OS of CRC patients with a low FFMI-CXI was significantly worse than that of CRC patients with a high FFMI-CXI (62.84% vs. 84.31%; log-rank p < 0.001) (Fig. 1). The survival curves for the two groups clearly separated. To investigate the impact of the CXI on OS under various conditions, Log-rank tests were conducted according to sex using Kaplan–Meier survival curves (Fig. 2A,B), TNM stage (Fig. 2C,D) and cachexia status (Fig. 2E,F). Our results suggested that a high FFMI-CXI was correlated with notably improved OS in both male and female cohorts, as well as among patients diagnosed with TNM stage III + IV disease, irrespective of cachexia status.
Fig. 1.
Kaplan–Meier curve of FFMI-based cachexia index in patients with colorectal cancer.
Fig. 2.
Comparison of OS between patients with low FFMI-based cachexia index and high FFMI-based cachexia index in (A) male; (B) female; (C) CRC patients with TNM stage I + II; (D) CRC patients with stage III + IV; (E) CRC patients without cachexia; (F) CRC patients with cachexia.
Correlation, interaction, stratified and sensitivity analyses
To assess the influence of numerous clinicopathological variables on the OS of patients, univariate Cox regression analyses were performed. A significant nonlinear relationship was observed between FFMI-CXI and patients’ survival (Supplemental Fig. S3). The FFMI-CXI was divided into 4 quartiles for men (1st quartile: FFMI-CXI < 156.25; 2nd quartile: 156.25 ≤ FFMI-CXI index < 284.26; 3rd quartile: 284.26 ≤ FFMI-CXI index < 451.52; 4th quartile: FFMI-CXI index ≥ 451.52) and women (1st quartile: FFMI-CXI < 148.34; 2nd quartile: 148.34 ≤ FFMI-CXI index < 312.08; 3rd quartile: 312.08 ≤ FFMI-CXI index < 512.29; 4th quartile: FFMI-CXI index ≥ 512.29). A high FFMI-CXI (HR = 0.45, 95% CI 0.33–0.63; p < 0.001) was associated with a significantly longer OS. CRC patients with TNM stage III + IV disease (HR = 4.25, 95% CI 2.64–6.86; p < 0.001) had a decreased OS (Supplemental Table 2). According to multivariate analysis, only the FFMI-CXI (HR 0.47, 95% CI 0.33–0.66; p < 0.001) and TNM stage (HR = 3.38, 95% CI 2.63–4.35; p < 0.001) were significantly associated with OS (Supplemental Table 2). According to the adjusted models, a low FFMI-CXI was confirmed to be an independent prognostic factor for patients with CRC. Each SD change in the FFMI-CXI resulted in a 22% change in the HR of prognosis in cancer patients (HR = 0.78, 95% CI 0.63–0.97; p = 0.026) in the total cohort (Table 2). Multivariate survival analysis also revealed that FFMI-CXI was consistently associated with survival in both men and women (Supplemental Table 3).
Table 2.
Association between the FFMI-based cachexia index and survival of patients with colorectal cancer.
| FFMI-based cachexia index | Model a | p-value | Model b | p-value | Model c | p-value |
|---|---|---|---|---|---|---|
| Continuous (per SD) | 0.8 (0.67–0.96) | 0.014 | 0.75 (0.62–0.91) | 0.003 | 0.78 (0.63–0.97) | 0.026 |
| Cutoff value | ||||||
| Low FFMI-CXI | Ref | Ref | Ref | |||
| High FFMI-CXI | 0.45 (0.33–0.63) | < 0.001 | 0.45 (0.32–0.63) | < 0.001 | 0.49 (0.34–0.7) | < 0.001 |
| Quartiles | ||||||
| Quartile 1 | Ref | Ref | Ref | |||
| Quartile 2 | 0.77 (0.53–1.11) | 0.162 | 0.55 (0.37–0.81) | 0.002 | 0.56 (0.37–0.84) | 0.006 |
| Quartile 3 | 0.56 (0.37–0.84) | 0.006 | 0.47 (0.31–0.72) | 0.001 | 0.48 (0.3–0.76) | 0.002 |
| Quartile 4 | 0.51 (0.33–0.79) | 0.002 | 0.39 (0.25–0.61) | 0.000 | 0.43 (0.26–0.71) | 0.001 |
| p for trend | 0.001 | < 0.001 | 0.001 | |||
Model a: No adjusted.
Model b: Adjusted for age, sex, BMI, TNM stage.
Model c: Adjusted for age, sex, BMI, TNM stage, surgery, radiotherapy, chemotherapy, hypertension, diabetes, smoking, drinking, family history.
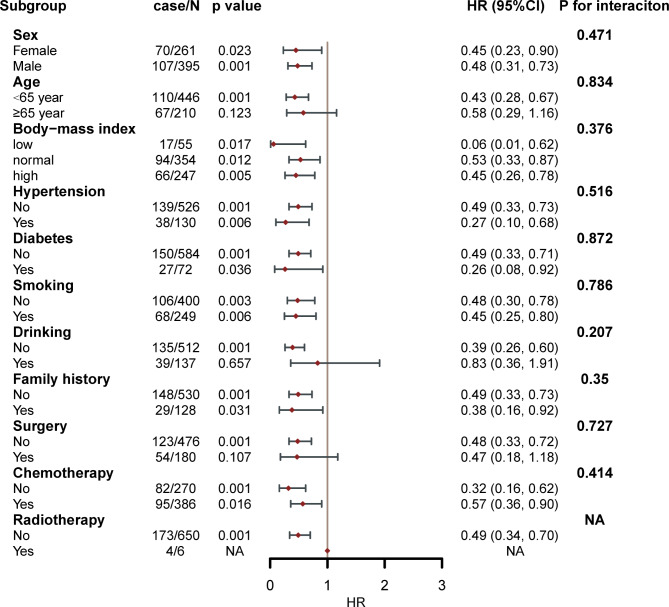
Based on the results of stratified analysis, the FFMI-CXI was found to be associated with stable OS in various stratifications, including sex (men vs. women), age (< 65 years vs. ≥65 years), BMI (< 18 kg/m², 18–24 kg/m², and > 24 kg/m²), hypertension (yes vs. no), diabetes (yes vs. no), smoking status (yes vs. no), family history (yes vs. no), and chemotherapy (yes vs. no) (Fig. 3). FFMI-CXI did not interact with any of the above covariates (all p values for interactions > 0.050). In addition, sensitivity analysis was performed following the exclusion of patients who experienced mortality beyond the 90-day timeframe. We found that a higher FFMI-CXI was strongly linked to a reduced risk of mortality (HR = 0.51, 95% CI 0.35–0.74, p < 0.001) (Supplemental Table 4).
Fig. 3.
The association between FFMI-based cachexia index and hazard risk of overall survival in various subgroups.
Logistic regression analysis
A total of 24 patients (3.66%) experienced 90-day outcomes. According to the multivariable logistic regression, low FFMI-CXI levels were an independent risk factor for 90-day outcomes (OR = 0.37, 95% CI 0.24–0.57; p < 0.001) in patients with CRC. The ORs in the Q2, Q3, and Q4 groups were 0.47, 0.38, and 0.34, respectively, with Q1 serving as the reference group (Supplemental Table 5). A total of 434 (66.16%) patients with CRC were diagnosed with malnutrition, and a PG-SGA score ≥ 4 and a low FFMI-CXI were found to be independent risk factors for malnutrition (OR = 0.36, 95% CI 0.24–0.55; p < 0.001) in patients with CRC. The ORs in the Q2, Q3, and Q4 groups were 0.47, 0.25, and 0.28, respectively, with Q1 serving as the reference group (Supplemental Table 6). Overall, 227 patients (34.60%) were diagnosed with cachexia according to Fearon’s criteria, and FFMI-CXI was an independent factor associated with cachexia (OR = 0.40, 95% CI 0.25–0.62, p < 0.001) (Supplemental Table 7). Moreover, 447 (68.14%) patients with CRC were hospitalized for more than seven days. A decrease in FFMI gradually increased the risk of prolonged hospitalization (OR = 0.60, 95% CI 0.41–0.89; p = 0.010). Using the Q1 group as a reference, the ORs in the Q2, Q3 and Q4 groups were 0.92, 0.69 and 0.69, respectively (Supplemental Table 8). There were 328 (50.0%) patients having hospitalization costs exceeded 17,201.45 yuan. Low FFMI-CXI was associated with increased hospitalization costs for patients with CRC (OR = 0.52, 95% CI 0.35–0.77; p = 0.001). The ORs of the Q2, Q3 and Q4 groups were 0.65, 0.46 and 0.42, respectively, compared with those of the Q1 group (Supplemental Table 9).
Evaluation of the nomogram
A nomogram was constructed based on the FFMI-CXI, sex and tumor stage (Supplemental Fig. S4). There was good agreement between the calibration curves derived from the nomogram and the observed outcomes among patients at 1, 3, and 5 years of OS (Supplemental Fig. S5). As compared to the TNM stage (C-index = 0.72, 95% CI 0.68–0.76), the nomogram’s C-index is significantly higher (0.75, 95% CI 0.72–0.79) (p < 0.001) (Supplemental Table 10). Therefore, using a FFMI-CXI-based nomogram as a guide may lead to greater net benefit in the case of medical intervention triggered at the same probability threshold.
Discussion
In this study of 656 patients, we first explored the prognostic significance of the FFMI-based CXI among individuals diagnosed with CRC. We found that a lower FFMI-based CXI was significantly associated with older age, hypertension, a more advanced TNM stage, malnutrition and high systemic inflammation, reflecting a more serious status of cancer. The Cox regression analysis and Kaplan‒Meier survival curve analysis conducted in this study demonstrated that CRC patients with high FFMI-CXI exhibited significantly prolonged survival compared to those with low FFMI-CXI. Furthermore, the FFMI-CXI is valuable for predicting 90-day outcomes, malnutrition, cancer cachexia, duration of hospitalization and associated healthcare expenditures. A low FFMI-CXI was found to be an independent predictor of the secondary outcomes mentioned above. It may be helpful for clinicians to use these findings in designing effective antitumor therapies. In high-risk cancer cachexia patients with a poor prognosis, FFMI-CXI can be employed as a screening test. FFMI-CXI may serve as a tool for identifying patients at high risk for cachexia and poor prognosis. Subsequently, tailored interventions such as nutritional support, exercise regimens, anti-inflammatory therapies, and adjustments to the intensity of anticancer treatments can be implemented accordingly. Throughout the treatment process, the surveillance of FFMI-CXI trajectories can also facilitate comprehension of the effectiveness of anticancer therapy in cancer patients. In such individuals, adjustments or enhancements to nutritional supplementation, exercise routines, anti-inflammatory interventions, and the intensity of anticancer treatments can be deliberately made if deemed necessary. Tracking the FFMI-CXI trajectory during treatment serves as an additional means to ascertain the effectiveness of anticancer therapy. In addition, our results suggested that patients characterized by high FFMI-CXI levels exhibited a decreased prevalence of cancer cachexia in accordance with Fearon’s criteria, although no significant difference was found, indicating that low FFMI-CXI representative cachexia may differ from the type diagnosed by Fearon et al.
Cachexia remains a challenging clinical condition. Inflammation significantly contributes to cachexia development, which is often accompanied by weight loss, a low BMI, and sarcopenia. Fearon’s criteria are extensively utilized in the diagnosis of cancer cachexia, wherein weight loss constitutes an indispensable criterion. Few studies have explored cachexia’s impact on the survival outcomes of CRC patients, with varying and inconsistent results. For instance, Thoresen et al.14 and Gannavarapu et al.15 showed that cachexia impairs the prognosis of colorectal cancer patients. A recent study by Shibata et al. concluded that cachexia was not a complete predictor of survival for colorectal cancer patients16. Moreover, findings from a cohort study conducted by Brown et al. suggest that maintaining stable body weight does not necessarily imply the absence of muscle loss in patients with CRC17. Consequently, there are still arguments regarding the diagnostic criteria for cachexia among CRC patients, particularly between clinical assessment approaches and the Fearon criteria18,19.
A comprehensive evaluation of sarcopenia, nutrition and systemic inflammation can be accomplished with the CXI. In previous studies, the L3-SMI has been found to be useful in determining the CXI in patients with CRC20,21. The CXI predicts OS better than cachexia, and it could be used as a prognostic indicator in CRC patients. However, the measurement of L3-SMI involves a complex process, which necessitates substantial time and effort20,22,23. Because the process of assessing L3-SMI requires extracting the original files from the system of CT scan and the aid of the software24. All measurements and calculations are performed by trained doctors. Also, dietitians are generally untrained to interpret scans. Therefore, CT images are not routinely used to detect low muscle mass in clinical practice25.
Previous studies attempted to modify CXI for easier use. Rather than using the L3-SMI, L3-psoas muscle index (PMI)20, urea–creatinine ratio (UCR)22, and hand grip strength (HGS)23 were used to modified CXI in order to predict the prognosis of CRC patients. Both PMI- and HGS-based CXIs have shown potential in predicting CRC prognosis. However, earlier studies20,23 did not compare the prognostic performance of the modified CXI with the traditional CXI. The UCR-modified CXI, based on blood markers, has been found to predict CRC prognosis only postoperatively, with the prognostic implications for stage IV CRC patients remaining unclear.
It was suggested that there was a strong correlation between the FFMI and SMI [skeletal muscle area (cm2)/height (m2)] measuring by CT scan on the level of L3 vertebrae (r = 0.997)26. Based on the findings of Marina et al.‘s study, BIA-measured FFMI showed strong positive correlations with both DXA-measured (r = 0.95) and BIA-measured (r = 0.96) appendicular skeletal muscle mass (ASM), regardless of age and obesity status27. Based on previous studies, the FFMI could be used as an alternative to the ASMI for measuring low muscle mass in sarcopenia patients. As an alternative, FFMI, which reflects skeletal muscle mass, bone, organs, and connective tissue, can be used for the clinical diagnosis of sarcopenia28. This is the first study to evaluate the CXI based on the FFMI by the BIA method using the InBody S10 analyzer.
The TNM stage serves as the predominant tool for evaluating both cancer prognosis and progression and is commonly accepted for guiding treatment. Nevertheless, its predictive validity and accessibility have been challenged due to exclusive evaluation of tumor characteristics, while neglecting host-related factors29. FFMI-CXI is an innovative measure of cachexia that is calculated as FFMI (kg/m2) × serum ALB (g/L)/NLR. The three parameters are both objective and readily obtainable via BIA, biochemical tests and routine peripheral blood. In patients with gastrointestinal cancer, the ALB concentration serves as a critical nutritional biomarker30. Hypoalbuminemia can indicate malnutrition induced by cancer and has an adverse effect on patients’ prognosis. The NLR, an inflammation-associated marker, is utilized for the determination of FFMI-CXI. Additionally, the NLR is acknowledged as a marker indicating systemic inflammation associated with CRC31–33. Consequently, the three objective indicators incorporated into the computational formula of FFMI-CXI serve as reflections of the skeletal muscle status, malnutrition, and systemic inflammatory response evident in individuals with cancer cachexia. Our study showed that the combination of TNM stages and FFMI-CXI could increase the precision of discriminating CRC patients with different prognoses.
According to the European Palliative Care Research Collaborative (EPCRC), three stages of cancer cachexia were distinguished: pre-cachexia, cachexia, and refractory cachexia1. Patients with refractory cachexia are unresponsive to anticancer treatment, and their life expectancy is less than three months. The EPCRC highlighted the importance of early detection and intervention during the reversible pre-cachexia and subsequent cachexia phases. Our findings demonstrate that low FFMI-CXI can predict poor survival in CRC patients with or without cachexia. In addition, FFMI-CXI effectively discriminated the prognosis of colorectal cancer patients at stage III + IV. The results showed that FFMI-CXI could serve as a valuable tool for initiating early interventions in individuals afflicted with cachexia.
Several limitations were associated with this study. First, this study was limited to elderly patients in relatively good health who could stand for 90 s on the device. As a result, elderly patients with significant frailty were excluded. Second, the CXI measurement method employed in this study diverged from that utilized in previous studies, and the cutoff value of the CXI based on the FFMI measured by CRC has not been validated. Third, Since SMI data are not available, FFMI-CXI has not yet been shown to be superior to CXI, even though it may be useful in this study. In addition, other known prognostic factors in CRC were not included as variables including the location of the primary tumor (left vs. right CRC) and BRAF gene mutation status, which could potentially influence the interpretation and generalizability of our findings. Future research should incorporate these prognostic factors to enhance the understanding and interpretation of FFMI-CXI’s role in CRC outcomes.
In conclusion, this study revealed that the FFMI-CXI can be used as a prognostic indicator in patients with CRC. And patients with low FFMI-CXI should be given more attention.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We acknowledge the support provided by the National Key Research and Development Program to Dr. Han-Ping Shi (Grants No. 2017YFC1309200 and No. 2022YFC2009600), as well as the funding received from the Beijing Municipal Science and Technology Commission (Grant No. SCW2018-06). We extend our appreciation to all project participants and the numerous members of the study teams situated across diverse study centers, whose contributions facilitated the conduct of this research endeavor.
Author contributions
All authors participated in formulating and designing the study. H.S. provided the funds and supervised the process. The initial version of the manuscript was authored by Y.Q. and H.X..T.L. and H.Z.prepared the structure of the research. Data was collected and analyzed by Y.Q., C.L., J.S., Z.B., and X.L. The manuscript was reviewed and edited by X.L., S.L., Y.C., X.Z., H.Z.J.S. All authors provided feedback on preceding manuscript versions and endorsed the final manuscript.
Data availability
The datasets produced and/or analyzed in the present study are not accessible to the public due to their proprietary nature as private databases. However, they can be obtained from the corresponding author upon reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Yating Qin and Hailun Xie.
References
- 1.Fearon, K. et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 12, 489–495 (2011). [DOI] [PubMed] [Google Scholar]
- 2.Ruan, G. et al. Prognostic value of systemic inflammation and for patients with colorectal cancer cachexia. J. Cachexia Sarcopenia Muscle. 14, 2813–2823 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jafri, S. H. R., Previgliano, C., Khandelwal, K. & Shi, R. Cachexia index in advanced non-small-cell lung cancer patients. Clin. Med. Insights Oncol. 9, (2015). CMO.S30891. [DOI] [PMC free article] [PubMed]
- 4.Porporato, P. E. Understanding cachexia as a cancer metabolism syndrome. Oncogenesis. 5, e200–e200 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xie, H. et al. Development and applicability of modified weight loss grading system in cancer: a real-world cohort study. J. Cachexia Sarcopenia Muscle. 14, 2090–2097 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Go, S. I., Park, M. J. & Lee, G. W. Clinical significance of the cachexia index in patients with small cell lung cancer. BMC Cancer. 21, 563 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goh, M. J. et al. Prognostic significance of cachexia index in patients with advanced hepatocellular carcinoma treated with systemic chemotherapy. Sci. Rep. 12, 7647 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsunaga, T. et al. Prognostic significance of the cachexia index in patients with unresectable advanced gastric cancer receiving palliative chemotherapy: a retrospective single-center study. Surg. Today. 54, 231–239 (2024). [DOI] [PubMed] [Google Scholar]
- 9.Okubo, S. et al. Evaluation of the cachexia index using a bioelectrical impedance analysis in elderly patients with Non-hodgkin’s lymphoma: a single-center prospective study. Ann. Hematol. 103, 823–831 (2024). [DOI] [PubMed] [Google Scholar]
- 10.Aleixo, G. F. P. et al. Bioelectrical impedance analysis for the assessment of Sarcopenia in patients with cancer: a systematic review. Oncologist. 25, 170–182 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang, F., Zhen, H., Wang, H. & Yu, K. Measurement of Sarcopenia in lung cancer inpatients and its association with frailty, nutritional risk, and malnutrition. Front. Nutr. 10, 1143213 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poisson, J. et al. Prevalence and prognostic impact of cachexia among older patients with cancer: a nationwide cross-sectional survey (NutriAgeCancer). J. Cachexia Sarcopenia Muscle. 12, 1477–1488 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ji, W. et al. The efficacy of fat-free mass index and appendicular skeletal muscle mass index in cancer malnutrition: a propensity score match analysis. Front. Nutr. 10, 1172610 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thoresen, L. et al. Nutritional status, cachexia and survival in patients with advanced colorectal carcinoma. Different assessment criteria for nutritional status provide unequal results. Clin. Nutr. 32, 65–72 (2013). [DOI] [PubMed] [Google Scholar]
- 15.Gannavarapu, B. S. et al. Prevalence and survival impact of pretreatment cancer-associated weight loss: a tool for guiding early palliative care. J. Oncol. Pract. 14, e238–e250 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shibata, M., Fukahori, M., Kasamatsu, E., Machii, K. & Hamauchi, S. A retrospective cohort study to investigate the incidence of cachexia during chemotherapy in patients with colorectal cancer. Adv. Ther. 37, 5010–5022 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown, J. C. et al. Weight stability masks changes in body composition in colorectal cancer: a retrospective cohort study. Am. J. Clin. Nutr. 113, 1482–1489 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van der Werf, A., van Bokhorst, Q. N. E., de van der Schueren, M. A. E., Verheul, H. M. W. & Langius, J. A. E. Cancer cachexia: identification by clinical assessment versus international consensus criteria in patients with metastatic colorectal cancer. Nutr. Cancer. 70, 1322–1329 (2018). [DOI] [PubMed] [Google Scholar]
- 19.Bruggeman, A. R. et al. Cancer cachexia: beyond weight loss. J. Oncol. Pract. 12, 1163–1171 (2016). [DOI] [PubMed] [Google Scholar]
- 20.Kamada, T. et al. Prognostic significance of the cachexia index in patients with stage I–III colorectal cancer who underwent laparoscopic surgery. Surg. Today. 53, 1064–1072 (2023). [DOI] [PubMed] [Google Scholar]
- 21.Wan, Q. et al. Prognostic value of cachexia index in patients with colorectal cancer: a retrospective study. Front. Oncol. 12, 984459 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yuan, Q. et al. Developing and validating a modified cachexia index to predict the outcomes for colorectal cancer after radical surgery. Eur. J. Clin. Nutr.10.1038/s41430-024-01469-x (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xie, H. et al. Hand grip strength-based cachexia index as a predictor of cancer cachexia and prognosis in patients with cancer. J. Cachexia Sarcopenia Muscle. 14, 382–390 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown, L. R. et al. Cachexia index for prognostication in surgical patients with locally advanced oesophageal or gastric cancer: multicentre cohort study. Br. J. Surg. 111, znae098 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gort-van Dijk, D. et al. Bioelectrical impedance analysis and mid-upper arm muscle circumference can be used to detect low muscle mass in clinical practice. Nutrients. 13, 2350 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Magri, V. et al. Correlation of body composition by computerized tomography and metabolic parameters with survival of nivolumab-treated lung cancer patients. Cancer Manag Res. 11, 8201–8207 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawakami, R. et al. Fat-free mass index as a surrogate marker of appendicular skeletal muscle mass index for low muscle mass screening in Sarcopenia. J. Am. Med. Dir. Assoc. 23, 1955–1961e3 (2022). [DOI] [PubMed] [Google Scholar]
- 28.Narumi, T. et al. Sarcopenia evaluated by fat-free mass index is an important prognostic factor in patients with chronic heart failure. Eur. J. Intern. Med. 26, 118–122 (2015). [DOI] [PubMed] [Google Scholar]
- 29.Argilés, J. M. et al. The cachexia score (CASCO): a new tool for staging cachectic cancer patients. J. Cachexia Sarcopenia Muscle. 2, 87–93 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gupta, A. et al. Preoperative malnutrition in patients with colorectal cancer. Can. J. Surg. 64, E621–E629 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li, Z., Zhao, R., Cui, Y., Zhou, Y. & Wu, X. The dynamic change of neutrophil to lymphocyte ratio can predict clinical outcome in stage I-III colon cancer. Sci. Rep. 8, 9453 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ming-Sheng, F. et al. Preoperative neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and CEA as the potential prognostic biomarkers for colorectal cancer. Can. J. Gastroenterol. Hepatol. 2022, 1–9 (2022). [DOI] [PMC free article] [PubMed]
- 33.Yamamoto, T., Kawada, K. & Obama, K. Inflammation-related biomarkers for the prediction of prognosis in colorectal cancer patients. Int. J. Mol. Sci. 22, 8002 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets produced and/or analyzed in the present study are not accessible to the public due to their proprietary nature as private databases. However, they can be obtained from the corresponding author upon reasonable request.